Journal of Plant Biology & Soil Health
Download PDF
Research Article
*Address for Correspondence: Suphiya Khan, Department of Bioscience and Biotechnology, Banasthali University, Tonk, Rajasthan, India - 304022, Tel: +91 9829926646; E-mail: suphiyakhan@gmail.com
Citation: Chaudhary K, Khan S. Physiochemical Characterization of Fluoride (F) Contaminated Soil and its Microbe-Assisted Bioremediation by Prosopis juliflora. J Plant Biol Soil Health. 2016;3(2): 8.
Citation: 2016 Chaudhary K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Biology & Soil Health | ISSN: 2331-8996 | Volume: 3, Issue: 2
Submission: 07 June, 2016 | Accepted: 17 September, 2016 | Published: 26 September, 2016
Physiochemical Characterization of Fluoride (F) Contaminated Soil and its Microbe-Assisted Bioremediation by Prosopis juliflora
Khushboo Chaudhary and Suphiya Khan*
- Department of Bioscience and Biotechnology, Banasthali University, Rajasthan, India
*Address for Correspondence: Suphiya Khan, Department of Bioscience and Biotechnology, Banasthali University, Tonk, Rajasthan, India - 304022, Tel: +91 9829926646; E-mail: suphiyakhan@gmail.com
Citation: Chaudhary K, Khan S. Physiochemical Characterization of Fluoride (F) Contaminated Soil and its Microbe-Assisted Bioremediation by Prosopis juliflora. J Plant Biol Soil Health. 2016;3(2): 8.
Citation: 2016 Chaudhary K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Biology & Soil Health | ISSN: 2331-8996 | Volume: 3, Issue: 2
Submission: 07 June, 2016 | Accepted: 17 September, 2016 | Published: 26 September, 2016
Abstract
Fluoride (F) contamination is a worldwide problem which has severe consequences on human and animal health. Physiochemical characteristics of F-polluted soil in different sites (Banasthali, Rajasthan, India) were tested. Soil pH (7 - 9.3), E.C (2.1 - 6.1 dSm-1), O.C (0.30% - 0.60%), N (11.4 - 53.4), P (3.3 - 5.8), K (106 - 117), S (1.3 - 8.3), Ca (11.7 - 14.2), Mg (1.8 - 11.7 kgha-1) and total Fe (3.6 - 5.6), Mn (1 - 2.4), Zn (0.30 - 2.6), Cu (0.70 - 4.1) and total F contents (9.6 - 11.0 mgkg-1) were observed. Prosopis juliflora is a hyperaccumulator plant, known and suitable species for phytoremediation of F polluted soil. A repeat experiment for confirmation using F contaminated soil with amendments of Pseudomonas fluorescence (P.F) and Pseudomonas aeruginosa (P.A) were applied to soil in small scale for 120 days. Moreover, total plant biomass increase (67.73% - 74.33%), chlorophyll reduced (50% - 34.67%) and mineral contents enhanced (46.38% - 58.68%) at 100 mgkg-1 were found as compared to the control. These two strains, increased bioaccumulation factor (BF) 0.22 - 2.89 and translocation factor (TF) 0.63 - 1.08 of plant, accumulated high amount of F in root. Comparative results (biomass, chlorophyll, mineral content, TF and BF) between P.F and P.A indicated that applications of Pseudomonas fluorescence significantly (P ≤ 0.05) impacted on the study of P. juliflora tolerance capacity. Results showed P. juliflora has good tolerance capacity when soil F concentration at 100 mg kg-1 with P.F - 108 cfu ml-1kg-1 of soil. In conclusion, Pseudomonas fluorescence identified in our study may be potentially used for phytoremediation purpose in field scale.Keywords
Fluoride; Phytoremediation; Prosopis juliflora; Pseudomonas fluorescence; Pseudomonas aeruginosaIntroduction
