Journal of Neurology and Psychology
Download PDF
Review Article
*Address for Correspondence: Alicia K. Smith, PhD, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 101 Woodruff Circle NE, Atlanta, GA 30322, USA, Tel: 404-712-9582; E-mail: alicia.smith@emory.edu
Citation: Ebot Enaw JO, Smith AK. Biomarker Development for Brain-Based Disorders: Recent Progress in Psychiatry. J Neurol Psychol. 2013;1(2): 7.
Copyright © 2013 Enaw JO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 1, Issue: 2
Submission: 09 September 2013 | Accepted: 26 November 2013 | Published: 30 November 2013
Reviewed & Approved by: Dr. David AS Kaufman, Department of Psychology, Saint Louis University, USA
Neuropsychiatric disorders are a leading cause of disability worldwide [4], though their biological basis generally remain unknown. Diagnosis of psychiatric disorders is based on the presence of characteristic symptoms and is guided by the Diagnostic and Statistical Manual (DSM) or the International Classification of Diseases (ICD). These criteria are periodically revised as more is learned about each disorder. Although this system has been widely used with a high degree of inter-rater reliability, it has achieved this at the expense of validity [5,6]. Notable limitations exist with respect to the diagnostic classification of individuals for research purposes. For example, symptom assessment can be subjective [7]. Symptoms can overlap, making it difficult to distinguish similar disorders from each other [8,9], and comorbidity is common. Additionally, criteria established for one ethnic or cultural group may not be applicable to others [10].
Biomarker Development for Brain-Based Disorders: Recent Progress in Psychiatry
James O Ebot Enaw and Alicia K. Smith*
- Department of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta, GA, USA
*Address for Correspondence: Alicia K. Smith, PhD, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 101 Woodruff Circle NE, Atlanta, GA 30322, USA, Tel: 404-712-9582; E-mail: alicia.smith@emory.edu
Citation: Ebot Enaw JO, Smith AK. Biomarker Development for Brain-Based Disorders: Recent Progress in Psychiatry. J Neurol Psychol. 2013;1(2): 7.
Copyright © 2013 Enaw JO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 1, Issue: 2
Submission: 09 September 2013 | Accepted: 26 November 2013 | Published: 30 November 2013
Reviewed & Approved by: Dr. David AS Kaufman, Department of Psychology, Saint Louis University, USA
Abstract
Biomarkers are biological measures that are indicative of a specific disorder, its severity or response to treatment. They are widely used in many areas of medicine, but biomarker development for brain-based disorders lags behind. Using examples from the field of psychiatry, this article reviews the concepts of biomarkers, challenges to their development and the recent progress along those lines. In addition to discussing historical biomarker candidates such as cortisol or catecholamine levels, we include progress from recent genetic, epigenetic, proteomic, neuroimaging and EEG studies. Successful identification of biomarkers will advance the field of psychiatry towards the goal of biological tests for diagnosis, symptom management and treatment response.Keywords
Biomarker; Brain; Psychology; Psychiatry; Neuroimaging; Cortisol; BDNF; GeneticIntroduction
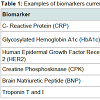
A biomarker is a biological measure that provides information about the state of a normal biologic process, pathogenic process, or pharmacologic response to an intervention [1]. In clinical practice, it must be reliable, reproducible, cost effective, and noninvasive [2,3]. For example, hemoglobin A1c (HbA1c) is measured in peripheral blood, and is widely used to assess glycemic control in patients with diabetes or those at high risk for developing diabetes; it is used both as a clinical diagnostic and for the development of new pharmaceutical treatments. In fact, biomarkers are used widely in a number of fields (Table 1), while others, including brain-based disorders, lag behind.Neuropsychiatric disorders are a leading cause of disability worldwide [4], though their biological basis generally remain unknown. Diagnosis of psychiatric disorders is based on the presence of characteristic symptoms and is guided by the Diagnostic and Statistical Manual (DSM) or the International Classification of Diseases (ICD). These criteria are periodically revised as more is learned about each disorder. Although this system has been widely used with a high degree of inter-rater reliability, it has achieved this at the expense of validity [5,6]. Notable limitations exist with respect to the diagnostic classification of individuals for research purposes. For example, symptom assessment can be subjective [7]. Symptoms can overlap, making it difficult to distinguish similar disorders from each other [8,9], and comorbidity is common. Additionally, criteria established for one ethnic or cultural group may not be applicable to others [10].
