Journal of Veterinary Science & Medicine
Download PDF
Research Article
*Address for Correspondence: Christelle Fontaine, Companion Animal Medical Department, Virbac, 13ème rue LID, BP 27, 06511 Carros Cedex, France, Tel: +33 (0)4 92 08 76 63; Fax: +33 (0)4 92 08 71 65; E-mail: l: christelle.fontaine@virbac.com
Citation:Schreiber P, Sanquer A, Martin V, Fontaine C, Gueguen S. Safety of Canigen® DHPPi/L(R) Vaccines for Pregnant Bitches and their Offspring. J Veter Sci Med. 2015;3(2): 6.
Copyright © 2015 Schreiber P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 08 October, 2015 | Accepted: 26 October, 2015 | Published: 30 October, 2015
Ethical approval
In the event of the death of a puppy a necropsy was performed to ascertain the cause of death as far as it was possible. The animal caretaker performed general observations of the female dogs and puppies on a daily basis. Additional clinical examinations were performed by the veterinarian if abnormal signs or behaviour were observed.
Safety for pregnant bitches
Vaccination site examination: After the first vaccine overdose administration, two vaccinated animals showed mild and transient swelling where the Leptospira component was injected. This reaction was detected respectively 2 and 3 days following the overdose injection and started to decrease after one day without any treatment. On the clinical examination at days 21, the swelling reactions could no longer be detected. Except for the swelling reaction observed in these two female dogs, no other local abnormalities could be observed in any bitch after any vaccine injection. There was no significant difference (p=0.22) in the incidence of local reactions between the two groups
Growth evaluation: On the first day post-whelping (DPW1), the median puppy body weights were 0.311 kg (Q25%: 280, Q75%: 347) for group V vs 0.326 kg (Q25%: 292, Q75%: 355) for puppies of group C. Puppies of both groups gained weight normally during their 2 first weeks of life, as presented in Figure 3.
General health parameters: Some puppies (1 for Group V and 2 for group C) exhibited signs of diarrhea within the ten first days of life and the entire litters were treated with an association of sulfamethoxazole and trimethoprim antibiotics daily for 6 days. No other abnormal clinical signs could be observed in any of the puppies.
Safety of Canigen® DHPPi/L(R) Vaccines for Pregnant Bitches and their Offspring
Paul Schreiber, Annaële Sanquer, Virginie Martin, Christelle Fontaine* and Sylvie Gueguen
- Companion Animal Medical Department, Virbac, 13ème rue LID, BP 27, 06511, Carros Cedex, France
*Address for Correspondence: Christelle Fontaine, Companion Animal Medical Department, Virbac, 13ème rue LID, BP 27, 06511 Carros Cedex, France, Tel: +33 (0)4 92 08 76 63; Fax: +33 (0)4 92 08 71 65; E-mail: l: christelle.fontaine@virbac.com
Citation:Schreiber P, Sanquer A, Martin V, Fontaine C, Gueguen S. Safety of Canigen® DHPPi/L(R) Vaccines for Pregnant Bitches and their Offspring. J Veter Sci Med. 2015;3(2): 6.
Copyright © 2015 Schreiber P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 08 October, 2015 | Accepted: 26 October, 2015 | Published: 30 October, 2015
Abstract
Introduction: Vaccination during pregnancy is usually not recommended with live vaccines, because of the risks of inducing disease in a pregnant female dog with an impaired immune system, and of infecting growing foetuses and neonates, especially with parvovirus. However, there are cases where pregnant female dogs could be vaccinated, for example an unknown pregnancy or during a disease outbreak in a shelter environment. The aim of this study was to assess the safety of a multivalent canine vaccine, Canigen® DHPPi/ L(R), in pregnant dogs.Materials and methods: A vaccine overdose was administered twice (approximately days 25 and 46 of gestation), to five multiparous pregnant beagle bitches. Five non-vaccinated pregnant beagle bitches served as negative controls.