Soil pollution by Fluoride (F) non-metal is one of the main worldwide problems. Several reports suggest that Fluoride (F) is an essential element for the normal growth of plants and in higher concentration, it is toxic for plants [1]. Seed germination and early seedling growth are important phases for the successful growth and survival of plants and these physiological parameters of plants are affected by F stress [2]. Several physiological and biochemical processes are known to be affected by F such as chlorosis and necrosis of leaf, low nutrient uptake, reduction of plant biomass, and enzymatic activities [3]. Higher soil salinity increases the catalase, peroxidase and superoxidase activity among tolerant and sensitive varieties of plant [4]. The relationship between antioxidants and salinity indicate that O-2 radical and H2O2 could play an important role in the mechanisms system [5].Fluoride pollution is spreading all over the world causing India to severely suffer from its effects [6]. According to survey, high concentration of F in drinking water has been found in South and North American countries (Italy, Holland, Mexico and Spain) [7]. In India, 17 out of 32 states are critically affected by high F concentration [8]. A state named Rajasthan in India, having arid to semi-arid environment, is severely affected from high F concentration. In India, about 20% of F concentration were found in the household water supply; out of these 10% was found in Rajasthan [9]. According to Saini et al. total F content in soil was higher level than normal ones Krashi Vigyan Kendra farm (127.56 μgg-1) and Banasthali (679.63 μgg-1) Rajasthan, India [10].
The conventional technologies (adsorption & biosorption, ionexchange, electrocoagulation, flotation and reverse osmosis) are too expensive so it is important to develop eco-friendly and more effective method to decontaminate soils. Recently plants using for F contamination has gained importance. Phytoremediation is low cost and effective technology used for degradation of contaminants [11]. The cost for conventional technology expected is “US$ 100,000 and 10,00000 per ha” [12], whereas the cost of phytoremediation was “US$ 60,000 and 100,000 per ha” [13]. Microorganisms are able to survive in extreme cold, heat and desert, salt conditions due to their potentially produced enzymatic activities, which allow to converting hazardous compounds into harmless compounds [14,15]. Different microorganism evolved mechanism for resistance to metal toxicity and adapted to live in the presence of soil pollutants [16,17]. Soil bacteria that were resistant to metals (Zn, Cu, and Pb) and found that a P-solubilizer (Bacillus megaterium HKP-1) and a K-solublizer (Bacillus mucilaginosus HKK-1) had a high resistance to heavy metal toxicity to Zn (250 mgl-1), Cu (100 mgl-1), and Pb (300 mgl-1) due to formation of endospores which are capable to lived in extreme condition [18]. Pseudomonas aeruginosa strain Wl-1 could tolerate up to 0.6 mM lead nitrate and also accumulated lead at 26.5 mgg-1 of dry cell biomass [19]. Bioremediation technology which is generally accepted and carried out on contaminated sites without affecting soil fertility metabolic pathway in microbes and is consequently a potential threats to the human health and also prevent environment from different variety of contaminants. Successful phytoremediation depends not only on the interaction of plants root with microbes but also on the bioavailability of heavy metals in soils.
In the present study of leguminous species, Prosopis juliflora hyperaccumulator plant promoted us to assess the potential use of this plant in the reclamation of F contaminated soils [20]. This is also naturally grown in Rajasthan (India) without showing any morphological distortions against the F stress. It is tolerant to very high temperature (45 °C). This tree can grow in different areas and the roots can enter to large depths in the soil. The objective was to investigate (a) soil characteristics of Banasthali, Tonk, Rajasthan, India (b) effect of microbial consortium on plant biomass, chlorophyll, mineral content of plant organs (c) check efficacy of phytoremediation in small scale of pot experiment under given microbial treatment.
