Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: Dr. Marlon Guerrero, MD, University of Arizona, Department of Surgery, 1501 N. Campbell Ave., Office 4327, Tucson, AZ 85724, USA, Tel: (520) 626-7946; Fax: (520) 626-7785; E-mail: mguerrero@surgery.arizona.edu
Citation: Shultz CL, Guerrero MA. Primary Thyroid Lymphoma: A Comprehensive Review of Contemporary Management. J Surgery. 2013;1(1):6.
Copyright © 2013 Shultz Cl, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 26 July 2013 | Accepted: 27 August 2013 | Published: 30 August 2013
Historically, primary thyroid lymphoma was considered a surgical disease with the therapeutic goal of resection and debulking before adjuvant treatment (radiotherapy with or without chemotherapy) [3,4]. However, diagnostic tools — such as fine-needle cytology and axial imaging — now yield higher predictive values, so surgical management is rarely required.
Primary thyroid lymphoma is more prevalent in women, with a male:female ratio of 1:1.3 to 1:7.6. It most often presents in the 5th or 6th decade of life (Table 2). Few epidemiologic studies have described any racial prevalence, though a comprehensive study of prognostic factors in the United States conducted by Graff-Baker et al. found the highest prevalence in white populations [4]. In addition, Graff-Baker et al. found that, geographically, most patients with this disease are from the West (49%) and Midwest (28%).
Chemotherapy alone has yielded a complete response in 77% to 100% of patients: the 5-year overall survival rates have ranged from 55% to 60%; the 5-year disease-free survival rates, from 40% to 65%. All of the studies that we reviewed used CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like chemotherapy (CVP: cyclophosphamide, vincristine, prednisone) in either 3-dose or 6-dose regimens (Table 3), though the 3-dose regimen is considered standard practice [28].
Primary Thyroid Lymphoma: A Comprehensive Review of Contemporary Management
Christopher L. Shultz and Marlon A. Guerrero*
- Department of Surgery, University of Arizona School of Medicine, Tucson, AZ, USA
*Address for Correspondence: Dr. Marlon Guerrero, MD, University of Arizona, Department of Surgery, 1501 N. Campbell Ave., Office 4327, Tucson, AZ 85724, USA, Tel: (520) 626-7946; Fax: (520) 626-7785; E-mail: mguerrero@surgery.arizona.edu
Citation: Shultz CL, Guerrero MA. Primary Thyroid Lymphoma: A Comprehensive Review of Contemporary Management. J Surgery. 2013;1(1):6.
Copyright © 2013 Shultz Cl, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 26 July 2013 | Accepted: 27 August 2013 | Published: 30 August 2013
Abstract
Primary thyroid lymphoma is an uncommon malignancy that constitutes fewer than 2% of all extranodal non-Hodgkin lymphomas (NHLs) and only 0.6% to 5% of all thyroid malignancies. Histologic subtypes include diffuse large B-cell lymphoma, mucosa-associated lymphoid tissue lymphomas, follicular lymphomas, and rarely Hodgkin lymphomas. The rarity of this disease precludes optimizing diagnostic and management modalities. This review provides an evaluation of the presentation of thyroid lymphoma and the current diagnosis, evaluation, and management of thyroid lymphoma.Introduction
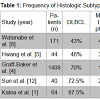
Primary thyroid lymphoma arises from the thyroid gland; the definition excludes malignancies originating from local invasion or distant metastasis. An uncommon malignancy, primary thyroid lymphoma constitutes fewer than 2% of all extranodal non-Hodgkin lymphomas (NHLs) [1] and only 0.6% to 5% of all thyroid malignancies [2]. Histologic subtypes include Diffuse Large B-cell lymphoma (DLBCL) (43% to 87% of cases), mucosa-associated lymphoid tissue (MALT) lymphomas (3% to 47%), follicular lymphomas, and other rare variants [2] (Table 1). Hodgkin thyroid lymphomas are relatively uncommon.Historically, primary thyroid lymphoma was considered a surgical disease with the therapeutic goal of resection and debulking before adjuvant treatment (radiotherapy with or without chemotherapy) [3,4]. However, diagnostic tools — such as fine-needle cytology and axial imaging — now yield higher predictive values, so surgical management is rarely required.
