Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: Mirela Roman, Institut Jules Bordet, Université Libre de Bruxelles,Rue Héger-Bordet, 1, 1000 – Bruxelles, Tel: 003225413164; Fax: 003225413131; E-mail: mirela-mariana.roman@bordet.be
Citation: Roman M, Nogaret JM, Fils JF, Bourgeois P. Seromas and Punctures after Complete Axillary Node Dissection for Breast Cancer: Differences between Mastectomy and Lumpectomy. J Surgery. 2015;3(2): 7.
Copyright © 2015 Roman M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 3, Issue: 2
Submission: 17 November 2015 | Accepted: 11 December 2015 | Published: 15 December 2015
Compared with the L group, the M group patients had significantly longer Dp (median = 26 days, but up to 449 days, vs. 11.5 days) and a higher Np (median = 4, but up to 21, vs. 2), VpTot (median = 1000 ml, but up to 9045 ml, vs. 300 ml), VpMp (median = 233 ml, but up to 705 ml, vs. 120 ml), VpMd (median = 32.4 ml, but up to 162 ml, vs. 17.9 ml), VdTot (median = 390 ml, but up to 1460 ml, vs. 227 ml), and VdMd ( 88,9 ml -but up to 212- vs. 60.4 ml).
We next examined which of the measured variables at baseline significantly predicted outliers, which were defined as patients having a high Np and a high post-operative VdTot; thus, patients in Clusters 1 and 2.In this whole series, these “outliers” were related as follows:
Seromas and Punctures after Complete Axillary Node Dissection for Breast Cancer: Differences between Mastectomy and Lumpectomy
Mirela Roman2,3*, Jean-Marie Nogaret3, Jean- Francois Fils4 and Pierre Bourgeois1,2
- 1Department of Nuclear Medicine, Institut Jules Bordet, Université Libre de Bruxelles
- 2Clinic-Unit of Lymphology, Institut Jules Bordet, Université Libre de Bruxelles
- 3Department of Mammo - Pelvic Surgery, Institut Jules Bordet, Université Libre de Bruxelles
- 4Ars Statistica, Belgium
*Address for Correspondence: Mirela Roman, Institut Jules Bordet, Université Libre de Bruxelles,Rue Héger-Bordet, 1, 1000 – Bruxelles, Tel: 003225413164; Fax: 003225413131; E-mail: mirela-mariana.roman@bordet.be
Citation: Roman M, Nogaret JM, Fils JF, Bourgeois P. Seromas and Punctures after Complete Axillary Node Dissection for Breast Cancer: Differences between Mastectomy and Lumpectomy. J Surgery. 2015;3(2): 7.
Copyright © 2015 Roman M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 3, Issue: 2
Submission: 17 November 2015 | Accepted: 11 December 2015 | Published: 15 December 2015
Abstract
Background:“Seromas” represent a frequent complication after complete axillary lymph node dissection (CALND) for breast cancer. The aims of this work were to analyze patients with seromas at our institution and to try to define patients at risk for such events.Methods:The medical reports of 223 women who underwent CALND after mastectomy (n=127) or lumpectomy (n=96) for breast cancer and who were followed in our institute were retrospectively reviewed to obtain the following: the characteristics (volume and duration) of the drained seromas; the number, volume, and duration of punctures performed after hospital discharge; the patient’s age and body mass index; the presence or absence of hypertension (HTA); the pT of the tumor, the TNM stage, the number of axillary lymph nodes removed (nLN), the number of positive LN, the associated treatments (the pre and post-operative chemotherapy or not); and whether or not there was an infection at the level of the breast and/or arm.
Results:Only 18.75% of the patients after lumpectomy and 9.45% after mastectomy did not have a puncture for seroma after hospital discharge. The patients who had a mastectomy with CALND had a significantly higher number of punctures (Np), longer duration, and higher volumes than those who had a lumpectomy. The risk of infection significantly increased with the Np.
Conclusion:This institutional survey highlights the problem of post-operative seromas and their related punctures. The seromas were statistically more frequent after mastectomy than lumpectomy. Therefore, in the future, neo-adjuvant approaches with conservative surgeries are recommended. Our analysis identified an abnormally high Np and/or total puncture volume (VpTot) as outliers.