Independent of diagnosis, the high cost of healthcare and lost productivity weigh heavily on individuals and their governments worldwide [11,12]. Despite the development of a variety of pharmacological treatments, the remission rate for those with psychiatric disorders remains low [13,14]. Because of these reasons, the field would benefit especially from identification and use of biomarkers that can be used to detect the first episode of a disease, chronic illness, symptom severity, treatment response or nonresponse (Figure 1). There are no biomarkers that are relevant for clinical practice in psychiatry or psychology, but over the past several years, we have seen remarkable progress towards this goal. As the field advances, it is important to review the key criteria a psychiatric biomarker should possess (Table 2).
Central Versus Peripheral Measures
Decades of research support that psychiatric disorders are brainbased, and many argue that the brain is the only place to look for biologically meaningful correlates of brain-based disorders. Studies of postmortem brains provide insight into how those with and without major depressive disorder (MDD) or with and without psychotic symptoms differ on a molecular and functional level [15,16]. Though such studies provide insight into the pathophysiology of MDD, the brain is not generally accessible for molecular testing. Unless the same biological differences are observable in accessible tissues, measures identified in postmortem brains are not practical for biomarker development.A recent study compared results from potential biomarkers measured in postmortem brain and those measured in serum [17]. Though not all molecules were comparable between central and peripheral measures, many were, particularly those that were involved in the inflammatory response. In fact, a number of peripheral systems interact with and respond to signals from the central nervous system (CNS) [18,19]. For example, peripheral inflammatory cytokines can access the brain and interact with numerous pathophysiologic domains relevant to psychiatric illness including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity and mood-relevant neurocircuitry [20,21]. Researchers with an interest in biomarker development often leverage these relationships by focusing on peripheral tissues that can be readily sampled.
Genetic and Epigenetic Biomarkers
Because of the relatively high heritability of a number of psychiatric disorders [22], genetic or epigenetic studies can provide insight into genes that relate to etiology, disease progression and treatment response. While sequence variants may increase risk for a psychiatric disorder, they will not make effective biomarkers because an individual’s genotype is fixed. However, gene expression patterns change over time, and mRNA levels have been associated with psychiatric disorders, symptoms and treatment response. For example, P11 (also known as S100A10) is an annexin II light chain protein that belongs to the S100 family [23,24]. Su and colleagues suggested that P11 expression in peripheral blood cells could be used to differentiate post-traumatic stress disorder (PTSD) from other psychiatric conditions including MDD, bipolar disorder (BPD) and schizophrenia (SCZ) [25]. In addition to full-length transcripts, there is also evidence to suggest that splice variants in peripheral blood mononuclear cells (PBMCs) delineate those with SCZ and BPD from each other and from controls [26]. Gene expression patterns are tissue-specific, but the expression patterns between blood and brain are reasonably correlated [27] suggesting that some expression profiles may not only serve as biomarkers but also reflect brain-based differences as well.Gene expression patterns cannot be detected in all tissues, but many epigenetic modifications can. Epigenetic marks, such as histone modifications, DNA methylation and miRNA, help to regulate gene expression [28-30]. High-throughput technologies have made epigenetic marks easier to assay, and numerous studies have described associations with psychiatric diagnoses, symptoms or treatments [31-34]. This promising area of research has been providing insight into biological mechanisms through which gene expression varies in response to the environment [35]. For example, exposure to antiepileptic medications can promote widespread changes in DNA methylation as well as other epigenetic modifications [36-39]. In addition to medication exposure, preclinical research using animal models and clinical research with human subjects [40-42] supports the hypothesis that exposure to early life stressors may result in DNA methylation changes in the glucocorticoid receptor (NR3C1), a hormone-activated factor that mediates many of the downstream effects of the stress hormone cortisol. These studies are helping to elucidate how environmental factors modify gene regulation to increase risk for some psychiatric disorders [43].
