Journal of Oral Biology
Download PDF
Research Article
*Address for Correspondence: Keiko Watanabe, Professor, Department of Periodontics, College of Dentistry, University of Illinois at Chicago, Chicago, IL, 60612-7212, USA, Tel: 312-996-0636; Fax: 312-996-0943; E-mail: keiko@uic.edu
Citation: Bhat UG, Watanabe K. Serpine1 Mediates Porphyromonas gingivalis Induced Insulin Secretion in the Pancreatic Beta Cell Line MIN6. J Oral Bio. 2015;2(1): 7.
Copyright © 2014 Watanabe et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 2, Issue: 2
Submission: 28 February 2015 | Accepted: 02 April 2015 | Published: 06 April 2015
Using clones 23 and C, we determined if Serpine1/SERPINE1 downregulation influences insulin secretion by GSIS. As shown in Figure 4, there was no difference between clone C and 23 with respect to insulin secretion under normoglycemia and hyperglycemia conditions without Pg stimulation. Addition of Pg to media upregulated insulin secretion in clone C but this upregulation did not occur in clone 23 in normoglycemic conditions (p<0.001) (Figure 4). Under hyperglycemic conditions, the mean insulin secretion was low in clone 23 compared to clone C in response to Pg stimulation but this reduction was statistically marginal (p=0.057).
Serpine1 Mediates Porphyromonas gingivalis Induced Insulin Secretion in the Pancreatic Beta Cell Line MIN6
Uppoor G. Bhat and Keiko Watanabe*
- Department of Periodontics, College of Dentistry, University of Illinois at Chicago, USA
*Address for Correspondence: Keiko Watanabe, Professor, Department of Periodontics, College of Dentistry, University of Illinois at Chicago, Chicago, IL, 60612-7212, USA, Tel: 312-996-0636; Fax: 312-996-0943; E-mail: keiko@uic.edu
Citation: Bhat UG, Watanabe K. Serpine1 Mediates Porphyromonas gingivalis Induced Insulin Secretion in the Pancreatic Beta Cell Line MIN6. J Oral Bio. 2015;2(1): 7.
Copyright © 2014 Watanabe et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 2, Issue: 2
Submission: 28 February 2015 | Accepted: 02 April 2015 | Published: 06 April 2015
Abstract
Periodontitis is an inflammatory disease resulting in destruction of gingiva and alveolar bone caused by an exuberant host immunological response to periodontal pathogens. Results from a number of epidemiological studies indicate a close association between diabetes and periodontitis. Results from cross-sectional studies indicate that subjects with periodontitis have a higher odds ratio of developing insulin resistance (IR). However, the mechanisms by which periodontitis influences the development of diabetes are not known. Results from our previous studies using an animal model of periodontitis suggest that periodontitis accelerates the onset of hyperinsulinemia and IR. In addition, LPS from a periodontal pathogen, Porphyromonas gingivalis (Pg), stimulates Serpine1 expression in the pancreatic beta cell line MIN6. Based on these observations, we hypothesized that a periodontal pathogen induces hyperinsulinemia and Serpine1 may be involved in this process.To test this hypothesis, we co-incubated Pg with the pancreatic beta cell line MIN6 and measured the effect on insulin secretion by MIN6 cells. We further determined the involvement of Serpine1 in insulin secretion by downregulating Serpine1 expression.
Our results indicated that Pg stimulated insulin secretion by approximately 3.0 fold under normoglycemic conditions. In a hyperglycemic state, Pg increased insulin secretion by 1.5 fold. Pg significantly upregulated expression of the Serpine1 gene and this was associated with increased secretion of insulin by MIN6 cells. However, cells with downregulated Serpine1 expression were resistant to Pg stimulated insulin secretion under normoglycemic conditions.
We conclude that the periodontal pathogen, Pg, induced insulin secretion by MIN6 cells and this induction was, in part, Serpine1 dependent. Thus, Serpine1 may play a pivotal role in insulin secretion during the accelerated development of hyperinsulinemia and the resulting IR in the setting of periodontitis.