Keywords
Seromas; Breast cancer; Axillary dissection; Mastectomy; Lumpectomy; Cluster analysisAbbreviations
BMI: Body Mass Index; CALND: Complete Axillary Lymph Node Dissection; Dp, Duration of puncture; HTA: Hypertension; L group: Lumpectomy group; LPA: Latent Profile Analysis; M group: Mastectomy group; nLN: number of axillary Lymph Nodes; Np: Number of punctures; VdTot: total discharge volume; VdMd: mean discharge drained per day; VpMd: mean puncture volume per day; VpMp: mean volume per puncture; VpTot: total puncture volume per day; pTNM: pathological examination of the size of the primary tumor “T”, of the regional lymph node “N” and of presence of distant metastasis “M”Introduction
The first mastectomy was carried out by Halsted in 1882 and since then surgeons have faced several problems such as necrosis of the skin flaps, breakdown of the wound, hematoma, infection, and seroma [1]. Among these, seroma formation is the most frequent postoperative complication seen after breast cancer surgery, with an incidence of 3% to 85% [2]. It is so common that many surgeons view seromas as an unavoidable nuisance rather than a serious complication [1,3]. It is unclear if their pathophysiogenesis is of lymphatic origin (also calledlymphocele) and/or “simply” related to inflammatory exudates, with various predictive factors being proposed. Seromas may require repeated and long lasting punctures, which can be complicated by infections that affect all patients’ quality of life. The aims of this work were to analyze the seroma situation at our institution and to try to define patients at risk for such events (formation of seromas, their punctures, and the related infections), especially comparing patients who underwent a mastectomy vs. a lumpectomy.Materials and Methods
From 02/2012 to 09/2014, we retrieved 223 women from our institutional database who had undergone complete axillary lymph node dissection (CALND) either after mastectomy (M group: n = 127) or lumpectomy (L group: n = 96) for breast cancer and who were followed in our institute. Their medical reports were reviewed to obtain the following: the number of punctures (Np) performed, their volumes (summed to obtain the total volume [VpTot] and the mean volume per puncture [VpMp]), the duration of the first and the last punctures reported (Dp) to obtain the “volume per day” (VpMd), the patient’ age and body mass index (BMI), the presence or absence of hypertension (HTA), the date and kind of surgery (mastectomy/lumpectomy), the pT and the TNM staging, the number of axillary lymph nodes removed (nLN), the number of positive LN, the associated treatments (chemotherapy, either preoperative, or postoperative) or not, the volumes of liquids drained after surgery and before hospital discharge (total drainage volume [VdTot] and mean drainage volume “per day” [VdMd]), and the occurrence or absence of infection at the level of the breast and/or arm. Our definition of seroma is “any collection of liquid requiring at least one puncture after hospital discharge”.Operative procedures
All surgical procedures were performed under general anesthesia by a specialist surgeon. The modified radical mastectomy was performed according to Madden [4], but with a transverse incision. The CALND was performed between standard anatomic borders. In case of a lumpectomy, two separate incisions were made. At the end of surgery, two suction drains were placed in the axilla and on the chest, respectively. No attempts were made to close the dead space in the axilla or the breast wound by additional measures.
Postoperative procedures
Drainage volumes were registered daily. Each drain was removed when their fluid production was less than 50 ml per day. Each patient was seen 1 week after discharge and then weekly or more frequently as needed. After the drain was removed, any clinically evident fluid collection in the axilla and/or in the breast wound was removed by percutaneous aspiration. The VpTot and Np were noted. Wound infection was defined as an inflamed wound with positive microbiology that needed antibiotic treatment.
Statistical analysis
Descriptive statistics are reported by group (M vs. L). For binary data, counts and percentages are presented and p-values were calculated using the Fisher’s exact test. The mean and standard deviation or median and interquartile range are reported, and p-values were calculated with the help of the Student T-Test or the Wilcoxon signer rank test, as appropriate. All statistical analyses were performed with R Software, version 3.0.1 (R Core Team, 2015 [5]).