Protein Biomarkers
Dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis is a hallmark of multiple psychiatric illnesses. As stress levels vary so too do the levels of cortisol, which can be assessed in saliva, urine, blood or hair. Changes in serum cortisol level have been reported in MDD as well as many other disorders [44,45]. Cortisol levels decrease in patients with schizophrenia [46]. They also decrease following treatment with an antipsychotic medication [47], and discontinuation of that antipsychotic increases cortisol levels again [48]. Some studies report an inverse relationship between PTSD severity and cortisol levels, while other report no difference [49] or even higher levels [50]. This may be because cortisol also associates with trauma exposure [51,52], a risk factor for depression and other psychiatric disorders [53], making it difficult to distinguish the molecular correlates of an experience that increases risk for a disorder from the disorder itself.Driven by discrepancies in the literature and reports that catecholamine levels also differentiate PTSD from controls, Young and Breslau reported that urinary catecholamines, but not cortisol, levels distinguish those with PTSD from trauma exposed individuals without PTSD or non-exposed subjects [54]. Previous studies have used urinary analysis of catecholamine neurotransmitters (norepinephrine and dopamine) to delineate those with and without depression [55,56] as well as those that respond to treatment [57,58]. Despite being easy to assay and consistent with our present understanding of psychopathology, the measurement of cortisol and catecholamines is not particularly useful in discriminating between disordered and healthy individuals, and thus these measures are not likely to be informative biomarkers.
It is difficult, if not impossible, for a single molecule to discriminate between all potential diagnoses, symptoms and treatment responses across the broad range of human psychopathology. Indeed biomarkers for SCZ have been consistently implicated in a variety of psychiatric disorders as well; the common link is that all of these putative biomarkers have an inflammatory or immune activation component [59,60]. Because of this, investigators have begun to focus on identifying a profile of multiple biological markers to increase predictive capabilities. A recent study compared serum from controls to patients with SCZ, MDD, euthymic BPD and Asperger’s Syndrome [61]. Examination of 51 analytes [62] distinguished the serum from the majority SCZs from those with other psychiatric disorders or controls. This study suggests that profiles of multiple biomarkers may be more successful than examination of a single molecule.
Moving beyond this approach, other investigators advocate the use of proteomic approaches to more comprehensively assess how proteins interact temporally and spatially in a particular disease state [16,63,64]. Because expression products can be extensively modified after translation, proteomic approaches provide insight beyond those offered by genetic or epigenetic measures. In a recent study, a shotgun proteomic experiment compared post-mortem tissue from 24 MDD patients and 12 controls [16]. A large number of proteins were identified that differed between the two groups, many of which had been implicated in other psychiatric disorders. In addition, the proteomic signature successfully differentiated MDD patients with and without psychotic symptoms. Proteomic approaches are applicable to all bodily fluids typically explored in biomarker studies and do so in an unbiased manner that may reveal novel markers [65]. However, to realize its potential to biomarker research, predictive differences detected in the brain must also be observable and predictive in peripheral tissues. Future studies in this area are likely to be highly promising.
Neuroimaging & Electroencephalography Biomarkers
Not all potential biomarkers require sampling of fluid or tissues. Magnetic Resonance Imaging (MRI), diffusion tensor imaging (DTI) and positron emission tomography (PET) provide a remarkable ability to detail the morphological and functional characteristics of the brain without being invasive. For example, a recent meta-analysis of neuroimaging studies identified a number of brain regions whose properties reflect clinically relevant outcomes in depressed subjects and may be appropriate biomarkers [66]. Higher activity levels in the anterior cingulate cortex (ACC) has been observed in patients with MDD and those that are more likely to respond to antidepressants [67,68]; the meta-analysis confirmed that higher baseline activity of the ACC is predictive of clinical improvement [66]. The subgenual ACC is a target for deep brain stimulation, a technique used to treat MDD patients who are resistant to other therapies [69,70].Similarly, higher baseline activation of the insula predicts poor clinical response in patients with MDD [66]. A study examining brain glucose metabolism with PET scans reported that subjects with lower glucose metabolism in the insula were more likely to respond to cognitive behavioral therapy while those with higher glucose metabolism in the insula were more likely to respond to ecitalopram [71]. While replication of this finding is necessary, it could dramatically reduce the time to identify an effective treatment. Neuroimaging studies have substantial potential to produced informative biomarkers for psychiatric disorders, but many studies have small samples sizes and limited power to detect between group differences. For this reason, meta-analyses will be essential as neuroimaging biomarkers move forward. Neuroimaging is promising, but its predictive value is currently limited. It is also quite expensive, limiting the likelihood that it will be adopted for widespread clinical use [72].