Keywords
Insulin secretion; Prophyromonas gingivalis; Serpine1; Beta cellsIntroduction
Periodontitis is a destruction of gingiva and alveolar bone that affects approximately 50% of population in the US [1]. This destruction is caused by an exuberant host inflammatory reaction to bacteria that reside in and around the gingival pocket. Without treatment, periodontitis can lead to loss of teeth.Results from epidemiological studies suggest that periodontitis is associated with insulin resistance (IR) and/or prediabetes [2-4]. However, the exact mechanism for this relationship, particularly the influence of periodontitis on IR, is not known. In addition, there are few animal studies that have investigated the effect of periodontitis on IR [5,6]. The investigation of factors that induce or accelerate the development of IR is important since IR/prediabetes is a condition which can be reversed to normal. Based on the odds ratios of subjects with periodontitis to have IR and the results from animal studies, periodontitis likely has a significant impact on the development of IR.
Results from our previous studies indicate that hyperinsulinemia develops when periodontitis is induced in rats and mice [7,8] and this finding is supported by results from studies that demonstrate that periodontitis promotes insulin resistance in a rat model in part via hyperinsulinemia [6,7].
Hyperinsulinemia occurs when insulin signaling downstream of the insulin receptor is impaired in insulin target organs. This signaling impairment is in large part due to proinflammatory cytokines. In response to insulin signaling impairment, and thus subsequent insensitivity to insulin, pancreatic beta cells produce higher levels of insulin (hyperinsulinemia). This is a widely accepted pathway of IR development. However, it has been proposed that hyperinsulinemia also causes IR [9,10]. Whether the hyperinsulinemia in animals with periodontitis is in response to impaired insulin signaling in insulin target organs and/or some other factor such as periodontal pathogens stimulating insulin secretion is not known. We proposed that periodontal pathogens or byproducts stimulate insulin secretion directly from beta cells and this may contribute to hyperinsulinemia.
We recently determined the direct effect of LPS from the periodontal pathogen, Porphyromonas gingivalis (Pg), on the beta cell line MIN6 in vitro. The results indicate that Pg LPS stimulates insulin secretion by MIN6 cells [11], further supporting the concept that periodontal pathogens influence IR. In addition, the results from a polymerase chain reaction array analysis identified a greater than 2-fold increase in Serpine1 gene expression by MIN6 cells upon stimulation with Pg LPS [11]. The Serpine1gene product SERPINE1, also known as Plasminogen activator inhibitor-1 (PAI1), is a serine protease inhibitor that inhibits tissue type and urokinase plasminogen activators (tPA and uPA respectively), thus functioning as an antifibrinolytic. Interestingly, it has been reported that subjects with periodontitis or diabetes have a higher concentrations of circulating SERPINE1 compared to control subjects [12-14]. However, whether there is a causal relationship between SERPINE1 and the association between periodontitis and IR is not known.
In order to elucidate the mechanism by which periodontitis influences insulin secretion, we determined the effect of Pg on insulin secretion and a possible involvement of Serpine1 on the Pg induced insulin secretion in the pancreatic beta-cell line MIN6.
Materials and Methods
MIN6 cell cultureMIN6 cells (passages 20-30) were grown in Dulbecco’s modified Eagle’s medium (DMEM, containing 25 mM glucose, 10% fetal bovine serum, 4 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mg/ml penicillin/streptomycin and 2 μl/liter of 2-mercaptoethanol). Cells were incubated at 37 °C in a 5% CO2 humidified incubator. The culture medium was changed every 3-4 days and cells passaged once a week.
P. gingivalis culture
P. gingivalis (strain W83) was grown anaerobically (85% N2,10% H2 and 5% CO2) in Gas Pak anaerobic containers (Becton Dickinson, Franklin Lakes, NJ) using Gas Pak EZ pouches (BD) at 37 °C. The bacteria were grown in 3% Bacto Todd Hewitt Broth (BD) supplemented with 10 μg/ml hemin and 1 μg/ml vitamin K1 (both from Sigma, St. Louis, MO). The cells were grown overnight and the approximate cell density was determined using a spectrophotometer at an optical density of 550 nm based on a standard curve established by colony formation on bacterial plates.