In this article, our aim is to provide a comprehensive review of contemporary management of primary thyroid lymphoma.
Methods
A review of the literature was performed using Medline and Pubmed databases to identify all studies published up to February 2013 involving thyroid lymphoma. The MeSH search terms used were “thyroid lymphoma”, “thyroidectomy”, “surgery”, “chemotherapy”, “radiation therapy”, and “chemoradiation therapy”. The above terms and their combinations were also searched as text words. We excluded studies involving cancers of follicular and parafollicular origin, as well as poorly differentiated, advanced differentiated, and anaplastic thyroid cancer.Results
Our search strategy yielded 35 studies related to the management of thyroid lymphoma and we included 35 studies that discussed the presentation, diagnosis, evaluation, and management of thyroid lymphoma.Clinical presentationPatients with primary thyroid lymphoma usually present with a rapidly enlarging neck mass [2]. The thyroid mass enlarges within 5 months (mean, 2.1 months) [5]. The mass is often firm; occasionally, it is fixed to adjacent structures [5]. Some patients have obstructive symptoms, including dyspnea, hoarseness, and dysphagia, though few patients (< 10%) report any pain or tenderness on palpation. Much less commonly, patients present with the so-called “B” symptoms of fever, night sweats, or weight loss (Table 2). Time interval from initial presentation to final diagnoses has not been reported.
Primary thyroid lymphoma is more prevalent in women, with a male:female ratio of 1:1.3 to 1:7.6. It most often presents in the 5th or 6th decade of life (Table 2). Few epidemiologic studies have described any racial prevalence, though a comprehensive study of prognostic factors in the United States conducted by Graff-Baker et al. found the highest prevalence in white populations [4]. In addition, Graff-Baker et al. found that, geographically, most patients with this disease are from the West (49%) and Midwest (28%).
Many epidemiologic studies of primary thyroid lymphoma have found an association with Hashimoto thyroiditis, with several patients presenting with either a past medical history or pathologic evidence of Hashimoto thyroiditis [1,2,5-8]. However, in a retrospective study of 24,553 patients with Hashimoto thyroiditis in Japan, Watanabe et al. found that only 0.56% went on to develop primary thyroid lymphoma, with an incidence of 15.6 patients per 10,000 person-years [8].
Diagnostic considerations
The initial diagnostic workup for suspected primary thyroid lymphoma is similar to the workup for other rapidly enlarging goiters, including complete blood count (CBC), serum Lactate Dehydrogenase (LDH), serum beta2-microglobulin, antithyroglobulin or antimicrosomal antibody, and thyroid function tests. The pattern of thyroid function associated with primary thyroid lymphoma is unclear, though 36% to 61% of patients may have hypothyroidism as measured by Thyroid-Stimulating Hormone (TSH) levels greater than 4.5 IU/liter [6,8]. Only 1% to 6% of patients present with decreased TSH levels (< 0.1 IU/liter) [6,8]. The specific mechanism underlying the compromised thyroid function has not been elucidated; it may be associated with underlying Hashimoto thyroiditis or with replacement of thyroid parenchyma by the lymphomatous process.
Measurement of serum LDH and beta2-microglobulincan also be helpful diagnostic indicators. However, only 23% to 47% of patients have elevated serum LDH levels [6,8], and only 24% have elevated serum beta2-microglobulin levels [6]. The lack of sensitivity and specificity of the 2 tests excludes them from being diagnostic for primary thyroid lymphoma, though they may still be useful in explicating the clinical picture. Moreover, in a retrospective study of 26 patients, Thieblemont et al. found that elevated serum LDH and beta2-microglobulin levels were associated with more DLBCL, suggesting that the 2 tests might be useful as a measure of disease aggression [6]. Serum LDH measurement as a prognostic indicator of response to treatment and of overall survival has been found to have no statistically significant value in multiple studies [1,5,9,10].