Electroencephalography (EEG) measures brain electrical activity via electrodes placed on the head. Oscillation frequencies associate with brain function and are classified by range: <4 Hz (delta), 4-8 Hz (theta), 8-12 Hz (alpha) and 12-30 Hz (beta). EEG is non-invasive, easy to administer, well tolerated, widely available and much less expensive. However, it has lower spatial resolution compared to neuroimaging methods. Despite this, EEG has widely been used in the diagnosis of epileptic seizures, and is being used to identify potential biomarkers of antidepressant response [73]. For example, studies suggest that baseline theta activity associates with treatment response in patients with MDD [74,75]. Treatment response may also associate with hyperactivity of the theta band in the anterior cingulate cortex [76,77], the region of the brain that associated with antidepressant response in neuroimaging studies [67,68]. The observed increased theta in the ACC may reflect increased metabolism in the ACC in response to effective treatment [78]. An increase in alpha wave activity has also been reported in depressed patients who are not on medication [75,79], and those who respond to SSRI treatment have detectable differences in alpha wave characteristics when compared to non-responders [80-82]. These observations support the idea that EEG measures may be informative for tracking treatment response. However, studies with limited power and uncertainty of findings in the light of comorbidity complicate the interpretation of these studies [83-85].
A Candidate Biomarker: BDNF
The literature is full of association studies linking psychiatric disorders to varying environmental exposures, genetic or molecular factors and physiological measurements or traits. However, few of these achieve the level of confidence and reproducibility required to investigate it as a biomarker. One example of a gene that has been evaluated as a potential biomarker is Brain-derived neurotrophic factor (BDNF), a small, basic protein with approximately 50% homology to other known neutrophins [86,87]. As a class, neutrophins have been implicated in the development and survival of sympathetic neurons and neural crest-derived sensory neurons [86,88]. BDNF supports existing neurons and promotes growth and differentiation of new ones [89,90]. It also supports in synaptic plasticity, which is involved for learning and memory [91]. Of relevance to its use as a biomarker, BDNF is expressed in the central and peripheral nervous systems at similar levels [92,93].Changes in BDNF expression or function have been implicated in numerous psychiatric disorders [94-96]. Serum levels of BDNF are lower in subjects with MDD than in healthy controls, but they increase following treatment with an antidepressant [97-100]. DNA methylation of the BDNF promoter in peripheral blood delineates MDD cases from controls without any history of psychopathology [34], and methylation of this region has been proposed as a biomarker for depression [15]. Similarly, BDNF has also been proposed as a biomarker for schizophrenia [97-100]. Serum BDNF levels are lower in schizophrenics and also correlate with both positive and negative symptoms [101]. However, BDNF cannot be considered specific biomarker if it predicts both disorders.
Because of the overlap in core symptoms between psychiatric disorders, many biomarker studies focus on symptoms that change with disease status. Indeed this strategy may be more practical for measuring the progression of an illness or for guiding treatment response. BDNF expression and function may simply represent an underlying symptom or intermediate phenotype common to both MDD and schizophrenia. BDNF responds to stress and changes in brain function [102]. Its levels are tightly correlated with the activity of multiple neurotransmitter systems and may be a final common pathway for some psychotropic medications [100,103]. Thus, while BDNF is promising as a biomarker, further studies will be necessary to define its specific utility.