Glucose stimulated insulin secretion (GSIS) and ELISA
Mid-log MIN6 cells (p24-28) were plated in 24 well plates at 2x105 cells/well in 1ml of low glucose (5.5 mM) DMEM and incubated at 37 °C and 5% CO2. After 24 hrs, media were removed and fresh low glucose DMEM was added. Pg from overnight cultures were washed with PBS and resuspended in low glucose DMEM. Amounts of the bacterial suspension were added to the wells to obtain MIN6 to Pg ratios of 1:100 and 1:200. The plates were then incubated overnight (16 hrs). The media were removed and the cells were washed twice with KRBH buffer (119 mM NaCl, 2.54 mM CaCl2.2H2O, 1.19mM KH2PO4, 4.74 mM KCl, 25 mM NaHCO3, MgCl2.6H2O, 10mM HEPES) containing 0.5% BSA. KRBH-0.5% BSA with 2.8 mM glucose was added to each well and incubated for 30 minutes. This minimal glucose solution was removed and replaced with KRBH-0.5% BSA with 5 mM or 25 mM glucose. Cells were re-incubated for an additional 2 hrs. 700 μl of supernatant was collected from each well and spun briefly (400 x g) to remove cell debris. GSIS was determined based on insulin concentrations in the supernatants determined using Mouse Insulin High Range ELISA kit from ALPCO (Salem, NH) according to the manufacturer’s protocol. The samples from duplicate wells for each time point were assessed for insulin concentration. Four independent experiments were carried out.
Methylene blue staining
The insulin concentration in each well was normalized to the cell number in the respective wells. Briefly, MIN6 cells were fixed in 100% ethanol and stained with 0.5 % methylene blue in 10% methanol for 15 minutes. The cells were washed 5 times with distilled water and the dye was extracted with 250 μl of 1N NaOH, transferred to a clear 96 well plate, and the OD of the dye solution measured at 600 nm using a plate reader. The OD of the dye in each well is proportional to the number of cells in the well. The insulin concentration in each well was divided by the respective OD of the dye from the well to obtain relative insulin secretion.
Total RNA extraction and purification
Mid log MIN6 cells (p26-28) were plated in 100 mm tissue culture dishes (1X107 cells) and incubated for 24 hrs. Media containing 2X109 Pg (1:200 MIN6 to Pg) was added and plates reincubated for 2, 4, 7, 16 or 24 hrs. After incubation media were removed and MIN6 cells immediately lysed in 4 ml of Trizol (Life Technologies, Carlsbad, CA). Control cells were also lysed at 0 and 24 hr time points. Total RNA was isolated according to the manufacturer’s protocol (Life Technologies, Carlsbad, CA) and was further purified by using a RNeasy Mini kit from Qiagen (Valencia, CA) according to the manufacturer’s protocol. RNA was quantitated using a Nano Drop 2000 spectrophotometer (Thermo Scientific, Pittsburgh, PA).
Genomic DNA elimination and cDNA synthesis
3 μg of total RNA was treated with 1 μl of 10X DNase buffer and 1 μl of RQ1 RNase free DNase I (Promega, Madison, WI) for 30 min at 37 °C, added 1ul of stop buffer (Promega) and incubated for 10 min at 65 °C in a 10 μl reaction mix. Reverse transcription was carried out in a 20 μl volume using high capacity cDNA Reverse Transcription kit (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol (25 °C for 10min, 37 °C for 2 hrs, 85 °C for 5 min).
Quantitative real-time PCR
1 μl of cDNA was amplified in a 25 μl total volume containing 12.5 μl of 2X SYBR Green PCR Mastermix (Life Technologies, Carlsbad, CA) and 400 nM each of the forward and reverse primers corresponding to the gene of interest. The Serpine 1 primers were custom synthesized by Integrated DNA Technologies (Coralville, IA). The sequence of primers (Integrated DNA Technologies, Coralville, IA) were 5’-GAC ACC CTC AGC ATG TTC ATC-3’ (Sense), 5’-AGG GTT GCA CTA AAC ATG TCA G-3’ (Antisense). The amplification was carried out using a Step One Plus real-timePCR system (Life Technologies, Carlsbad, CA) using the following conditions: 1 cycle of 95°, 10 min and 40 cycles of 95°, 15s and 60°, 1 min. The homogeneity of the amplification products were ascertained by melting curve analysis. The results were normalized by simultaneous amplification of the house keeping gene, hypoxanthine phosphoribosyltransferase1 (HPRT) and comparing the CT values. The relative expressions of the mRNA was estimated by the ΔΔCT method (quantity of mRNA= 2-ΔΔCT).