Fine-needle aspiration (FNA) has become the initial cytologic tool for diagnosing rapidly enlarging neck masses. In a clinicopathologic study and outcome analysis in 64 patients, Katna et al. found that, overall, FNA yielded a definitive diagnosis of primary thyroid lymphoma in only 28% of patients [1]. However, the yield has varied widely in other clinical studies: from 4% [3] to 90% [11]. Furthermore, thyroid lymphoma can be difficult to distinguish confidently from florid Hashimoto thyroiditis. Given the limitations of FNA, patients thought to possibly have thyroid lymphoma should undergo core needle biopsy, or, rarely, open biopsy. Hemithyroidectomy was performed diagnostically in 22-25% of primary thyroid lymphoma cases [3,12]. However, thyroid lobectomy or thyroidectomy is rarely indicated for diagnoses but may be appropriate under certain acute life-threatening circumstances [1,7,13,14]. It is unclear how many diagnoses of primary thyroid lymphoma are made incidentally in patients who receive thyroid surgery, as surgical intent is rarely reported. However, a case study of 17 primary thyroid lymphoma patients in Malaysia reported that in only 3 of 9 patients who underwent surgery was lymphoma unsuspected [13].
The advent of molecular techniques, such as immunohistologic staining and flow cytometry of biopsy samples, has allowed for greater diagnostic accuracy. Correctly identifying and differentiating histologic subtypes is imperative for assessing both ideal treatment options and prognosis. Molecular techniques help pinpoint immunoglobulin-specific binding to cell-surface markers and exploit differences in expression profiles. It is known that nearly all large B-cell lymphomas are CD19-, CD20-, and CD45- positive, whereas MALT lymphomas express surface immunoglobulin and are CD5-, CD10-, and CD23-negative [15]. Other useful markers such as Thryoglobulin, CD3, CD43, CD45RA, CD45RB, CD45RO, Bcl-2, Kappa r, and Lambda r have been described previously [2]. Assays may also be helpful in differentiating between Hashimoto thyroiditis and MALT lymphomas, which historically has been difficult to do by cytologic techniques alone, given lymphocytichomogeneity and small sample sizes [13,15]. The cytologic challenges of differentiating between Hashimoto thyroiditis and MALT lymphomas may be related to pathogenesis. Some authors have suggested that prolonged autoimmune antigenic stimulation may be responsible for the development of lymphocytic changes [5,16], and that underlying MALT lymphomas in patients with DLBCL may even imply a transformation from 1 subtype to the other [2].
Gene expression profiling studies have enabled division of heterogeneous DLBCL into 2 major cell-of-origin phenotypes according to the classification method of Hans et al.: germinal center B-cell-like (GCB) and activated B-cell-like (ABC) subgroups [17]. Prognostic differences between the 2 subgroups have been studied, with a relative consensus that a GCB diagnosis is associated with higher overall survival rates [1,8,16]. Yet in a retrospective univariate analysis of 37 patients, no single individual marker was associated with a significant prognostic difference [1].
Few clinical studies have assessed any genetic associations and/or mutations in patients with primary thyroid lymphoma. In a retrospective case series of 16 patients with DLBCL, 12 had an abnormal karyotype [16]. Of those 12 patients, 2 had t(8;14)(q24;q32) mutations, and 4 had 3q27 translocations [16]. Currently, the absence of a clear genetic pattern in patients with primary thyroid lymphoma makes genetic testing cost-ineffective and of little diagnostic value.