Future Directions
Psychiatric disorders are very complex and their causes are multifactorial [104-106]. Psychiatric diagnoses are inherently made on clinical grounds based on the presence of a particular group of signs and symptoms that result in distress and/or functional impairment. For biomarkers to become a realizable goal, the field must move beyond its current reliance on diagnosis. One way to do this is to identify endophenotypes, originally defined as an internal phenotype discovered by biochemical test or microbiological test [107]. In contrast to other potential traits of interest, endophenotypes should also be heritable and co-segregate with a disorder [108]. This approach has been useful in providing trait markers of psychiatric and other medicals disorders; however, the underlying etiology of endophenotypes may still be heterogeneous [109].Identification of discrete behavioral or functional characteristics may result in more rigorous study design and greater reproducibility, both for basic and clinical research. The Research Domain Criteria (RDoC) project represents a substantial effort towards accomplishing that goal. RDoC is a concerted effort to classify psychopathology based on distinct observable behavior or neurobiological measures, even if these basic dimensions are common across multiple traditionally defined diagnoses. Efforts such as the RDoC initiative will reduce the heterogeneity in classifying subjects for basic and clinical research, potentially leading to more precise and replicable studies.
In addition to alternate systems of classification, new technological approaches are being leveraged for biomarker studies. For example, magnetoencephalography (MEG) can provide dynamic information on brain activity, but this approach has not been utilized as extensively as EEG or neuroimaging. MEG signals have been used to successfully categorize subjects with different neurological, psychiatric and medical illensses including multiple sclerosis, Alzheimer’s disease, schizophrenia, chronic alcoholism, Sjogren’s syndrome and facial pain. In this study, each illness could be distinguished from each other and from healthy controls with a high degree of confidence [110]. Since that initial study, this approach has been applied to autism [111-113], Alzheimer’s disease [114], as well as migraine and other pain syndromes [115,116]. However, it has been only sparsely applied to studies of psychiatric disorders and treatment response [117,118]. As MEG and other noninvasive technologies are developed and adopted, we are likely to learn more about how healthy brain activity is altered in the context of psychiatric illness.
Conclusions
The need for biomarkers in the field of psychiatry is clear, but progress towards their development has been limited. With the recent advances in high-throughput biological assays and neuroimaging techniques, it seems that the field is on the forefront of a breakthrough that will translate research findings into reliable clinical tests. In all likelihood, development of a panel of biomarkers or a strategy that combines neuroimaging and peripheral measures may be more productive than reliance on a single measure or technique. Clinically informative biomarkers will likely also be limited in scope to a highly specific group of patients or type of treatment. As the field of psychiatry achieves a greater understanding of the molecular or functional signatures of an individual’s diagnosis, relapse, treatment and recovery, access to mental health screening and services will become more widely available.References
- Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89-95.
- Holsboer F (2008) How can we realize the promise of personalized antidepressant medicines? Nat Rev Neurosci 9: 638-646.
- Peedicayil J (2008) Epigenetic biomarkers in psychiatric disorders. Br J Pharmacol 155: 795-796.
- Yach D, Hawkes C, Gould CL, Hofman KJ (2004) The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA 291: 2616-2622.
- Parquet PJ (1995) [Is DSM IV of value in training of psychiatrists?]. Encephale 21: 59-62.
- Hyman SE (2010) The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol 6: 155-179.
- Wakefield JC (2010) Misdiagnosing normality: Psychiatry’s failure to address the problem of false positive diagnoses of mental disorder in a changing professional environment. J Ment Health 19: 337-351.
- Kirkpatrick B (2013) Understanding the physiology of schizophrenia. J Clin Psychiatry 74: e05.
- Friedrich F, Aigner M, Fearns N, Friedrich ME, Frey R, et al. (2013) Psychosis in neurosyphilis - clinical aspects and implications. Psychopathology.
- Strakowski SM, Keck PE Jr, Arnold LM, Collins J, Wilson RM, et al. (2003) Ethnicity and diagnosis in patients with affective disorders. J Clin Psychiatry 64: 747-754.
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, et al. (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 718-779.
- Smith K (2011) Trillion-dollar brain drain. Nature 478: 15.
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, et al. (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209-1223.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905-1917.
- Schneider B, Prvulovic D (2013) Novel biomarkers in major depression. Curr Opin Psychiatry 26: 47-53.
- Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, et al. (2012) Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2: e87.
- Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, et al. (2012) Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One 7: e46368.
- Eskandari F, Webster JI, Sternberg EM (2003) Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther 5: 251-265.
- Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24-31.
- Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732-741.
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, et al. (2009) Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 65: 296-303.
- Bienvenu OJ, Davydow DS, Kendler KS (2011) Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med 41: 33-40.
- The Pink sheet. Chevy Chase, MD: F-D-C Reports. pp. v.
- Gerke V, Weber K (1985) The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J 4: 2917-2920.
- Su TP, Zhang L, Chung MY, Chen YS, Bi YM, et al. (2009) Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. J Psychiatr Res 43: 1078-1085.
- Glatt SJ, Chandler SD, Bousman CA, Chana G, Lucero GR, et al. (2009) Alternatively spliced genes as biomarkers for Schizophrenia, bipolar disorder and psychosis: a blood-based spliceome-profiling exploratory study. Curr Pharmacogenomics Person Med 7: 164-188.
- Sullivan PF, Fan C, Perou CM (2006) Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 141B: 261-268.
- Bonasio R, Tu S, Reinberg D (2010) Molecular signals of epigenetic states. Science 330: 612-616.
- Feil R, Fraga MF (2012) Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13: 97-109.
- Mattick JS, Taft RJ, Faulkner GJ (2010) A global view of genomic information--moving beyond the gene and the master regulator. Trends Genet 26: 21-28.
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, et al. (2011) Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 156B: 700-708.
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, et al. (2010) Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A 107: 9470-9475.
- Dempster E, Viana J, Pidsley R, Mill J (2013) Epigenetic studies of schizophrenia: progress, predicaments, and promises for the future. Schizophr Bull 39: 11-16.
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, et al. (2011) DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One 6: e23881.
- Latham KE, Sapienza C, Engel N (2012) The epigenetic lorax: gene-environment interactions in human health. Epigenomics 4: 383-402.
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, et al. (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20: 6969-6978.
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, et al. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276: 36734-36741.
- Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, et al. (2004) The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 45: 737-744.
- Smith AK, Conneely KN, Newport DJ, Kilaru V, Schroeder JW, et al. (2012) Prenatal antiepileptic exposure associates with neonatal DNA methylation differences. Epigenetics 7: 458-463.
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, et al. (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7: 847-854.
- Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, et al. (2007) The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci 27: 1756-1768.
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, et al. (2008) Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3: 97-106.
- Rutten BP, Mill J (2009) Epigenetic mediation of environmental influences in major psychotic disorders. Schizophr Bull 35: 1045-1056.
- Reus VI, Joseph M, Dallman M (1983) Regulation of ACTH and cortisol in depression. Peptides 4: 785-788.
- Gillespie CF, Nemeroff CB (2005) Hypercortisolemia and depression. Psychosom Med 67: S26-S28.
- Ryan MC, Sharifi N, Condren R, Thakore JH (2004) Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology 29: 1065-1070.
- Markianos M, Hatzimanolis J, Lykouras L (1999) Switch from neuroleptics to clozapine does not influence pituitary-gonadal axis hormone levels in male schizophrenic patients. Eur Neuropsychopharmacol 9: 533-536.
- Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC (2005) Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacology 30: 1532-1538.
- Young EA, Breslau N (2004) Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch Gen Psychiatry 61: 394-401.
- Lindley SE, Carlson EB, Benoit M (2004) Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol Psychiatry 55: 940-945.
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, et al. (2006) Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40: 550-567.
- Bremner D, Vermetten E, Kelley ME (2007) Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis 195: 919-927.
- Brietzke E, Kauer Sant’anna M, Jackowski A, Grassi-Oliveira R, Bucker J, et al. (2012) Impact of childhood stress on psychopathology. Rev Bras Psiquiatr 34: 480-488.
- Young EA, Breslau N (2004) Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch Gen Psychiatry 61: 394-401.
- Roy A, Pickar D, Douillet P, Karoum F, Linnoila M (1986) Urinary monoamines and monoamine metabolites in subtypes of unipolar depressive disorder and normal controls. Psychol Med 16: 541-546.
