Journal of Microbiology & Microbial Technology
Download PDF
Research Article
*Address for Correspondence: Sarkar S, Manager, Quality Assurance, Metro Dairy Limited, Barrackpore, Barasat Link Road, Subhasnagar, Neelgunj Bazar, Kolkata - 700121, West Bengal, India, E-mail: drsurajitsarkar@yahoo.co.in
Citation: Sarkar S. Effect of Nisin on Technological and Microbiological Characteristics of Stirred Yoghurt. J Microbiol Microb Technol 2016;1(1): 6.
Copyright © 2016 Sarkar S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Microbiology & Microbial Technology | Volume: 1, Issue: 1
Submission: 21 April, 2016 | Accepted: 11 June, 2016 | Published: 15 June, 2016
Reviewed & Approved by: Dr. Hridayesh Prakash, Department of Biochemistry, University of Hyderabad, India
Effect of Nisin on Technological and Microbiological Characteristics of Stirred Yoghurt
Sarkar S*
- Quality Assurance, Metro Dairy Limited, Barrackpore, Kolkata,West Bengal, India
*Address for Correspondence: Sarkar S, Manager, Quality Assurance, Metro Dairy Limited, Barrackpore, Barasat Link Road, Subhasnagar, Neelgunj Bazar, Kolkata - 700121, West Bengal, India, E-mail: drsurajitsarkar@yahoo.co.in
Citation: Sarkar S. Effect of Nisin on Technological and Microbiological Characteristics of Stirred Yoghurt. J Microbiol Microb Technol 2016;1(1): 6.
Copyright © 2016 Sarkar S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Microbiology & Microbial Technology | Volume: 1, Issue: 1
Submission: 21 April, 2016 | Accepted: 11 June, 2016 | Published: 15 June, 2016
Reviewed & Approved by: Dr. Hridayesh Prakash, Department of Biochemistry, University of Hyderabad, India
Abstract
Purpose of this paper: Growing worldwide inclination towards healthful foods has compelled food processors to search for natural preservatives to enhance the shelf-life and extend the market reach. Limited shelf-life of cultured milk products arising from post-acidification could be enhanced with the application of bacteriocins such as nisin. Application of nisin in stirred yoghurt may retain its desirable properties during extended shelf-life.Design/Methodology/Approach: Stirred yoghurt containing nisin was obtained by culturing cow milk, heated at 95 °C / 5 min with 3% Yoghurt-YH-3 incubated at 42 ± 1 °C for 5 h and then stirred for incorporating 25 RU/ml nisin, followed by further 1 h incubation. Effect of incorporation of nisin on technological (titratable acidity, diacetyl and acetoin content, volatile acidity and extent of proteolysis and microbiological (total viable microbial contents, coliform count, yeast and mould, lipolytic organism and proteolytic organisms) and antibacterial characteristics of stirred yoghurt were assessed during 40 days of storage at 10 - 15 °C.
Findings: Incorporation of nisin in yoghurt induced lower acid and volatile acid development, but a significantly higher proteolytic activity as well as diacetyl + acetoin production. Nisin exhibitedantagonism against viable population but ineffective to yeast,mould and proteolytic organisms in yoghurt Nisin containing yoghurt exhibited lower degree of antagonism against various pathogenic test organisms, which progressive increased during storage.
Keywords
Nisin; Yoghurt; Antibacterial activityIntroduction
Growing consumer’s inclination towards health foods has attracted yoghurt owing to its prophylactic properties and its consumption has increased significantly throughout the world including UK and USA [1-3]. To extend the market reach, shelf-life of yoghurt and other cultured milk products could be enhanced by adopting diverse techniques such as bacteriocins (nisin, MicrogardTM, natamycin), LP-system, high pressure treatment, post-production heat-treatments (thermization, microwave heating), UV irradiation, carbonization etc. [4].Lactic acid bacteria (LAB) and their metabolites may have an important role towards microbiological quality and shelf-life of various fermented milk products [5]. With the objective of enhancing the shelf-life, inclusion of nisin producing strains of Lactococcus lactis subsp. lactis during the manufacture of cultured milk products, may not be advantageous due to their slower rate of acid production, limited proteolytic activity, inability to ferment sucrose and more proneness to bacteriophage [6,7].