Imaging
The use of ultrasound has become routine in the assessment of thyroid masses. However, primary thyroid lymphoma lacks a unique appearance on ultrasound, so ultrasound in such patients is most effective when used in conjunction with a biopsy [18,19]. Ultrasound is in adequate in terms of primary thyroid lymphoma staging, which should be reserved for magnetic resonance imaging (MRI); if MRI is unavailable, then computed tomography (CT) should be used in order to sufficiently assess the presence and extent of extranodal involvement in the neck, mediastinum, and space below the diaphragm [18].
The use of 18F-Flurodeoxygluoce positron emission tomography (FDG-PET) in staging and evaluation of response has been well established for a variety of malignancies [20]. Studies of PET imaging in primary thyroid lymphoma are currently limited but have shown some benefits in differentiating primary thyroid lymphoma from chronic thyroiditis [21] as well as advantages over CT imaging alone in assessing metabolic response and disease recurrence [22]. The relative paucity of data and potential utility of PET in managing patients with primary thyroid lymphoma necessitates further study.
Management
Monotherapy: The multiple histologic subtypes of primary thyroid lymphoma make uniform treatment impossible. Historically, surgery - total or subtotal thyroidectomy or partial thyroid lobectomy - was considered standard practice in the management of primary thyroid lymphoma [3,14]. The role of surgery, however, has been diminished, thanks to improvements in diagnosis and treatment [3,13].
Before the introduction of radiation therapy in the 1950s, surgical management alone for primary thyroid lymphoma conferred poor outcomes, with 5-year overall survival (5-year OS) rates of 20% [23]. The tendency was to attempt surgical resection whenever a tumor was small or confined to the thyroid; only when the lymphoma was of the inoperable extrathyroidal stage was radiotherapy alone resorted to [24].
The debate over the utility of surgery dates back to the 1970s. Devine et al. found that combining surgical resection with radiotherapy to treat lymphoma offered no significant survival advantage [24]. In contrast, Rossl et al. advocated total tumor excision, whenever possible, as well as postoperative radiation [25].
More recent retrospective case reports continue to muddy the waters, with 5-year overall survival rates ranging from 33% [26] to 100% [6]. In a case study of 6 patients with the MALT lymphoma subtype of primary thyroid lymphoma, Thieblemont et al. found that all 6 had acomplete response after surgery alone, with a 5-year overall survival rate of 100% [6]. However, it is well documented that MALT lymphomas patients have a better prognosis [1,2,4-6,8]: this earlystage subtype has an indolent course and reflects an “ideal scenario” applicable to only a small subset of patients whose outcomes should not be extrapolated to all patients with primary thyroid lymphoma.
Contemporary studies analyzing outcomes after surgery alone are scarce. Many institutions have accepted combined modality treatment as the mainstay, rightfully abandoning surgery alone. Future studies assessing treatment in patients with the low-grade, early-stage MALT lymphoma subtype may marshal evidence for the utility of surgery alone.
Radiation therapy alone has yielded a complete response in 67% to 97% of patients: the 5-year overall survival rates have ranged from 69% to 93%; the 5-year disease-free survival rates, from 63% to 78% (Table 3). Despite such favorable outcomes, Doria et al. reported an overall 37.1% relapse rate with radiation therapy alone [27], making the case for systemic therapy as an adjuvant to local treatment. Doria et al. did not assess specific radiation therapy dosing and protocols. One study recommends doses of 24 to 40Gy and clinical target volumes comprising the thyroid bed, bilateral cervical node levels 3, 4, and 6, and upper mediastinal lymph nodes [28].
Chemotherapy alone has yielded a complete response in 77% to 100% of patients: the 5-year overall survival rates have ranged from 55% to 60%; the 5-year disease-free survival rates, from 40% to 65%. All of the studies that we reviewed used CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like chemotherapy (CVP: cyclophosphamide, vincristine, prednisone) in either 3-dose or 6-dose regimens (Table 3), though the 3-dose regimen is considered standard practice [28].