Materials and Methods
Soil characteristicsRandom soil samples were collected from a depth of 0-10 cm from nearby areas of Braham Mandir, Botanical Garden and Krashi Vigyan Kendra regions of Banasthali University (26°60’ N 75°54’ E) Tonk, Rajasthan, India. Samples were stored in cold temperature at 4 °C and brought to a soil testing laboratory. The soil sample were analyzed for pH, electrical conductivity (E.C), organic carbon (C), nitrogen (N), phosphorus (P), potassium (K), available DTPA (diethylenetriaminepentaacetic acid) extractable iron (Fe), manganese (Mn), zinc (Zn), copper (Cu) and fluoride (F). The organic carbon was estimated by Walkley method [21]. Available N was estimated by alkaline permanganate method [22]. Available phosphorus was extracted by using Olsen’s reagent and estimated through spectrophotometer after developing the blue color by ascorbic method. Available K was extracted with neutral ammonium acetate and estimated by flame photometer [23]. The DTPA extractable Fe, Mn, Zn and Cu were estimated by using atomic absorption spectrophotometer (AAS). The total F content was estimated by alkali fusion-ion selective method [24].
Small-scale pot experiment
Prosopis juliflora seeds were collected from Central Arid Zone Research Institute (CAZRI), Jodhpur (Rajasthan) India. Seeds were surface sterilized with 10% H2SO4 for 15 min and rinsed with millipore water. Seeds were germinated in plastic pots with 1 kg of sterilized soils. Each pot received six seeds which were placed at 1 cm depth. Pots were rearranged in the greenhouse chamber. The F contaminated soil of pot experiment was performed artificially given NaF of different concentration 25, 50, and 75 and 100 mgkg-1 in soil. Other set of experiment was subjected to a different type of inoculation: PF 8904 (Pseudomonas fluorescence), and PA 1934 (Pseudomonas aeruginosa) strains obtained from Microbial Tissue Culture Collection (MTCC) Chandigarh together with F concentration. Three replicates were used for each F level inoculation type treatment and without microbial treatment. Bacterial strain suspension (108 cfu ml-1) in nutrient broth was used for the inoculation, by spraying them on soil surfaces, after 10 days of germination [25]. To the control pots, 10 ml of sterile distilled millipore water was added (Table 1). Plants were harvested after 120 days, washed with tap water and deionized sterile water for further analysis. Plant biomass (roots and shoot length) was determined using measuring scale.Photosynthesis pigments
Fresh experimental plant leaves were taken for the determination of photosynthetic pigments e.g. Total Chlorophyll (total Chl), Chlorophyll a (Chl a) and Chlorophyll b (Chl b). The leaves were cut into tiny pieces; and homogenized 100 mg material in 5 ml of chilled 100% acetone by grinding the leaves of control and stressed seedlings in a pre-cooled mortar and pestle until the powdered materials becomes completely non-green. The homogenate was centrifuged for 5 min at 3000 rpm at 4 °C in a cooling centrifuge. The pellet was discarded and the supernatant was re-adjusted to 5 ml acetone. To 1.6 ml of the supernatant, 0.4 ml double distilled water was added. The chlorophyll content absorbance of the resulting supernatant was recorded at 645 and 663 nm using a double beam UV-Vis spectrophotometer as given below [26]:
Chl a (mg/l) = 12.7 (A663) - 2.69 (A645)
Chl b (mg/l) = 22.9 (A645) - 4.68 (A663)
Total Chl (a and b) (mg/l) = a + b
The above formula expressed the pigments content in mgg-1 fw of sample.
Mineral content of plant organs (root, shoot and leaves)
The plants were harvested after 120 days of inoculation and analyzed for further experiments. The whole plants were washed with distilled water, separate out into root, shoot and leaf than ovendried for 72 h at 70 °C to determine the contents of plant organs. The plant organs (root, shoot and leaf) were analyzed for Nitrogen - N, Phosphorus - P, Potassium - K, Iron - Fe, Manganese - Mn, Zinc - Zn and Cooper - Cu. The samples were analyzed (except N) by taking 1 g materials digesting in di-acid mixture Zn, and (9:4 ratio of nitric and perchloric acids) using standard analytical methods. N was estimated by Kjeldahl method [27], whereas available P was extracted by using Olsen’s reagent and estimated through spectrophotometer after developing the blue color by ascorbic method and K was analyzed by flame photometer [28]. The micronutrients (Fe, Mn, Zn and Cu) were analyzed by using atomic absorption spectrophotometer [28].