Nisin has more than one mechanisms of antimicrobial action depending on several factors such as structural properties of target bacteria and exhibits antimicrobial activity against many species of Gram positive bacteria but not Gram negative bacteria due to their outer membrane barriers. Antibacterial mechanisms of nisin may be due to passage through cell wall and interaction with lipid II [8,9]. Nisin was inhibitory to both Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus and storage studies revealed prolonged inhibitory action towards the former organism [10].
It has been identified that nisin in pure form is not used for food processing owing to its very high specific activity and consequently JECFA (Joint FAO/WHO Expert Committee on Food Additives) specified that commercial nisin concentrates should possess at least 2.25% active nisin [11]. Nisaplin, a commercial preparation of bacteriocin nisin, is widely being used in dairy industries to attain three main objectives such as to combat an existing spoilage problem, permitting low heat-processing conditions and formulating new products [12-16].
Toxicological studies have revealed that nisin is safe for human consumption as it is inactivated by pancreatin and alphachymotrypsin, not detected in human saliva after 10 min of consumption, does not interfere with intestinal microbiota and has been designated as GRAS (Generally Recognised As Safe) for use as an anti-microbial [17-21]. Scientific Committee on Food (SCF) have approved an average daily intake of 0.13 mg/kg body weight and has been permitted for diverse food products in USA, Australia, South Africa, Russia and India [19,22]. In the present investigation an attempt has been made to evaluate the effect of inclusion of nisin on technological and microbiological characteristics of stirred yoghurt.
Materials and Methods
Type of milkAutoclaved reconstituted skim milk (0.5% fat and 7.64% SNF) was used for starter propagation as well as for evaluating their metabolic activities. For yoghurt manufacture fresh cow milk (3.0% fat, 8.5% SNF), obtained from local herds situated in Nadia district, West Bengal, India were used.
Source and maintenance of starter cultures
Freeze dried cultures of Yoghurt-YH-3, S. thermophilus, L. delbrueckii subsp. bulgaricus W and Bifidobacterium bifidum NDRI were obtained from National Collection of Dairy Cultures, National Dairy Research Institute, Karnal, India. All the cultures were maintained in plain sterile skim milk, except for B.bifidum NDRI in sterile skim milk fortified with 1% dextrose and 1% yeast extract [23].
Source and maintenance of pathogens
Virulent pathogenic strains of Bacillus cereus, Shigella dysenteriae (National Collection of Dairy Cultures, National Dairy Research Institute, Karnal, India), Salmonella typhi, three strains (03, 018, 078) of Escherichia coli and two strains (P1, P3) of Salmonella typhimurium (Department of Veterinary Microbiology, West Bengal University of Animal and Fishery Sciences, Nadia, India) were maintained on nutrient agar slants (Hi-Media, Bombay, India) by weekly propagations and were activated by three successive transfers at 24 h intervals in nutrient broth (Hi-Media, Bombay, India).
Source of Nisaplin
Nisaplin brand nisin with an activity of 1,000,000 IU/ g, obtained from Aplin and Barrett Ltd., England was added to inoculated milk after 3 h and 5 h of incubation at recommended concentration 25 RU/ml [24].
Preparation of stirred yoghurt
Stirred yoghurt was obtained by culturing cow milk, heated at 95 °C/5 min with 3% Yoghurt-YH-3 incubated at 42 ± 1 °C for 5 h and then stirred for incorporating 25 RU/ml nisin, followed by further 1 h incubation. Stirred yoghurt, stored at 15 ± 1 °C was subjected to various technological as well as microbiological attributes at intervals of 0, 5, 10, 15, 20, 25, 30, 35 and 40 days.
Analytical Techniques
Technological attributesMetabolic activities of various starter combinations and technological attributes of stirred yoghurt were evaluated on the basis of titratable acidity, diacetyl and acetoin content, volatile acidity and extent of proteolysis [25-28].