The addition of rituximab, a chimeric anti-CD20 immunoglobulin G1 monoclonal antibody, to CHOP improved overall survival to 92%, as compared with 71% with CHOP alone (P=0.06) [29]. Few studies have assessed outcomes associated with the addition of rituximab to CHOP in patients with primary thyroid lymphoma specifically. Arguably, the therapeutic benefits of rituximab found in patients with other DLBCL lymphomas [30,31] can be applied to patients with DLBCL primary thyroid lymphoma as well, providing patients tolerate the adverse effects.
Combined Modality Treatment: According to retrospective case studies, the combination of radiation therapy and chemotherapy has yielded the most favorable outcomes, as compared with any single modality [1,7,14,27,29,32]. In a retrospective study of 87 patients, Onal et al. found a 5-year overall survival rate of 92% in such patients, as compared with only 61% in those treated with a single modality; the 5-year disease-free survival rate was also significantly higher in patients on both radiation therapy and chemotherapy (hazard ratio, 4.2; P= 0.03) [29].
In our review of the literature, we found a complete response in 85% to 92% of patients on both radiation therapy and chemotherapy: overall survival rates have ranged from 45% to 93%; the 5-year disease-free survival rates, from 77% to 93% (Table 3).
The combination of surgery and postoperative radiation therapy has yielded comparable complete response rates, but with a lower 5-year overall survival rate of 44% (Table 3).
The few studies that have assessed the efficacy of a combination of chemotherapy and surgery have reported complete response rates of 67% to 72% and a 5-year overall survival rates of 58% (Table 3), ruling this combined modality inappropriate for patients with primary thyroid lymphoma.
The current literature suggests that the combination of radiation therapy and chemotherapy is most appropriate for patients with primary thyroid lymphoma — a conclusion substantiated by the relative reduction in the relapse rate associated with this particular combined modality treatment [27]. In a series of 211 patients, Doria et al. found a relapse rate of only 7.7% in patients on both radiation therapy and chemotherapy, as compared with 37.1% with radiation therapy alone and 43% with chemotherapy alone [27].
In patients with primary thyroid lymphoma, the supplementation of surgery remains controversial. Pyke et al. reported no significant difference, either in the complete response rate or in the overall survival rate, between patients who underwent substantial debulking followed by adjuvant therapy versus patients with diagnostic biopsy results who underwent adjuvant therapy alone [33]. Likewise, Meyer-Rochow et al. reported no significant difference, in Kaplan-Meier disease-free survival curves, between patients who underwent thyroid resection versus patients with diagnostic open-biopsy results [3]. In contrast, Graff-Baker et al. reported data supporting an association between surgery and/or radiation and improved survival for patients with stage I MALT lymphomas, DLBCL, and small-lymphocytic variants per histologic tests [4].
Interestingly, Mian et al. found a clear trend, in the Kaplan-Meier curve, toward better overall survival in patients who first underwent surgical debulking followed by radiation therapy and chemotherapy [10].
Given the well-established operative risks (including recurrent laryngeal nerve palsy and hypoparathyroidism) associated with thyroid resection, surgery should be limited to patients who need an open biopsy after failure of diagnostic FNA and to those who need palliation of compressive symptoms [14]. 8-19% of patients treated surgically required tracheostomy for management of compressive airway symptoms or direct tracheal invasion [12,14,29].
Discussion
A significant limitation of our review is the inconsistency, due primarily to the subtypes of primary thyroid lymphoma, in the patient populations among the various studies. Very few of the studies we analyzed stratified outcomes by both histologic subtype and treatment modality [6,10,16,34] — a significant constraint, as different histologic subtypes may indicate different treatment modalities.Many studies have reported a more favorable prognosis for MALT lymphoma patients than for DLBCL patients [1,2,4-6,8]. In the largest primary thyroid lymphoma study, to date (of 1,408 patients), Graff-Baker et al. found that the DLBCL subtype carried a 4.87-fold higher hazard ratio than the MALT lymphoma type [4]. Furthermore, Hwang et al. found a much higher complete response rate in MALT lymphoma patients (94%) than in DLBCL patients (53%) (P < 0.01) [5]. These findings support the utility of an accurate histologic diagnosis and the need for future studies assessing outcomes by histologic subtype.