Determination of F
The bioaccumulation and translocation factor were measured according to Niu et al. [29]. A total F content in plant organs and remaining soil was calculated by alkali fusion-ion selective technique [30].(BF = {F concentration in shoot} / {F concentration in soil})
(TF = {F concentration in shoot} / {F concentration in root})
Statistical analysis
Statistical analysis were determined according to analysis of variance (ANOVA) test and significant T-test was computed using Standard Statistical Package (SPSS 17.0) to examine difference between each treatment at P ≤ 0.05 and all graphs was prepared by OriginPro 8.5 software.
Result and Discussion
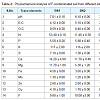
Soil characteristicsThis paper incorporates the results of present studies on preliminary investigation of physiochemical characteristics of the soil and samples sites areas of Braham Mandir, Botanical Garden and Krashi Vigyan Kendra regions of Banasthali University (26°60’ N 75°54’ E) Tonk Rajasthan, India (Table 2 and Figure 1). The concentrations of macronutrients was higher as per the Banasthali standard for F in soils: pH were (7 - 9.3), E.C (2.1 - 6.1 dSm-1), O.C (0.30% - 0.60%), total N (11.4 - 53.4), P (3.3 - 5.8), K (106 - 117), S (1.3 - 8.3), Ca (11.7 -4.2) and Mg (1.8 - 11.7) kgha-1 that were highly alkaline soil in both summer and winter season. Meanwhile the concentrations of micronutrients were of moderate levels as follows: Fe (3.6 - 5.6 mgkg-1), Mn (1 - 2.4 mgkg-1), Zn (0.3 - 2.6 mgkg-1), Cu (0.7 - 4.1 mgkg-1) and F (9.6 - 11.00 mgkg-1). F-polluted soil is just because of use phosphorus fertilizers, which contain more than 1.5% Fluorine. Polluted soil generally influences human well being by direct contact with soil or vegetation through inhalation of soil contaminants which have vaporized and move into the food chain. Rajasthan soil is generally highly alkaline, have poor soil structure and low infiltration capacity so they have a hard calcareous layer and nfavorable for agriculture land in desert region. Alkaline soil have trouble with nutrient mobility in soil and total 16 elements should be available within the soil for good plant growth (nitrogen, phosphorus and potassium are the most important elements for plant growth).
Growth parameters
The effect of microbes on plant growth was observed under different amendments (P.F and P.A) (Figure 2). Plant did not showed promoting effects on root, shoot length (Figure 2- 1,2) and biomass production (Figure 2- 3,4,5,6) of P. juliflora under given microbes treatment (Figure 2). Although the length and biomass of plant tissues were significantly (p ≤ 0.05) affected at 100 mgkg-1 F. P. juliflora did not show any toxic symptom when plant under normal stress condition but if we were to go above more than 100 mgkg-1 than it showed chlorosis and necrosis effect on plant due to more accumulated in tissue part and no translocation of F. Then the P.F and P.A significantly (p ≤ 0.05) increased both root length (12.76 - 23.96 cm) and the root biomass (fresh weight from 0.247 to 0.288 g and dry weight from 0.203 to 0.293 g). P.F performed better as compared to P.A in case of root parameters and P.F promoted shoot length (10.43 to 18.53 cm) and the shoot biomass (fresh weight from 0.269 to 0.349 g and dry weight from 0.195 to 0.322 g) of plants under F stress condition as compared to P.A. However, P.A increased both the root length (17.26 to 19.36 cm) and the root biomass (fresh weight from 0.248 to 0.284 g and dry weight from 0.204 to 0.283 g) in comparison to control. Meanwhile, P.A increased both the shoot length (10.60 to 16.03 cm) and the shoot biomass (fresh weight from 0.259 to 0.346 g and dry weight from 0.260 to 0.291 g) was significantly increased but did not as P.F. However, F stress was decreased root length, shoot length and also affected plant biomass under 100 mgkg-1 F stresses. However, microbial consortium treatment has increased plant biomass as compared to control due to the bioavailability of mineral content in soil. P.F have tendency to increase F mobility in soil therefore, its formed complex with cation (P, Al, Fe and Cu) because of F have more electronegativity power to interact with other metal. However, these phenomenon’s have important phases for enhanced plant biomass.Chlorophyll contents
Figure 2: Effect of microbial consortium on plant growth and biomass under F-contaminated soil (1 - root length, 2 - shoot length, 3 - root dry weight, 4 - shoot dry weight, 5 - root fresh weight, 6 - shoot fresh weight and P.F - Pseudomonas fluorescence, P.A - Pseudomonas aeruginosa significantly indicated p value*).