We used the R package “mclust” [6] to perform a latent profile analysis (LPA) of the next variables: Np, total volume, duration of punctures, puncture volume, and mean volume, to mathematically detect outliers. The LPA is a method for analyzing the relationships among continuous manifest data [7-10] and tries to find how many clusters of observations/patients may be found based on collected data. The Bayesian Information Criteria (BIC) are used to determine how many clusters have to be retained: the higher the BIC, the better the fit of the model.
Based on the obtained clustering, outliers were defined and characterized by means of logistic regression and its associated ROC curve.
Finally, we investigated whether the Np was significantly different in the two groups emerging from the LPA (normal values vs. outliers). A Wilcoxon signed rank test was performed because the residuals of the linear model were not normally distributed.
Results
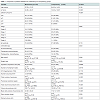
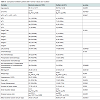
In the whole series, only 30 of 223 (13.45%) patients did not have a puncture after hospital discharge with the statistical analysis suggesting a borderline difference between the M group (9.45%) and L group (18.75%).The M group and L group showed no statistical differences with regard to the following characteristics: right and left side of surgery, age, weight, HTA, nLN removed, the pN positive status and a borderline difference with regard to the body mass index (BMI) and the drain duration (Table 1). There was a significantly difference between groups M and L patients with regard to size of the tumor (pT), TNM staging and the administration or not of one chemotherapy (Table 1).
Compared with the L group, the M group patients had significantly longer Dp (median = 26 days, but up to 449 days, vs. 11.5 days) and a higher Np (median = 4, but up to 21, vs. 2), VpTot (median = 1000 ml, but up to 9045 ml, vs. 300 ml), VpMp (median = 233 ml, but up to 705 ml, vs. 120 ml), VpMd (median = 32.4 ml, but up to 162 ml, vs. 17.9 ml), VdTot (median = 390 ml, but up to 1460 ml, vs. 227 ml), and VdMd ( 88,9 ml -but up to 212- vs. 60.4 ml).
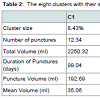
Among the 193 patients with at least one puncture after hospital discharge, the Np, VpTot, Dp, VpMp, and VpMd were used to draw one LPA model. The BIC criteria indicated that the solution with eight clusters (group of patients with same characteristics of their “punctures”) had a better fit to the data.
The data in Table 2 indicate that clusters (patient groups) 1 and 2 were dominated by a high Np, VpTot, Dp, VpMp, and VpMd. Clusters 3 and 4 had a moderate Np for a high VpTot. Clusters 5 and 7 had a lower Np (around three) and there was a higher VpTot in cluster 5 (397) than cluster 7 (274). Clusters 6 and 8 had only one puncture and there was a higher VpTot in cluster 6 (185) compared with cluster 8 (37).
We next examined which of the measured variables at baseline significantly predicted outliers, which were defined as patients having a high Np and a high post-operative VdTot; thus, patients in Clusters 1 and 2.In this whole series, these “outliers” were related as follows:
- In univariate analysis, the outliers were related to the nLN, VdTot, the VdMd, and to having a mastectomy.
- In multivariate analysis, the outliers were related only to having a mastectomy and the nLN, but with an AUC of only 0. 7219 (Figure 1).
The risk of infection statistically significantly increased with the Np (Figure 2). The Wilcoxon-signed rank test (W = 1219.5, p-value = 0.003058) indicated that patients in the infection group had a higher Np (median=5.5, q25=2, q75=6) than patients without infection (median=3, q25=5, q75=11).