Transduction with serpine 1 and control shRNA lentiviral particles and confirmation of serpine 1 expression by qPCR and western blotting
Mid-log MIN6 cells (p24) were plated with high glucose DMEM containing serum and antibiotics into 12 well plates at a density of 5X105 cells/ml/well and incubated for 24 hours at 37 °C and 5% CO2. Media was replaced with 1ml of fresh medium containing 5 μg/ml of polybrene. The Serpine 1 (cat# sc36180-V) and control (cat# sc108080) shRNA lentiviral particles (both from Santa Cruz Biotechnology, Dallas, TX) were thawed at room temperature and added to cultures in separate wells (100 μl, containing approximately 5X105 virus particles). After 24 hours of incubation, the culture medium was replaced with fresh medium without polybrene. The cells were allowed to grow to approximately 80-90 % confluent (3 days), and then trypsinized and re-plated in 100 mm tissue culture plates at various low dilutions in culture medium containing 5 μg/ ml of puromycin (Life Technologies, Grand Island, NY) for clonal selection. The media were replaced with fresh puromycin containing medium every 3-4 days until distinctly visible resistant colonies were formed. Several colonies of Serpine1- and control virus-transduced cells were selected and expanded. The expression of shRNA in the clones was assayed by RT-qPCR and western blotting. Serpine1 gene expression was confirmed by RT-qPCR as described above.
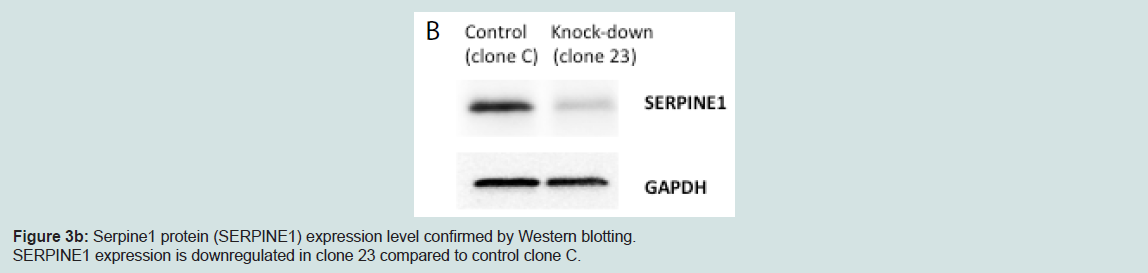
To confirm the level of Serpine1 protein expression, cell lysates from clone #23 and control clone were prepared in RIPA buffer [50mM Tris–HCl (pH 7.5), 150 mM NaCl, 1mM EDTA, 1% IGEPAL (Sigma)] supplemented with protease inhibitor tablet (Complete Mini, Cat# 11836153001, Roche Diagnostics, Manheim, Germany), 10 μm/ml of phosphatase inhibitor cocktail II (cat# 524625, EMD Millipore, Billerica, MA), 5 μl/ml of 10 mM NaVO4 and 20 μl/ml of 50 mM phenylmethyl sulfonyl fluoride. Lysates were sonicated and incubated on ice for 20 min. Following centrifugation, aliquots ofthe supernatant were stored at -85 °C until assays were conducted. SERPINE1 was detected using PAI1 antibody (cat# 11907S, Cell Signaling Technology, Danvers, MA) as primary and HRPconjugated anti-rabbit IgG (cat# 170-6515, Bio-Rad Laboratories, Inc., Hercules, CA) as secondary antibody. GAPDH (cat# 2118S, Cell Signaling Technology, Danvers, MA) was used as a loading control. Signal was detected using Super Signal West Dura substrate (cat# 34075, Thermos Scientific, Rockford, IL) and signals were quantified using ChemiDoc XRS+ imaging system (Bio-Rad Laboratories, Inc., Hercules, CA).