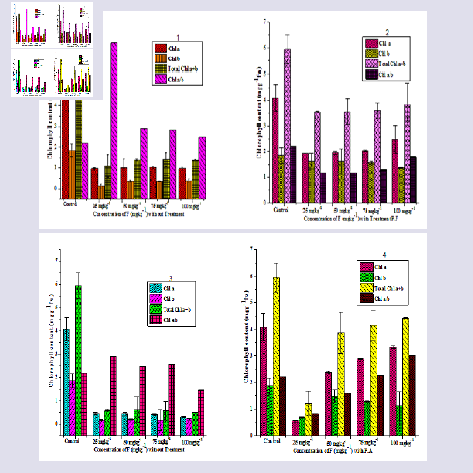
The chlorophyll content of P. juliflora leaves were reduced under F stress as compare to control but increased when microbes treatment has given as Chl a from (3.329 mgg-1 fw), Chl b from (1.101 mgg-1 fw), total chl a + b (4.431 mgg-1 fw) and Chl a/b (3.021 mgg-1 fw) (Figure 3). Heavy metal stresses are known to be interfering in chlorophyll contents through the direct inhibition of enzyme activity which effects on the plant nutrients. The chlorophyll substance was reduced under copper contaminated soil in Triticum aestivum cv. Brassica oleracea [31,32]. In previous research, it was found out that chlorophyll biosynthesis reduced and inhibit chlorophyll. P.F affect on chlorophyll content was highly reduced at the transient phase (stationary state) approximately 90 days. After that crucial period, plant was not affected under given P.F treatment as compared with P.A and F stress at 120 days.Mineral content
The analysis of N, P and K in root, shoot and leaves of P. juliflora were given (Figure 4). The N, P and K amount significantly increased (p = 0.98) when microbial consortium level was increased with enhanced F concentration but decreased when only with F concentration (Figure 4). The N, P and K concentration in root under P.F treatment was found to be (19.78%, 21% and 78%) higher in comparison to the treatment with P.A in Prosopis juliflora. However the N, P and K levels was affected by different F treatments (25, 50, 75 and 100 mgkg-1), but has only enhanced when the microbes treatment has given to the plant due to the biosynthesis of enzymes which has helpful under stress. Thus Cd can inhibit the activity of enzymes related with phosphorus metabolism. The Increasing chromium concentrations from 10-40 mgkg-1 in the soil decreased the N, P, K, and Fe contents of Brassica juncea shoot system [33]. Significant decreases in shoot K contents of white lupine (Lupinus albus) with 18 and 45 μM Cd treatments were also reported [34]. However, Fe, Mn, Zn and Cu concentration in root significantly increased (p = 1.00) with microbial treatment. The mean value of micronutrients Fe, Mn, Zn and Cu were analyzed separately (41.98%, 81.76%, 47.30% and 51.48%).The nutrient (N, P and K) present in shoot under P.F treatment was 68.04%, 20.39% and 87.25% which significantly increased value (p = 0.98). The available micronutrients ranges were also enhanced in case of Fe, Mn, Zn and Cu (94.94%, 92.5%, 68.88% and 74.37%). Meanwhile, in leave N, P and K was low ranged (45.86%, 39.06% and 55.55%) as compare to root and shoot due to negative effects of F. The available micronutrient ranges in leave of Fe, Mn, Zn and Cu was 63.33%, 51.97%, 78.12% and 52.59%. Different kinds of nutrients were involved including calcium, zinc, magnesium, phosphorus, potassium, iron and cooper [35]. Heavy metals such as Cu, Zn, Al, Cd, Ni, Hg and As has negative effects on macronutrient and micronutrients [9]. Heavy metals bind to all calcium binding