- Roy A, Linnoila M, Karoum F, Pickar D (1986) Urinary excretion of free tyramine and of norepinephrine and its metabolites in unipolar depressed patients. Biol Psychiatry 21: 221-224.
- Mooney JJ, Schatzberg AF, Cole JO, Kizuka PP, Salomon M, et al. (1988) Rapid antidepressant response to alprazolam in depressed patients with high catecholamine output and heterologous desensitization of platelet adenylate cyclase. Biol Psychiatry 23: 543-559.
- Kotzailias N, Marker M, Jilma B (2004) Early effects of paroxetine on serotonin storage, plasma levels, and urinary excretion: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 24: 536-539.
- Fessel WJ, Solomon GF (1960) Psychosis and systemic lupus erythematosus: a review of the literature and case reports. Calif Med 92: 266-270.
- Goldberg RB (2009) Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 94: 3171-3182.
- Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, et al. (2012) Identification of a biological signature for schizophrenia in serum. Mol Psychiatry 17: 494-502.
- Schwarz E, Izmailov R, Spain M, Barnes A, Mapes JP, et al. (2010) Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights 5: 39-47.
- Reckow S, Gormanns P, Holsboer F, Turck CW (2008) Psychiatric disorders biomarker identification: from proteomics to systems biology. Pharmacopsychiatry 41: S70-S77.
- Patel S (2012) Role of proteomics in biomarker discovery and psychiatric disorders: current status, potentials, limitations and future challenges. Expert Rev Proteomics 9: 249-265.
- Tambor V, Fucikova A, Lenco J, Kacerovsky M, Rehacek V, et al. (2010) Application of proteomics in biomarker discovery: a primer for the clinician. Physiol Res 59: 471-497.
- Fu CH, Steiner H, Costafreda SG (2013) Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis 52: 75-83.
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, et al. (1997) Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8: 1057-1061.
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, et al. (1999) Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res 91: 127-139.
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, et al. (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45: 651-660.
- Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1: 1106-1107.
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, et al. (2013) Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 70: 821-829.
- Iosifescu DV (2011) Electroencephalography-derived biomarkers of antidepressant response. Harv Rev Psychiatry 19: 144-154.
- Harden CL (2002) The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology 59: S48-S55.
- Iosifescu DV, Greenwald S, Devlin P, Mischoulon D, Denninger JW, et al. (2009) Frontal EEG predictors of treatment outcome in major depressive disorder. Eur Neuropsychopharmacol 19: 772-777.
- Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER (1996) Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord 39: 175-184.
- Mulert C, Juckel G, Bruninmeler M, Karch S, Leicht G, et al. (2007) Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci 38: 78-81.
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, et al. (2001) Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158: 405-415.
- Pizzagalli DA, Oakes TR, Davidson RJ (2003) Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology 40: 939-949.
- Pollock VE, Schneider LS (1990) Quantitative, waking EEG research on depression. Biol Psychiatry 27: 757-780.
- Knott V, Mahoney C, Kennedy S, Evans K (2000) Pre-treatment EEG and it's relationship to depression severity and paroxetine treatment outcome. Pharmacopsychiatry 33: 201-205.
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, et al. (2001) Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry 49: 416-425.
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, et al. (2008) Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry 63: 1171-1177.
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, et al. (2000) Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J Abnorm Psychol 109: 797-802.
- Reid SA, Duke LM, Allen JJ (1998) Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology 35: 389-404.
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, et al. (2000) Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology 41: 31-37.
- Barde YA (1989) Trophic factors and neuronal survival. Neuron 2: 1525-1534.
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, et al. (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341: 149-152.
- Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science 237: 1154-1162.
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, et al. (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374: 450-453.
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677-736.
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, et al. (2010) BDNF function and intracellular signaling in neurons. Histol Histopathol 25: 237-258.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ (1998) Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37: 1553-1561.
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, et al. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14: 347-353.
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, et al. (2003) Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 8: 592-610.
- Tsankova N, Renthal W, Kumar A, Nestler EJ (2007) Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 8: 355-367.
- Angelucci F, Brene S, Mathe AA (2005) BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 10: 345-352.