Statistical analysis
Statistical analysis was performed using a paired Student’s T-test with a significance level of p<0.05.
Results
Relative insulin secretion increased with increased number of P. gingivalis to MIN6 cell ratios in both normo- and hyperglycemic conditionsRelative insulin secretion was measured by GSIS analysis. Insulin secretion increased relative to an increasing number of Pg to MIN6 ratio (Figure 1). This increase was statistically significant when comparing secretion of control (no Pg) to secretion using a 1:200 cell to Pg ratio (p<0.001) under both normo- and hyperglycemic conditions, and for control vs. a 1:100 cell to Pg ratio under normoglycemic conditions.
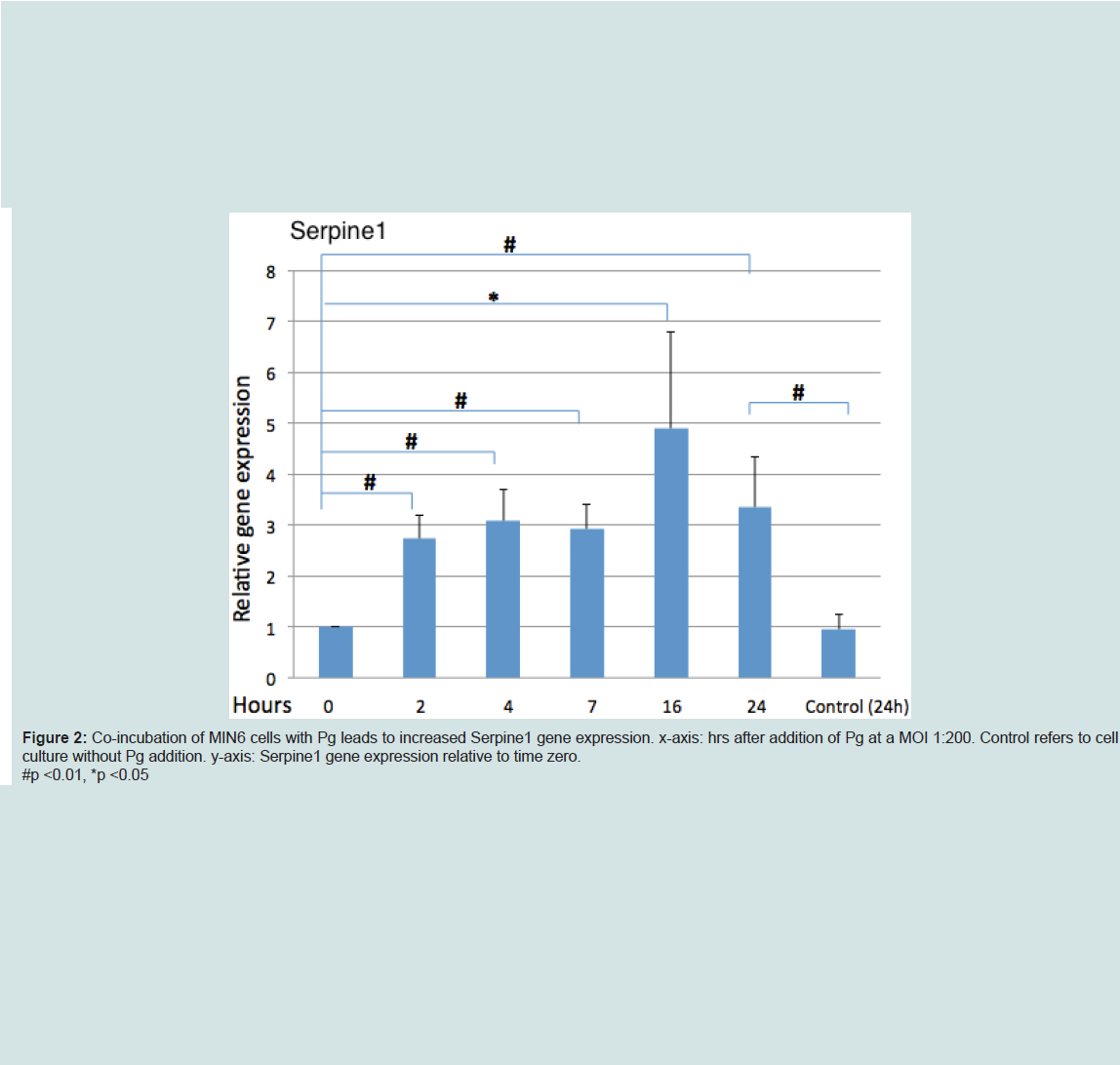
P. gingivalis upregulated serpine1 gene expression
Analysis of Serpine1 gene expression by MIN6 cells co-incubated with Pg (MOI of 1:200 cell to Pg) revealed an increased expression relative to time zero under normoglycemic conditions at all time points (Figure 2).