Another limitation of our review is the prevailing lack of consensus regarding chemotherapeutic agents and radiation techniques. Most studies used CHOP or CHOP-like regimens, but outcomes may have been distorted by the addition of rituximab or bleomycin and by the use of other modalities such as CVP [1,10,16,26,29,33]. Radiation therapy also varied in terms of exposure fields, dosage, and frequency, possibly sullying interpretation of outcomes [1,6,10,16,26,29,33,34].
Conclusion
Lymphoma arising primarily from the thyroid gland is rare. The rarity of thyroid lymphoma precludes the implementation of prospective randomized trials to determine the optimal treatment modality. However, with the limited data available, multimodality treatment provides the best opportunity to achieve increased survival.Acknowledgements
Thank you to Dr. Mary Knatterud, Ph.D. for editing and reviewing this article.References
- Katna R, Shet T, Sengar M, Menon H, Laskar S, et al. (2013) Clinicopathologic study and outcome analysis of thyroid lymphomas: Experience from a tertiary cancer center. Head Neck 35: 165-171.
- Derringer GA, Thompson LD, Frommelt RA, Bijwaard KE, Heffess CS, et al. (2000) Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol 24: 623-639.
- Meyer-Rochow GY, Sywak MS, Reeve TS, Delbridge LW, Sidhu SB (2008) Surgical trends in the management of thyroid lymphoma. Eur J Surg Oncol 34: 576-580.
- Graff-Baker A, Roman SA, Thomas DC, Udelsman R, Sosa JA (2009) Prognosis of primary thyroid lymphoma: demographic, clinical, and pathologic predictors of survival in 1,408 cases. Surgery 146: 1105-1115.
- Hwang YC, Kim TY, Kim WB, Shong YK, Yi KH, et al. (2009) Clinical characteristics of primary thyroid lymphoma in Koreans. Endocr J 56: 399-405.
- Thieblemont C, Mayer A, Dumontet C, Barbier Y, Callet-Bauchu E, et al. (2002) Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab 87: 105-111.
- Ruggiero FP, Frauenhoffer E, Stack BC Jr (2005) Thyroid lymphoma: a single institution’s experience. Otolaryngol Head Neck Surg 133: 888-896.
- Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, et al. (2011) Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24553 patients with Hashimoto’s disease. Br J Haematol 153: 236-243.
- Pedersen RK, Pedersen NT (1996) Primary non-Hodgkin’s lymphoma of the thyroid gland: a population based study. Histopathology 28: 25-32.
- Mian M, Gaidano G, Conconi A, Tsang R, Gospodarowicz MK, et al. (2011) High response rate and improvement of long-term survival with combined treatment modalities in patients with poor-risk primary thyroid diffuse large B-cell lymphoma: an International Extranodal Lymphoma Study Group and Intergruppo Italiano Linfomi study. Leuk Lymphoma 52: 823-832.
- Gupta N, Nijhawan R, Srinivasan R, Rajwanshi A, Dutta P, et al. (2005) Fine needle aspiration cytology of primary thyroid lymphoma: a report of ten cases. Cytojournal 2: 21.
- Sun TQ, Zhu XL, Wang ZY, Wang CF, Zhou XY, et al. (2010) Characteristics and prognosis of primary thyroid non-Hodgkin’s lymphoma in Chinese patients. J Surg Oncol 101: 545-550.
- Sarinah B, Hisham AN (2010) Primary lymphoma of the thyroid: diagnostic and therapeutic considerations. Asian J Surg 33: 20-24.