- Brunoni AR, Lopes M, Fregni F (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11: 1169-1180.
- Sen S, Duman R, Sanacora G (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64: 527-532.
- Satomura E, Baba H, Nakano Y, Maeshima H, Suzuki T, et al. (2011) Correlations between brain-derived neurotrophic factor and clinical symptoms in medicated patients with major depression. J Affect Disord 135: 332-335.
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, et al. (2011) Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 16: 1088-1095.
- Chen da C, Wang J, Wang B, Yang SC, Zhang CX, et al. (2009) Decreased levels of serum brain-derived neurotrophic factor in drug-naive first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl) 207: 375-380.
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S (2010) Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig 7: 251-256.
- Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, et al. (2012) Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev 36: 198-205.
- Dick DM (2011) Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol 7: 383-409.
- Levinson DF, Shi J, Wang K, Oh S, Riley B, et al. (2012) Genome-wide association study of multiplex schizophrenia pedigrees. Am J Psychiatry 169: 963-973.
- Middeldorp CM, de Moor MH, McGrath LM, Gordon SD, Blackwood DH, et al. (2011) The genetic association between personality and major depression or bipolar disorder. A polygenic score analysis using genome-wide association data. Transl Psychiatry 1: e50.
- Gottesman, II, Shields J (1973) Genetic theorizing and schizophrenia. Br J Psychiatry 122: 15-30.
- Gottesman, II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636-645.
- Flint J, Munafo MR (2007) The endophenotype concept in psychiatric genetics. Psychol Med 37: 163-180.
- Georgopoulos AP, Karageorgiou E, Leuthold AC, Lewis SM, Lynch JK, et al. (2007) Synchronous neural interactions assessed by magnetoencephalography: a functional biomarker for brain disorders. J Neural Eng 4: 349-355.
- Coskun MA, Loveland KA, Pearson DA, Papanicolaou AC, Sheth BR (2013) Interaction of Finger Representations in the Cortex of Individuals with Autism: A Functional Window into Cortical Inhibition. Autism Res.
- McFadden KL, Hepburn S, Winterrowd E, Schmidt GL, Rojas DC (2012) Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry 12: 213.
- Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, et al. (2010) MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res 3: 8-18.
- Zamrini E, Maestu F, Pekkonen E, Funke M, Makela J, et al. (2011) Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis 2011: 280289.
- Pahapill PA, Zhang W (2013) Restoration of Altered Somatosensory Cortical Representation With Spinal Cord Stimulation Therapy in a Patient With Complex Regional Pain Syndrome: A Magnetoencephalography Case Study. Neuromodulation.
- Chen WT, Lin YY, Wang SJ (2013) Headache frontiers: using magnetoencephalography to investigate pathophysiology of chronic migraine. Curr Pain Headache Rep 17: 309.
- Boutros NN, Galloway MP, Ghosh S, Gjini K, Bowyer SM (2013) Abnormal coherence imaging in panic disorder: a magnetoencephalography investigation. Neuroreport 24: 487-491.
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, et al. (2010) Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology 35: 1415-1422.
- Clyne B, Olshaker JS (1999) The C-reactive protein. J Emerg Med 17: 1019-1025.
- Bleyer JM (1979) Glycohemoglobin (HbA1c): a new blood sugar test and its clinical importance. R I Med J 62: 131-133.
- Gabbay KH, Hasty K, Breslow JL, Ellison RC, Bunn HF, et al. (1977) Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab 44: 859-864.
- Press MF, Jones LA, Godolphin W, Edwards CL, Slamon DJ (1990) HER-2/neu oncogene amplification and expression in breast and ovarian cancers. Prog Clin Biol Res 354A: 209-221.
- Slamon DJ (1990) Studies of the HER-2/neu proto-oncogene in human breast cancer. Cancer Invest 8: 253.
- Shell WE, Sobel BE (1976) Biochemical markers of ischemic injury. Circulation 53: I98-106.
- Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, et al. (1991) Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 87: 1402-1412.
- Foreback CC (1991) Biochemical diagnosis of myocardial infarction. Henry Ford Hosp Med J 39: 159-164.