Cells with serpine1 knockdown resisted Pg stimulated insulin secretion
We generated MIN6 clones which have knockdown Serpine1 expression following transduction of Serpine1 shRNA and control clones which were transduced with scrambled shRNA. Clones with minimal expression of Serpine1 (clone #23) in terms of both RNA (Figure 3a) and protein (Figure 3b) levels and clones with higher expression of Serpine1 (clone C) were selected for further study.
Discussion
We previously investigated the effect of Pg LPS on insulin secretion using the pancreatic beta cell line MIN6 [11]. The results indicated that Pg LPS augments insulin secretion from MIN6 cells under a normoglycemic, but not hyperglycemic conditions and that Serpine 1 expression increases in response to Pg LPS. In the current study, we investigated the effect of whole Pg on insulin secretion and determined the impact of Serpine 1 expression on insulin secretion under normo- and hyperglycemic conditions. The results indicated that Pg augmented insulin secretion not just under normoglycemic conditions, but also in cells maintained in a hyperglycemic state when the number of bacteria per MIN6 cells was increased (MOI 1:200). Furthermore, Serpine 1 expression was upregulated by Pg and cells with downregulated Serpine gene expression were resistant to Pg stimulated insulin secretion under normoglycmeic conditions.The results from a study by Madianos et al. indicate that Pg FDC381 multiplies and persists within human oral epithelial cells in vitro and also that 50% of Pg in culture media survives for at least 2 hrs after initiating incubation [15]. In the current study we did not determine if Pg invaded and persisted within MIN6 cells. Thus, we cannot be certain if direct Pg contact with MIN6 cells or Pg byproducts released by Pg during co-incubation were the cause for increased insulin secretion. Assuming that byproducts or products including but not limited to LPS may be responsible for the augmented secretion of insulin, an understanding of which by-products are involved may be of benefit as a potential therapy in diabetic subjects.
As a first step to determine the mechanisms by which Pg augments insulin secretion, we investigated Serpine1 which is known to be increased in plasma of subjects with metabolic impairment/diseases such as insulin resistance [16], metabolic syndrome [17], Type 1 [18] and Type 2 [13,14] diabetes mellitus. Serpine1 regulates various physiological processes that require proteolytic activity such as complement activation, inflammation, angiogenesis, cell signaling, migration, apoptosis, neoplasia and viral pathogenesis [19,20]. Elevated levels of SERPINE1 in plasma are also associated with moderate and severe periodontitis [12]. Interestingly, treatment of severe periodontitis by tooth extraction reduces systemic levels of SERPINE1 significantly [21]. This suggests that inflammation may be the cause of the increased plasma levels of SERPINE1 in subjects with periodontitis.
SERPINE1 is predominantly produced by the endothelium and adipose tissue, but is also secreted by human pancreatic islets upon glucose stimulation in vitro [22]. Based on the results from our current study, SERPINE1 was produced by the beta cell line MIN6 upon stimulation with Pg under normoglycemic conditions. It has been shown that LPS from Pg induces SERPINE1 production in human gingival fibroblasts via activation of NF-kB and the MAP kinases, ERK/p38/JNK [23]. In gingival fibroblasts, Serpine1 expression increases during the first 8 hrs and declines to baseline following 10 hrs incubation with Pg LPS [23]. In the current study, a decline in gene expression occurred after 16 hrs, but at 24 hrs, gene expression was still statistically elevated compared to baseline. This difference in response may be due to the different types of cells studied or due, in part, to the presence of Pg byproducts other than LPS.