Discussion
Serous fluid collections in the axillary dead space or over the anterior chest wall are known as seromas, and represent the most common complication following breast cancer surgery [2]. Definitions of seromas are highly variable [11-15]. However, the problem might be considered only taking into account the out hospital patients period, the “real” practical problems. Our definition of seroma is “any collection of liquid requiring at least one puncture after hospital discharge”. That is the reason why in our material and methods, we focused mainly on the problem of the punctures and the other related problems. In our series, only 13.4% of the patients did not have a puncture after hospital discharge. Therefore, we tried to identify smaller groups of patients characterized by “unusual” patterns related to their seromas. The statistical approach used in this article, namely defining outliers with a mathematically driven clustering method, LPA, is relatively original [16] with one other report based only on breast cancer patients [17]. With this approach, we identified 13.45% of our patients as “outliers”, characterized by a high Np (six or higher) and a high post-operative VdTot (≥ 2250 ml). These outliers are especially interesting for future studies.Several risk factors and predictors for seromas have been proposed: age, breast size, HTA, size of the tumor, nLN removed, pathological nodal status, number of positive LNs, previous surgical biopsy, the use of heparin or tamoxifen the use of preoperative chemotherapy, and whether intraoperative lymphatic channel ligation was done or not [11,15,18-22]. However, this literature, on the problem of seroma formation and their related consequences (punctures and infections), although relatively abundant, seems limited to the immediate postoperative period (before hospital discharge) [22-25]. In addition, few papers specifically analyzed the problem of punctures after hospital discharge [26,27]. With the evolution toward more and more conservative surgical approaches for patients who undergo CALND, the data on the difference (if any) between mastectomy and lumpectomy also appear “contradictory” [22,24,28].
The studies from Lumachi et al. and Vinton et al. considered that seroma formation after mastectomy is more common than after lumpectomy [22,24]. Petrek et al. predicted that modified radical mastectomy would be associated with greater fluid formation than axillary dissection [26], because additional fluid is the result of the mastectomy flap dissection. On the other hand, it was unclear to Burak et al. why lumpectomy/axillary dissection is associated with high rates of seroma formation [28]; in addition, Bonnema et al. found no higher incidence of seromas after modified radical mastectomy compared with lumpectomy and axillary node dissection [23]. In our study, we observed that the duration and volume of postoperative fluid formation are significantly longer and higher for the patients who had a modified radical mastectomy than those who had a breast conserving surgery with CALND.
With respect to axillary dissection, several studies reported that the nLN does not influence seroma formation [22,28-30] whereas others showed that the nLN influenced seroma formation [15,26,31]. On the contrary, our results showed that patients with a higher nLN and patients having a mastectomy are more vulnerable and are at higher risk of being an outlier (patients with a high Np and a high postoperative VdTot). The specific problem of punctures after hospital discharge was only analyzed by Petrek et al. [26], but they reported that there was no statistically significant difference between “only axillary dissections” and “modified radical mastectomy”. In contrast, the present data reveal a significantly higher Np, Dp, VpTot, VpMd, VdMd, and VdTot in the group of patients who had mastectomy.
In univariate analysis, the outliers were also related in our series to the VdTot and the VdMd.
The meta-analysis by Kuroi et al. was inconclusive for total drainage volume, total drainage volume during the initial 5 postoperative days or total [20].
In multivariate analysis, our outliers were related only to having a mastectomy and the nLN. Th e ROC curve and the AUC (0.7219) indicate a moderate fit of the model to the data. Therefore, the classification error using this model remains important, indicating that other variables not taken into account may play a key role in the prediction of outliers, as defined above.
The frequency of wound infections in patients treated for breast cancer varies in the literature from 1% -13% for lumpectomy [24,32] to 4%-18% for modified radical mastectomy [33,34]. However, the definition of infection in literature is different in every study. For instance, Vinton et al. considered wound infection as an erythema treated empirically with antibiotics and these authors reported no difference between mastectomy (15% out of 387) and lumpectomy (13% out of 173) (both with axillary dissection) [24]. If the wound infections are more precisely defined as an inflamed wound with positive cultures that needed antibiotic treatment, Petrek et al. reported no infection [26], but this study (although based on a smaller group than Vinton et al. [23]) and Vinton et al. found no difference between mastectomy (5% for Vinton and 7% [9/127] in our series) and lumpectomy (6% for Vinton and 4% [4/96] in our series) [24]. However, the incidence of wound infection was much higher in patients who had a higher Np in our study.
With respect to adjuvant treatment, a retrospective study by Say et al. demonstrated that pre- or postoperative radiation therapy does not affect seroma formation in patients who have undergone radical mastectomy [35]. In our study the majority of the patients had a postoperative radiotherapy. With respect to chemotherapy, one study found that neoadjuvant chemotherapy was associated with development of postoperative seromas [21] whereas, others showed that seroma formation is not influenced by the preoperative chemotherapy [36,37] and more, Broadwer et al. showed that preoperative chemotherapy decreased the incidence of seroma formation by almost 50 per cent in women undergoing M [38]. Our analysis concludes that the use of preoperative chemotherapy did not influence seroma formation.