Results: No abnormal systemic reactions were observed in any of the bitches following the vaccine injections. All pregnancies went to term in both groups. The vaccinated female dogs gave birth to 44 puppies in total, which corresponds to a median litter size of 9 puppies (first quartile: 7, third quartile: 9). In the control group, the total number of puppies was 40, corresponding to a median litter size of 8 puppies (first quartile: 7, third quartile: 8). No significant differences were observed between the groups regarding the number of live puppies at whelping (p = 0.67) and 14 days later (p = 0.75). Vaccination during regnancy did not alter the general health parameters or growth rate of the puppies.
This study confirmed the safety of Canigen® DHPPi/L(R) vaccine, even in the extreme conditions of the repeated administration of overdoses during pregnancy. Canigen® DHPPi/L(R) is therefore a safe multivalent vaccine for pregnant bitches and their offspring.
Keywords
Canigen; Vaccine; Pregnancy; Safety; Dog; FemaleAbbreviations
MLV: Modified Live Vaccine; CDV: Canine Distemper Virus; CAV: Canine Adenovirus; CPV: Canine Parvovirus; CPiV: Canine Parainfluenza Virus; DPW: Day Post-Whelping; MDA: Maternally Derived Antibodies; Group V: Vaccinated Group; Group C: Unvaccinated Control Group; Q25%: First Quartile; Q75%: Third QuartileIntroduction
To vaccinate a dog is a common act for veterinarians, aiming to protect an individual animal against one or several infectious diseases and also to provide optimum “herd immunity” that minimizes the likelihood of an infectious disease outbreak [1]. There are several types of vaccines: modified live vaccines (MLV), inactivated vaccines, subunit vaccines and recombinant vaccines. Among them, the MLV are the only ones containing the live native agents. The advantage of the MLV is that the live strains multiply [2] in the animal and stimulates both antigen specific T and B lymphocytes [3]. This type of vaccine is more effective [4] and induces a quicker immune response [2] than inactivated vaccines. Because of these properties, MLV are recommended for the routine canine “core” vaccines: canine distemper virus (CDV), canine adenovirus (CAV), and canine parvovirus (CPV) [1]. During the vaccine development, studies are performed to ensure that the attenuated MLV strains cannot revert to virulence. Canigen® DHPPi/L(R) is a multivalent vaccine composed of MLV cores strains and canine parainfluenza virus (CPiV) with inactivated Leptospira and rabies fractions. After use of millions of doses in the field, there is no doubt regarding the extreme safety of Canigen® DHPPi/L and Canigen® DHPPi/LR for dogs when used according to label instructions.However, even if vaccination of pregnant animals is usually not recommended, there are cases where pregnant female dogs could be vaccinated such as during a disease outbreak in a shelter environment [1], or in the case of an unknown pregnancy. Pregnancy is a particularly sensitive physiological status [5] that could involve increased risks for the mother [6], but more importantly for the foetuses [7,8], especially with parvovirus [9,10]. Indeed, as MLV strains replicate, they could theoretically contaminate the multiplying cells in the tissues of growing foetuses and cause developmental problems [11,12] or even revert to virulence [13].
The purpose of this study was therefore to assess the safety of a multivalent MLV-containing vaccine, Canigen® DHPPi/L(R), when administered to pregnant female dogs, for the female herself and her progeny.
Materials and Methods
The study was approved by the Ethical Review Committee of Virbac, France, under the reference number EU-ERC/201410-02.
Animals and study protocols
Ten healthy, multiparous, pregnant eagle bitches, aged five years or less, were selected for inclusion. Only bitches that did not have a history of problems with previous pregnancies (abortions, stillbirth, foetal abnormality or neonatal abnormality) were included. The selected dogs were identified by tattoo numbers. Pregnancy of about 25±3 days duration was confirmed prior to inclusion by ultrasound examination and visualisation of the embryonic vesicles which were counted and measured, when possible. The trial was performed in field conditions, in a breeding colony. The animals were fed ad libitum on a commercial diet for adult dogs and a diet suitable for puppies was added in the whelping facility. They had free access to water.
Non-inclusion criteria included any systemic antibiotic or corticoid treatment received in the 6 weeks before the beginning of the study. Localised treatments such as otic and ophthalmic preparations were permitted as were routine deworming treatments.