sites on the cell surface at low pH (4.5). It works with calcium absorption and uptake calcium by roots [36]. High amount of heavy metals were exposed to the inhibition of calcium. Inhibit the influx of calcium ion at 100 mM aluminium (Al) [37]. Aluminium ions interfere with the action of guanosine 5’ triphosphate (GTP) binding protein as well as the inhibit calcium ion uptake by binding verapamil-specific channel [38]. The uptake of calcium ions was decreased in beech plants due to the combination of nitrogen and aluminium at high concentration [9]. Under aluminum treatment the concentration of calcium is unaffected in shoots because aluminium enters the plant cells through calcium channel [39]. Potassium channel influx inhibit due to the toxicity of heavy metals like Aluminium. Active pathway involvement of potassium uptake is also inhibited by high concentration of aluminium [40]. Durum wheat is more tolerant against aluminium toxicity but potassium ion concentration was decreased in wheat under aluminium concentration [41]. Concentration decreased of magnesium ions in roots and shoots by increasing the concentration of heavy metals [42]. Iron concentration decreased at a pH of 4.5 when they were treated with different concentration of heavy metals and also significantly affected root growth [43]. Therefore, it may be calcium channel and active pathway interferes with the action of F with P.F. to increase mineral content with specific channel like GTP and Verapamil. Therefore, P.F has positive effect on mineral content. It seems that F has combative interactions especially at high concentrations with mineral content in plant, which this led to decreased mineral level.
Figure 4: Effect of microbes (P.F and P.A) on different fluoride concentration ( 25, 50, 75 and 100 mgkg-1) on root (1 - 2), shoot (3 - 4), leaf (5 - 6), mineral contents of P. juliflora - A - control, B - 25 mgkg-1, C - 25 mgkg-1 + P.F, D - 50 mgkg-1, E - 50 mgkg-1 + P.F, F - 75 mgkg-1, G - 75 mgkg-1+ P.F, H - 100 mgkg-1, I - 100 mgkg-1 + P.F, same set of experiments conducted with PA (P.F - Pseudomonas fluorescence, P.A - Pseudomonas aeruginosa significantly indicated p value*).
Fluoride accumulation
The bioaccumulation factor (BF) values were 0.12 - 3.30 at different microbial consortium amendments (P.F and P.A) with various F concentrations. The P. juliflora showed a translocation factor (T.F) which was 0.51 - 1.08 in (Figure 5). The ratio of translocation factor was greater than 1 mean hyperaccumulator plant [44]. The result of the present investigation showed that microbial consortium had high ability and increased the efficiency of F accumulation. Plant growth promoting bacteria deserve a special attention because they can directly improve the phytoremediation process by increasing the metal solubility through altering soil pH, release of chelators (e.g. organic acids, siderphores), and they also help to increased metal mobility for high accumulation in plant organ [45].