An interesting role for Serpine1 was determined using a Serpine-/- obesity mouse model (ob/ob) system in which animals were fed a high fat diet or high fat diet with high sucrose [24,25]. Results from these studies indicate that knocking down Serpine1 expression improves hyperglycemia. For example, knockout mice fed a high fat diet have reduced diet-induced obesity, hyperglycemia and hyperinsulinemia compared to wild type mice fed a high fat diet [24]. Similarly, Tamura et al. demonstrated that SERPINE1 deficiency improved hyperinsulinemia and insulin resistance in obese Serpine1 knockout mice. Collectively, the data suggest a close link between increased SERPINE1 expression, hyperinsulinemia and diabetes [25]. Furthermore, PPARγ and adiponectin, key molecules controlling lipid metabolism and insulin sensitivity, are significantly decreased in WT animals fed a HF diet compared to Serpine1-/- animals [24]. Thus, Serpine1-/- animals have a lower level of plasma insulin due to improved adipose tissue metabolism. In the current study, Pg directly influenced the insulin secretion by MIN6 cells and Serpine1 was involved in upregulation of insulin secretion. However, the exact mechanism(s) by which Serpine1/SERPINE1 is involved is not clear. Collectively, results from the mice studies and our previous studies suggest that SERPINE1 may influence hyperinsulinemia via multiple pathways.
In summary, our results suggest that a bacterium associated with periodontal disease leads to increased insulin secretion by a beta cell line when the cells and bacteria are co-incubated. It is not clear how a periodontal pathogen or its byproducts from the oral cavity reach distant organs although with respect to byproducts, systemic delivery is certainly plausible [26]. It has been shown that bacteremia can occur following dental treatment or even tooth brushing [27-29]. In addition, swallowed periodontal pathogens can reach and alter the gut microbiota and result in increased LPS transport to the liver [30]. Thus there is a distinct possibility that periodontal pathogens such as Pg can increase insulin secretion by pancreatic beta cells either directly or via byproducts in vivo. Although in our experiments we used high bacteria to MIN6 ratios, the experimental time was short and the effect achieved in animals/patients may be evident over an extended period in the case of periodontitis which is a chronic condition. In conclusion, our results indicated that Pg may have a significant contribution to the development of hyperinsulinemia in the setting of periodontitis and Serpine1 may play a role in mediating insulin secretion.
Acknowledgements
MIN6 cells were kind gift from Dr. Donald Steiner at the University of Chicago. This study was supported by the NIH R01DE021405(KW).References
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, et al. (2012) Prevalence of periodontitis in adults in the United States: 2009-2010. J Dent Res 91: 914-920.
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, et al. (2004) The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res 83: 485-490.
- Benguigui C, Bongard V, Ruidavets JB, Chamontin B, Sixou M, et al. (2010) Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol 37: 601-608.
- Zadik Y, Bechor R, Galor S, Levin L (2010) Periodontal disease might be associated even with impaired fasting glucose. Br Dent J 208: E20-24.
- Colombo NH, Shirakashi DJ, Chiba FY, Coutinho MS, Ervolino E, et al. (2012) Periodontal disease decreases insulin sensitivity and insulin signaling. J Periodontol 83: 864-870.
- Su Y, Wang D, Xuan D, Ni J, Luo S, et al. (2013) Periodontitis as a novel contributor of adipose tissue inflammation promotes insulin resistance in a rat model. J Periodontol 84: 1617-1626.
- Watanabe K, Petro BJ, Shlimon AE, Unterman TG (2008) Effect of periodontitis on insulin resistance and the onset of type 2 diabetes mellitus in Zucker diabetic fatty rats. J Periodontol 79: 1208-1216.