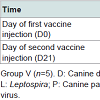
The bitches were randomly allocated to receive vaccination (Group V, n=5) or remain unvaccinated as controls (Group C, n=5). In order to be compliant with the European Pharmacopeia requirements in force when performing the study, overdoses of vaccine were administered through the recommended primary vaccination scheme. Group V bitches were vaccinated by subcutaneous injection twice at a 3 week interval, on day 0 and day 21 as shown in Table 1. The vaccines were administered to several specified sites (left and right sides of the shoulders, of half-way between shoulder and hip, and of the hips), with 2 ml per injection site to limit the local impact of high injection volumes.
The vaccines used were Canigen® DHPPi/L (Virbac, France) and Canigen® DHPPi/LR (Virbac, France). The freeze-dried part of both vaccines, containing the live attenuated strains of CDV, CAV type 2, CPV and CPiV; was reconstituted with either:
• Sterile water for injection, or
• The liquid fraction of Canigen® DHPPi/L containing a suspension of inactivated Leptospira canicola and Leptospira icterohaemorrhagiae or
• The liquid fraction of Canigen® DHPPi/LR containing a suspension of inactivated Leptospira canicola and Leptospira icterohaemorrhagiae and rabies. The vaccine vials were stored at 5±3 °C.
Monitoring
In the vaccination phase, female dogs were monitored at the time of vaccination, 4 hours after vaccination and then daily during the 4 consecutive days for signs of abnormal local and systemic reactions. General parameters such as bodyweight, rectal temperature, heart and respiratory rates were recorded. The general status was also evaluated by the observation of the following parameters: behaviour, ocular mucosa, dehydration, lymph nodes, appetite and faeces aspect of dogs. The time of occurrence, nature - pruritus, swelling nodules, size and duration of local reactions appearing at vaccination were recorded. Ultrasound examination was repeated approximately five days after the first vaccination to confirm the pregnancy diagnosis.
Immediately after whelping, the number of live, still born and mummified pups delivered by each bitch was recorded. Puppies were weighed at one, three, seven, ten and fourteen days of age as mentioned in Table 2. The veterinarian performed additional thorough clinical examinations of the puppies at the ages of 1 and 14 days. A specific evaluation of the umbilical region and recording of eye opening were performed in addition to the general health parameters evaluated during the clinical examination of the pregnant female dogs.
Statistical tests
Statistical analyses were performed with the software S-PLUS. The proportion of bitches presenting local or systemic reactions associated with vaccination was compared between the vaccinated and the non-vaccinated groups using a Fisher’s exact test.
The median number of live puppies delivered per bitch on day zero post-whelping (DPW0) and the median number of live puppies reaching 14 days of age was compared between the vaccinated and non-vaccinated control animals using a Wilcoxon rank sum test.
The level of significance was set at p ≤ 0.05.
Results
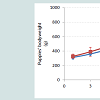
General health parameters: No abnormal systemic reactions were observed in any of the bitches following the vaccine injections. All individual rectal temperatures were within the normal physiological range. The time course of the group medians of the rectal body temperatures following the administration of the vaccine overdoses is presented in Figure 1.
Safety for puppies
Survival rates: All pregnancies went to term in both groups. The vaccinated female dogs gave birth to 44 puppies in total, which corresponds to a median litter size of 9 puppies (first quartile (Q25%): 7, third quartile (Q75%): 9). In the control group, the total number of puppies was 40, with a median litter size of eight puppies per female dog (Q25%: 7, Q75%: 8).
Two puppies were stillborn, one in each group, representing a stillbirth rate of 2.27% for the vaccinated group and 2.50% for the control group.
One puppy belonging to the group of vaccinated bitches died because of accidental evisceration by the bitch after birth at DPW0. The median number of live puppies per female dog at DPW0 remained unchanged in both groups, but the Q25% for the control group was reduced to 6. During the following 14 days, 1 other pup from group V and three puppies from group C died.