- Sippel RS, Gauger PG, Angelos P, Thompson NW, Mack E, et al. (2002) Palliative thyroidectomy for malignant lymphoma of the thyroid. Ann Surg Oncol 9: 907-911.
- Mack LA, Pasieka JL (2007) An evidence-based approach to the treatment of thyroid lymphoma. World J Surg 31: 978-986.
- Niitsu N, Okamoto M, Nakamura N, Nakamine H, Bessho M, et al. (2007) Clinicopathologic correlations of stage IE/IIE primary thyroid diffuse large B-cell lymphoma. Ann Oncol 18: 1203-1208.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, et al. (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275-282.
- Takashima S, Nomura N, Noguchi Y, Matsuzuka F, Inoue T (1995) Primary thyroid lymphoma: evaluation with US, CT, and MRI. J Comput Assist Tomogr 19: 282-288.
- King AD, Ahuja AT, King W, Metreweli C (1997) The role of ultrasound in the diagnosis of a large, rapidly growing, thyroid mass. Postgrad Med J 73: 412-414.
- Tagliabue L, Del Sole A (2013) Appropriate use of positron emission tomography with [(18)F]fluorodeoxyglucose for staging of oncology patients. Eur J Intern Med [Epub ahead of print].
- Nakadate M, Yoshida K, Ishii A, Koizumi M, Tochigi N, et al. (2013) Is 18F-FDG PET/CT Useful for Distinguishing Between Primary Thyroid Lymphoma and Chronic Thyroiditis? Clin Nucl Med 38: 709-714.
- Basu S, Li G, Bural G, Alavi A (2009) Fluorodeoxyglucose positron emission tomography (FDG-PET) and PET/computed tomography imaging characteristics of thyroid lymphoma and their potential clinical utility. Acta Radiol 50: 201-204.
- Welch JW, Chesky VE, Hellwing CA (1958) Malignant lymphoma of the thyroid. Surg Gynecol Obstet 106: 70-76.
- Devine RM, Edis AJ, Banks PM (1981) Primary lymphoma of the thyroid: A review of the mayo clinic experience through 1978. World J Surg 5: 33-38.
- Rossi R, Cady B, Meissner WA, Sedgwick CE, Werber J (1978) Prognosis of undifferentiated carcinoma and lymphoma of the thyroid. Am J Surg 135: 589-596.
- Vigliotti A, Kong JS, Fuller LM, Velasquez WS (1986) Thyroid lymphomas stages IE and IIE: comparative results and multimodality treatment. Int J Radiat Oncol Biol Phys 12: 1807-1812.
- Doria R, Jekel JF, Cooper DL (1994) Thyroid Lymphoma The Case for Combined Modality Therapy. Cancer 73: 200-206.
- Beasley MJ (2012) Lymphoma of the thyroid and head and neck. Clin Oncol (R Coll Radiol) 24: 345-351.
- Onal C, Li YX, Miller RC, Poortmans P, Constantinou N, et al. (2011) Treatment results and prognostic factors in primary thyroid lymphoma patients: a rare cancer network study. Ann Oncol 22: 156-164.
- Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, et al. (1998) Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 92: 1927-1932.
- Sehn LH (2012) Paramount prognostic factors that guide therapeutic strategies in diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Prog 2012: 402-409.
- Ha CS, Shadle KM, Medeiros LJ, Wilder RB, Hess MA, et al. (2001) Localized non-Hodgkin lymphoma involving the thyroid gland. Cancer 91: 629-635.
- Pyke CM, Grant CS, Habermann TM, Kurtin PJ, van Heerden JA, et al. (1992) Non-Hodgkin’s lymphoma of the thyroud: is more than biopsy necessary? World J Surg 16: 604-609.
- Laing RW, Hoskin P, Hudson BV, Hudson GV, Harmer C, et al. (1994) The significance of MALT histology in thyroid lymphoma: a review of patients from the BNLI and Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 6: 300-304.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, et al. (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25: 579-586.