- Watanabe K, Iizuka T, Adeleki A, Pham L, Shlimon AE, et al. (2011) Involvement of Toll-like receptor 4 in alveolar bone loss and glucose homeostasis in experimental periodontitis. J Periodontal Res 46: 21-30.
- Gavin JR 3rd, Roth J, Neville DM Jr, de Meyts P, Buell DN (1974) Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sic USA 71: 84-88.
- Rizza RA, Maandarino LJ, Genest J, Baker BA, Gerich JE (1985) Production of insulin resistance by hyperinsulinemia in man. Diabetologia 28: 70-57.
- Bhat UG, Ilievski V, Unterman TG, Watanabe K (2014) Porphyromonas gingivalis lipopolysaccharide (LPS) upregulates insulin secretion from pancreatic beta cells line MIN6. J Periodontol 85: 1629-1636.
- Bizzarro S, van der Velden U, ten Heggeler JM, Leivadaros E, Hoek FJ, et al. (2007) Periodontitis is characterized by elevated PAI-1 activity. J Clin Periodontol 34: 574-580.
- Pandolfi A, Cetrullo R, Polishuck, MM, Alberta MM, Calafiore A, et al. (2001) Plasminogen activator inhibitor type 1 increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol 21: 1378-1382.
- Lyon CJ, Hsueh WA (2003) Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med 115: 62S-68S.
- Madianos PN, Papanou PN, Nannmark U, Dahlen G, Sandros J (1996) Porphyromonas gingivalis FDC381 multiplies and persists within human oral epithelial cells in vitro. Infect Immun 64: 660-664.
- Bastard JP, Pieroni L, Hainque B (2000) Relationship between plasma plasminogen inhibitor 1 and insulin resistance. Diabetes Metab Res Rev 16: 192-201.
- Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, et al. (2007) Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr Metab 21: 4-28.
- Adly AA, Elbarbary NS, Ismail EA, Hassan SR (2014) Plasminogen activator inhibitor-1 (PAI-1) in children and adolescents with type 1 diabetes mellitus: relation to diabetic micro-vascular complications and carotid intima media thickness. J Diabetes Complications 28: 340-347.
- Richardson J, Vishwanathan K, Lucas A (2006) Serpins, the vasculature and viral therapeutics. Front Biosci 11: 1042-1056.
- Wilkins-Port CE, Freytag J, Higgin SP, Higgins PJ (2010) PAI-1: A multifunctional SERPIN with complex roles in cell signaling and migration. Cell Commun Insights 3: 1-10.
- Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, et al. (2006) Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res 85: 74-78.
- Schrimpe-Rutledge AC, Fontes G, Gritsenko MA, Norbeck AD, Anderson DJ, et al. (2012) Discovery of novel glucose-regulated proteins in isolated human pancreatic islets using LC-MS/MS-bases proteomics. J Proteome Res 11: 3520-3532.
- Na HS, Lim EJ, Jeong SY, Ryu MH, Park MH, et al. (2014) Plasminogen activator inhibitor type 1 expression induced by lipopolysaccharide of Porphyromonas gingivalis in human gingival fibroblast. J Microbiol 52: 154-160.
- Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, et al. (2004) Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 53: 336-346.
- Tamura Y, Kawao N, Yano M, Okada K, Matsuo O, et al. (2014) Plasminogen activator inhibitor-1 deficiency ameliorates insulin resistance and hyperlipidemia but not bone loss in obese female mice. Endocrinology 155: 1708-1717.
- Herzberg MC, Weyer MW (1998) Dental plaque, platelets, and cardiovascular diseases. Ann Periodontol 3: 151-160.
- Lockhart PB, Bremman MT, Sasser HC, Fox PC, Paster BJ, et al. (2008) Bacteremia associated with toothbrushing and dental extraction. Circulation 117: 3118- 3125.
- Daly CG, Mitchell DH, Highfield JE, Grossberg DE, Stewart D (2001) Bacteremia due to periodontal probing: a clinical and microbiological investigation. J Periodontol 72: 210-214.
- Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B (2005) Bacteremia following periodontal procedures. J Clin Periodontol 32: 708-713.
- Arimatus K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, et al. (2014) Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4: 482.