At day 14 post-whelping (DPW14), the median number of live puppies per female remained unchanged for the vaccinated group, but the Q25% was decreased from 7 to 6. In the control group however, the median number of live puppies was decreased to 6, without any modification of the Q25% and Q75% as presented in Figure 2. The statistical analysis of the results did not demonstrate any significant difference between the vaccinated group and the control group regarding the number of live puppies at DPW0 (p = 0.67) or DPW14 (p = 0.75). Stillbirths excluded, the overall mortality rates for the puppies at DPW14 were 4.65% (3/44 puppies) for group V compared to 7.69% (4/40 puppies) in the control group.
Discussion
According to the current international vaccination guidelines of dogs and cats, to vaccinate a pregnant animal is not recommended, except in a disease outbreak situation and if the pregnant female has, either, never been vaccinated before or if her vaccination history is unknown [1]. In this extreme situation, if it is not possible to ensure the protection of the pregnant animal and the growing foetuses by use of strict hygiene measures and isolation, the benefit:risk ratio will often remain in favour of vaccination even if no safety and efficacy data are available. The female dogs used in this study were vaccinated every year, as part as their annual prophylactic programme. As could be expected, serological assays performed just before the beginning of the study (before vaccination), revealed that all included bitches had still antibodies against CDV, CAV-2 and CPV (data not shown), meaning that the bitches had not yet lost their protection against the core diseases. The presence of antibodies, at the latest one year post vaccination in this study, was expected as core MLV provide a protection for at least 3 years in dogs [1]. Using annually vaccinated and still-protected pregnant female dogs in this study provides a useful answer to general practitioners who may accidentally vaccinate a female dog with an unknown pregnancy status.Intentional vaccination of a pregnant female dog is usually not primarily with the objective of protecting the female dog against infectious diseases. The main benefits, but also risks, would be indeed for the puppies. On one side, several live organisms are able to cross the placental barrier and infect the foetuses. Infection could possibly induce embryonic resorption, foetal death, stillbirth of term foetuses [88,14,15], or developmental issues [12]. On the other side, for diseases such as parvovirus, the risk of infection could be higher in shelter environments than in private pet household [16]. In the majority of cases, the conventional vaccination protocol in non-pregnant bitches can ensure an adequate transfer of maternally derived antibodies (MDA) in subsequent offspring. However, in some circumstances, vaccination of a pregnant female is expected to increase the level of MDA transferred to at least some of the neonates [18,19]. In any case, a primary vaccination protocol starting at 6 or 8 weeks of age with two injections 3 to 4 weeks apart and a last injection at 16 weeks of age should ensure the active immunisation of all puppies independently of the level of MDA [11,18].
In line with what was observed in other species [20,21], no relevant side effect susceptible to impair the gestation course was observed. Despite the administration of an overdose, the transient local swelling reactions reported in the study could be a consequence of a “normal” inflammatory reaction and are in accordance with the effects of Canigen DHPPi/L mentioned on the vaccine’s summary of product characteristics.
In this study, the MLV overdose was equivalent to ten times the normal dose to increase the risk of the living vaccine strains over passing the immune system and infecting the foetuses. Except for the doses used, the vaccination protocol was the official complete protocol for dog primary vaccination with Canigen®vaccines in France.
The vaccine overdose administrations during pregnancy did not negatively impact the puppies outcome in the study. The number of puppies per litter was even higher in the vaccinated group compared to the non-vaccinated group, which can explain the slightly lower median body weight on DPW1 [22]. In addition, the puppies did not show any abnormal clinical signs and grew normally during the two weeks after birth. MLV spread from the dam was either completely absent or did not induce any disease in the neonates.
The absence of relevant side effects after the administration of Canigen® DHPPi/L(R) overdoses in this study is reassuring regarding the high safety of this modified live and inactivated combo vaccine when administered by accident to a pregnant female dog.
This study demonstrated that vaccination of pregnant female dogs with Canigen® DHPPi/L(R) is safe for the dam, growing foetuses and neonates. No relevant systemic reactions or increased risk of local reactions were observed in the vaccinated dams following vaccination. The reproductive parameters (number of live puppies at whelping and 14 days after) were not altered by the use of this MLV containing vaccine during pregnancy. Vaccination during pregnancy did not alter the general health parameters and growth rate of the puppies. Canigen® DHPPi/L(R) is therefore a safe multivalent vaccine even if used in pregnant bitches.