As regards tumor characteristic, the data on the association between axillary lymph node status, tumor size and seroma formation were inconclusive [22,[26,30,[39-43]. No such association was identified by the present study.
To minimize seroma formation and their punctures, our results support the use of:
a) Neoadjuvant chemotherapy or hormonal therapy. The preoperative chemotherapy reduces the size of the primary tumor and lymph node metastases in up to 80% of patients, often rendering these patients candidates for breast conservation therapy [44].
b) Sentinel lymph node biopsy, which is associated with significantly less seroma formation than conventional axillary dissection [45].
Conclusions
This institutional survey highlights the problem of post-operative “seromas” and their related punctures. These were statistically more frequent after mastectomy than lumpectomy; therefore, neoadjuvant approaches with conservative surgeries should be favored. Our analysis also defined an abnormally high Np and/or VpTot as“outliers”.References
- 1. Aitken DR, Minton JP (1983) Complications associated with mastectomy. Surg Clin North Am 63: 1331-1352.
- 2. Kumar S, Lal B, Misra MC (1995) Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb 40: 292-294.
- 3. Tadych K, Donegan WL (1987) Postmastectomy seromas and wound drainage. Surg Gynecol Obstet 165: 483-487.
- 4. Madden JL (1965) Modified radical mastectomy. Surg Gynecol Obstet 121: 1221-1230.
- 5. R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 6. Fraley C, Raftery AE, Murphy TB, Scrucca L (2012) mclust Version 4 for R: Normal mixture modeling for model-based clustering, classification, and density estimation. Technical Report No. 597, Department of Statistics, University of Washington.
- 7. Muthén BO (2001) Latent variable mixture modeling. In: Marcoulides GA, Schumacker RE (Eds.), New Developments and Techniques in Structural Equation Modeling. Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 1-33.
- 8. Muthén B (2004) Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. The Sage Handbook of Quantitative Methodology for the Social Sciences.
- 9. Muthén B, Muthén LK (2000) Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res 6: 882-891.
- 10. Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling 14: 535-569.
- 11. Watt-Boolsen S, Nielsen VB, Jensen J, Bak S (1989) Postmastectomy seroma. A study of the nature and origin of seroma after mastectomy. Dan Med Bull 36: 487-489.
- 12. Watt-Boolsen S, Jacobsen K, Blichert-Toft M (1988) Total mastectomy with special reference to surgical technique, extent of axillary dissection and complications. Acta Oncol 27: 663-665.
- 13. Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F (1994) Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg 81: 856-859.
- 14. Agrawal A, Ayantunde AA, Cheung KL (2006) Concepts of seroma formation and prevention in breast cancer surgery. ANZ J Surg 76: 1088-1095.
- 15. Bryant M, Baum M (1987) Postoperative seroma following mastectomy and axillary dissection. Br J Surg 74: 1187.
- 16. Kim HJ, Barsevick AM, Beck SL, Dudley W (2012) Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: a secondary analysis. Oncol Nurs Forum 39: E20-E30.
- 17. Kim HJ, Abraham I, Malone PS (2013) Analytical methods and issues for symptom cluster research in oncology. Curr Opin Support Palliat Care 7: 45-53.
- 18. Willett WC, Rockhill B, Hankinson SE, Hunter DJ, Colditz GA (2000) Epidemiology and nongenetic causes of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK (Editors). Diseases of the Breast. 2nd edn. Philadelphia: Lippincott Williams & Wilkins, pp. 175-220.
- 19. Akinci M, Cetin B, Aslan S, Kulacoglu H (2009) Factors affecting seroma formation after mastectomy with full axillary dissection. Acta Chir Belg 109: 481-483.
- 20. Kuroi K, Shimozuma K, Taguchi T, Hirohisa I, Hiroyasu Y, et al. (2006) Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol 36: 197-206.
- 21. Woodworth PA, McBoyle MF, Helmer SD, Beamer RL (2000) Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg 66: 444-450.