Conclusion
Present study demonstrated that Pseudomonas fluorescence improve abilities of P. juliflora phytoremediation efficiency, mineral content and increase plant biomass as compare to the control. Results suggested that F had negative impact on chlorophyll pigments and mineral content of P. juliflora. Fluoride has highly interactions especially at high concentrations with mineral content in plant especially with P, Fe and Cu, which led to decrease the mineral levels in P. juliflora. Based on the results soil nutrients contents are sensitive to F concentration and it should be considered F contaminated soil. P. juliflora need the microbial treatment in soil to decline F toxicity. However, this work could be applied in the field scale and can be commercialized using microbial treatment with F. Pseudomonas fluorescence (P.F) for the use for remediation, counter balance nutrients content and could contribute to sustainable agriculture especially in areas where F contaminated problem in soil and water.Acknowledgement
Authors are deeply thankful to Prof. Aditiya Shastri for generous financial support to complete this research work and Bioinformatics Centre of Banasthali University, Newai (Rajasthan) India for computional work and also funding support from “MHRD project on center for excellence on Water and Energy” under training and research in frontiers areas of science and technology (FAST 5-5/2014 TS VII). We also acknowledge the Ms Swati Agarwal and Ms Sami Husain for language corrections.References
- Weinstein LH, Davison A (2004) Fluorides in the environment: effects on plants and animals. CABI, UK.
- Sabal D, Khan TI, Saxena R (2006) Effect of sodium fluoride on cluster bean (Cyamopsis Tetragonoloba) seed germination and seedling growth. Fluoride 39: 228-230.
- Chakrabarti S, Patra PK (2013) Effect of fluoride on superoxide dismutase activity in four common crop plants. Fluoride 46: 59-62.
- Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)-differential responses in salt-tolerant and sensitive varieties. Plant Sci 165: 1411-1418.
- Chookhampaeng S (2011) The effect of salt stress on growth, chlorophyll content proline content and antioxidant enzymes of pepper (Capsicum annuum L.) seedling. Eur J Sci Res 49: 103-109.
- Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater 137: 456-463.
- Mella S, Molina X, Atalah E (1994) Prevalence of endemic dental fluorosis and its relation with fluoride content of public drinking water. Rev Med Chil 122: 1263-1270.
- Vikas C, Kushwaha R, Ahmad W, Prasannakumar V, Reghunath R (2013) Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environ Earth Sci 68: 289-305.
- Hossain MA, Ashrafuzzaman M, Hossain AK, Ismail MR, Koyama H (2014) Role of accumulated calcium in alleviating aluminum injury in wheat plants. Sci World J 457187: 1-6.
- Saini P, Khan S, Baunthiyal M, Sharma V (2013) Mapping of Fluoride endemic area and assessment of F(-1) accumulation in soil and vegetation. Environ Monit Assess 185: 2001-2008.
- Memon AR, Schroder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res Int 16: 162-175.
- Russel M, Colgazier EW, English MR (1991) Hazardous waste remediation: the task ahead. University of Tennesse, Waste Management Research And Education Institute, Knoxville, TN, USA.
- Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109: 1427-1433.
- Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals--concepts and applications. Chemosphere 91: 869-881.
- Singh AK, Dhanjal S, Cameotra SS (2014) Surfactin restores and enhances swarming motility under heavy metal stress. Colloids Surf B Biointerfaces 116: 26-31.
- Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51: 730-750.
- Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, et al. (2010) Culturable bacteria from Zn- and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108: 1471-1484.
- Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140: 124-135.
- Naik MM, Pandey A, Dubey SK (2012) Pseudomonas aeruginosa strain WI-1 from Mandovi estuary possesses metallothionein to alleviate lead toxicity and promotes plant growth. Ecotoxicol Environ Saf 79: 129-133.
- Saini P, Khan S, Baunthiyal M, Sharma V (2012) Organ-wise accumulation of fluoride in Prosopis juliflora and its potential for phytoremediation of fluoride contaminated soil. Chemosphere 89: 633-635.
- Walkley A, Black IA (1934) An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37: 29-38.
- Subbiah BV, Bajaj JC (1962) A soil test procedure for assessment of available nitrogen in rice soils. Curr Sci 31: 196-198.
- Schollenberger CJ, Simon RH (1945) Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci 59: 13-24.