References
- Vaccination Guidelines Group, Day MJ, Horzinek MC, Schultz RD (2010) WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract 51: 1-32.
- American Animal Hospital Association (AAHA) Canine Vaccination Task Force, Welborn LV, DeVries JG, Ford R, Franklin RT, et al. (2011) 2011 AAHA canine vaccination guidelines. J Am Anim Hosp Assoc 47: 1-42.
- Greene CE, Schultz RD (2006) Immunoprophylaxis. In infectious diseases of the dog and cat, (3rd edn). Saunders Elsevier, 1070-1119.
- Digangi BA, Levy JK, Griffin B, Reese MJ, Dingman PA, et al. (2012) Effects of maternally-derived antibodies on serologic responses to vaccination in kittens. J Feline Med Surg 14: 118-123.
- Morein B, Blomqvist G, Hu K (2007) Immune responsiveness in the neonatal period. J Comp Pathol 137 Suppl 1: S27-S31.
- Sappenfield E, Jamieson DJ, Kourtis AP (2013) Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol 2013: 752852.
- Day MJ (2007) Immune system development in the dog and cat. J Comp Pathol 137 Suppl 1: S10-S15.
- Wilbur LA, Evermann JF, Levings RL, Stoll IR, Starling DE, et al. (1994) Abortion and death in pregnant bitches associated with a canine vaccine contaminated with bluetongue virus. J Am Vet Med Assoc 204: 1762-1765.
- Haun L, Kwan N, Hollier LM (2007) Viral infections in pregnancy. Minerva Ginecol 59: 159-174.
- Thiry E (2002) Virologie clinique du chien et du chat. Editions du point veterinaire, Maisons-Alfort, France.
- Krakowka S, Hoover EA, Koestner A, Ketring K (1977) Experimental and naturally occurring transplacental transmission of canine distemper Virus. Am J Vet Res 38: 919-922.
- Aeffner F, Ulrich R, Schulze-Rückamp L, Beineke A (2006) Cerebella hypoplasia in three sibling cats after intrauterine or early postnatal parvovirus infection. Dtsch Tierarztl Wochenschr 113: 403-406.
- Salsbury D (1976) Vaccine-induced blue-eye: another old wives tale that should be laid to rest. Vet Med Small Anim Clin 71: 1397-1398.
- Hashimoto A, Hirai K, Suzuki Y, Fujimoto Y (1983) Experimental transplacental transmission of canine herpesvirus in pregnant bitches during the second trimester of gestation. Am J Vet Res 44: 610-614.
- Carmichael LE, Schlafer DH, Hashimoto A (1991) Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet 81: 151-171.
- Levy J (2009) Outbreak intervention: canine and feline parvovirus. Proceedings of the SEVC South Eur Vet Conf.
- Cave TA, Thompson H, Reid SW, Hodgson DR, Addie DD (2002) Kitten mortality in the United Kingdom: a retrospective analysis of 274 histopathological examinations (1986 to 2000). Vet Rec 151: 497-501.
- Poulet H (2007) Alternative early life vaccination programs for companion animals. J Comp Pathol 137 Suppl 1: S67-S71.
- Hur J, Lee JH (2012) Development of a novel live vaccine delivering enterotoxigenic Escherichia coli fimbrial antigens to prevent post-weaning diarrhea in piglets. Vet Immunol Immunopathol 146: 283-288.
- Ellsworth MA, Brown MJ, Fergen BJ, Ficken MD, Tucker CM, et al. (2003) Safety of a modified-live combination vaccine against respiratory and reproductive diseases in pregnant cows. Vet Ther 4: 120-127.
- Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, et al. (2014) Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 32: 7057-7064.
- Scantlebury M, Butterwick R, Speakman JR (2001) Energetics and litter size variation in domestic dog Canis familiaris breeds of two sizes. Comp Biochem Physiol A Mol Integr Physiol 129: 919-931.