- 22. Lumachi F, Brandes AA, Burelli P, Basso SM, Iacobone M, et al. (2004) Seroma prevention following axillary dissection in patients with breast cancer by using ultrasound scissors: a prospective clinical study. Eur J Surg Oncol 30: 526-530.
- 23. Bonnema J, van Geel AN, Ligtenstein DA, Schmitz PI, Wiggers T (1997) A prospective randomized trial of high versus low vacuum drainage after axillary dissection for breast cancer. Am J Surg 173: 76 -79.
- 24. Vinton AL, Traverso LW, Jolly PC (1991) Wound complications after modified radical mastectomy compared with tylectomy with axillary lymph node dissection. Am J Surg 161: 584-588.
- 25. Andeweg CS, Schriek MJ, Heisterkamp J, Roukema JA (2011) Seroma formation in two cohorts after axillary lymph node dissection in breast cancer surgery: does timing of drain removal matter? Breast J 17: 359-364.
- 26. Petrek JA, Peters MM, Nori S, Knauer C, Kinne DW, et al. (1990) Axillary lymphadenectomy: A prospective, randomized trial of 13 factors influencing drainage, including early or delayed arm mobilization. Arch Surg 125: 378-382.
- 27. Tejler G, Aspegren K (1995) Complications and hospital stay after surgery for breast cancer: a prospective study of 385 patients. Br J Surg 72: 542-544.
- 28. Burak WE Jr, Goodman PS, Young DC, Farrar WB (1997) Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. J Surg Oncol 64: 27-31.
- 29. Somers RG, Jablon LK, Kaplan MJ, Sandler GL, Rosenblatt NK (1992) The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer. A prospective randomized trial. Ann Surg 215: 146-149.
- 30. Medl M, Mayerhofer K, Peters-Engl C, Mahrhofer P, Huber S, et al. (1995) The application of fibrin glue after axillary lymphadenectomy in the surgical treatment of human breast cancer. Anticancer Res 15: 2843-2845.
- 31. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, et al. (2007) Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology group trial Z0011. J Clin Oncol 25: 3657-3663.
- 32. Siegel BM, Mayzel KZ, Love SM (1990) Level I and II axillary dissection in the treatment of early-stage breast cancer. An analysis of 259 consecutive patients. Arch Surg 125: 1144-1147.
- 33. Feigenberg Z, Zer M, Dintsman M (1977) Comparison of postoperative complications following radical and modified radical mastectomy. World J Surg 2: 207-211.
- 34. Hayes JA, Bryan RM (1984) Wound healing following mastectomy. Aust N Z J Surg 54: 25-27.
- 35. Say CC, Donegan W (1974) A biostatistical evaluation of complications from mastectomy. Surg Gynecol Obstet 138: 370-376.
- 36. Forouhi P, Dixon JM, Leonard RC, Chetty U (1995) Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br J Surg 82: 79-82.
- 37. Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK (2003) Seroma formation following breast cancer surgery. Breast J 9: 385-388.
- 38. Broadwater JR, Edwards MJ, Kuglen C, Hortobagyi GN, Ames FC, et al. (1991) Mastectomy following preoperative chemotherapy. Strict operative criteria control operative morbidity. Ann Surg 213: 126-129.
- 39. Loo WT, Chow LW (2007) Factors predicting seroma formation after mastectomy for Chinese breast cancer patients. Indian J Cancer 44: 99-103.
- 40. Kumar S, Lal B, Misra MC (1995) Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb 40: 292-294.
- 41. Browse DJ, Goble D, Jones PA (1996) Axillary node clearance: who wants to immobilize the shoulder? Eur J Surg Oncol 22: 569-570.
- 42. Petrek JA, Peters MM, Cirrincione C, Thaler HT (1992) A prospective randomized trial of single versus multiple drains in the axilla after lymphadenectomy. Surg Gynecol Obstet 175: 405-409.
- 43. Schuijtvlot M, Sahu AK, Cawthorn SJ (2002) A prospective audit of the use of a buttress suture to reduce seroma formation following axillary node dissection without drains. Breast 11: 94-96.
- 44. Schwartz GF, Hortobagyi GN (2004) Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26-28, 2003, Philadelphia, Pennsylvania. Cancer 100: 2512-2532.
- 45. Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, et al. (2005) Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol 23: 4312-4321.