- Linger P, Mussig J, Fishcher H, Kobert J (2002) Industrial hemp (Cannabis Sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 16: 33-42.
- Marques AP, Pires C, Moreira H, Rangel AO, Castro PM (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42: 1229-1235.
- Arnon DI (1949) Copper enzymes in isolated chloroplast: polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1-15.
- Baethgen WE, Alley MM (1989) A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digest. Commun Soil Sci Plant Anal 20: 961-969.
- Bhargava BS, Raghupathi HB (1993) Analysis of plant material for macro-micronutrients. In: Tandon HL (Ed.), Methods of analysis of soil, plants, water, and fertilizers. Fertilizers Development And Consultation Organization, New Delhi, India.
- Zhi-xin N, Sun LN, Sun TH, Li YS, Wang H (2007) Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. J Environ Sci (China) 19: 961-967.
- McQuaker NR, Gurney M (1977) Determination of total fluoride in soil and vegetation using an alkali fusion-selective ion electrode technique. Anal Chem 49: 53-56.
- Lanaras T, Moustakas M, Symeonldis L, Diomantoglou S, Karataglis S (1993) Plant metal content, growth responses and some photosynthetic measurements of field-cultivated wheat growing on ore bodies enriched in Cu. Physiol Plant 88: 307-314.
- Chatterjee J, Chatterjee C (2000) Phototoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109: 69-74.
- Stobart AK, Griffiths WT, Ameen-bukhari I, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 63: 293-298.
- Sharma DC, Pant RC (1994) Chromium uptake and its effects on certain plant nutrients in maize (Zea mays L. cv. Ganga 5). J Environ Sci Health A Environ Sci Eng Toxicol 29: 941-948.
- Ribeiro MA, de Almeida AA, Mielke MS, Gomes FP, Pires MV, et al. (2013) Aluminum effects on growth, photosynthesis, and mineral nutrition of cacao genotypes. J Plant Nutr 36: 1161-1179.
- Mariano ED, Keltjens WG (2005) Long term effects of aluminum exposure on nutrient uptake by maize genotypes differing in aluminum resistance. J Plant Nutr 28: 323-333.
- Nichol BE, Oliveira LA, Glass A, Siddiqi MY (1993) The effect of aluminum on the influx of calcium, potassium, ammonium, nitrate and phosphate in an aluminum-sensitive cultivar of barley (Hordeum vulgare L.). Plant Physiol 101: 1263-1266.
- Rengel Z, Elliott DC (1992) Mechanism of aluminum inhibition of net Ca uptake by amaranths protoplasts. Plant Physiol 98: 632-638.
- Bengtsson B, Asp H, Jensen P (1994) Uptake and distribution of calcium and phosphorus in beech (Fagus sylvatica) as influenced by aluminum and nitrogen. Tree Physiol 14: 63-73.
- Liu K, Luan S (2001) Internal aluminum block of plant inward K(+) channels. Plant Cell 13: 1453-1465.
- Zsoldos F, Vashegyi A, Bona L, Pecsvaradi A, Szegletes Z (2000) Growth of and potassium transport in winter wheat and durum wheat as affected by various aluminum exposure times. J Plant Nutr 23: 913-926.
- Huang J, Bachelard EP (1993) Effects of aluminum on growth and cation uptake in seedlings of Eucalyptus mannifera and Pinus radiata. Plant Soil 149: 121-127.
- Moustakas M, Ouzounidou G, Lannoye R (1995) Aluminum effects on photosynthesis and elemental uptake in an aluminum-tolerant and non-tolerant wheat cultivar. J Plant Nutr 18: 669-683.
- Gupta S, Banerjee S, Mondal S (2009) Phytotoxicity of fluoride in the germination of paddy (Oryza sativa) and its effect on the physiology and biochemistry of germinated seedlings. Fluoride 42: 142-146.
- Rajkumar M, Ae N, Prasad MN, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28: 142-149.