Microbiological attributes
Microbiological attributes of stirred yoghurt were evaluated on the basis of total viable microbial contents using nutrient agar (Hi-Media, Bombay, India), coliform count using violet red bile agar (Hi-Media, Bombay, India), yeast and mould using potato dextrose agar (Hi-Media, Bombay, India), lipolytic organism using tributyrin agar (Hi-Media, Bombay, India) and proteolytic organisms using milk agar (Hi-Media, Bombay, India) adopting the methods of Bureau of indian standards [25]. Antibacterial spectrum of the product was estimated by modified cup agar assay technique [29].
Statistical analysis
Results obtained in the present investigation were analysed statistically by the method of Snedecor and Cochran [30].
Result and Discussion
Effect of nisin on metabolic activities of yoghurt culturesResults related to effect of incorporation of nisin (25 RU/ml) at the end of 3 h and 5 h of incubation at 42 ± 1 °C on rate of acid development, volatile acidity and post-acidification during storage at 15 ±1 °C for 96 - 120 h of Yoghurt-YH-3 and S. thermophilus + L. delbrueckii subsp. bulgaricus W in association with B. bifidum NDRI have been proclaimed in Table 1
For both sets of yoghurt cultures, an increase in inoculum from (3 - 6%) did not exhibited any significant increase (P > 0.05) in rate of acid production and volatile acidity up to 6 h of incubation, however higher (P < 0.05) post-acidification being noted for higher inoculum. Addition of nisin induced slower rate of acid production as well as volatile acid production, which may be attributed to inhibition of yoghurt cultures by nisin. However, Shahani demonstrated higher degree of acidification by both S. thermophilus (0.92 vs. 0.94% lactic acid) and L. delbrueckii subsp. bulgaricus (1.88 vs. 1.95% lactic acid) in presence of nisin [31]. Inhibitory activity of nisin on S. Thermophilus and L. delbrueckii subsp. Bulgaricus have already been demonstrated [32,33]. Time of inclusion of nisin did not revealed any significant effect (P > 0.05) on extent of acidification up to 6 h of incubation, but late addition of nisin (after 5 h) exhibited pronounced retarded acid development during subsequent prolonged storage (120 h), indicating preservative effect of residual nisin. Fowler and McCann registered retention of 28.57% nisin activity after 24 h of storage at 22 °C [34]. Higher volatile acid production by all culture combinations denoted with late inclusion of nisin after 5 h may be ascribed to better metabolic activities of yoghurt cultures prior to addition of nisin.
No significant (P > 0.05) change in extent of acidification and volatile acid production by either sets of yoghurt cultures could be noticed with the inclusion of B. bifidum NDRI, indicating no symbiotic effect between them, hence their inclusion during the manufacture of stirred yoghurt was not suggested. Titratable acidity produced by yoghurt cultures in association with B. bifidum (0.67 - 0.73% lactic acid) was higher (0.61% lactic acid) than those noted for freshly prepared probiotic yoghurt but lower than reported values (0.89 - 1.10% and 0.55% lactic acid, respectively) for bifidus yoghurt and dietetic yoghurt containing B. bifidum [35-38]. Inclusion of B. bifidum exhibited better acid production in association with L. delbrueckii subsp. bulgaricus than with S. Thermophilus, however no considerable deviation noted with either combined L. delbrueckii subsp. bulgaricus + S. Thermophilus or Yoghurt or Yoghurt - YH - 3 [36,39]. Similarly, with regard to flavour profiles of yoghurt, associated growth of B. bifidum with S. thermophilus + L. delbrueckii subsp. bulgaricus induced lower contents of acetaldehyde, ethanol, diacetyl and volatile acidity, however slight improvement denoted in association with Yoghurt-YH-3 [39,40]. Due to least postacidification (0.69% lactic acid after 120 h of storage at 15 ± 1 °C) and highest volatile acid production (2.3 ml 0.1 N NaOH/ 50 mg curd after 6 h of incubation at 42 ± 1 °C), 3% Yoghurt YH-3 and nisin incorporation time after 5 h of incubation were finalized for the manufacture of stirred yoghurt containing nisin.
Effect of nisin on technological attributes of stirred yoghurt
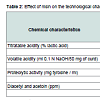
Results pertaining to effect of incorporation of nisin (25 RU/ml) on the technological attributes of stirred yoghurt have been proclaimed in Table 2. Incorporation of nisin in yoghurt induced lower acid development in contrast to samples without nisin (0.41 - 0.68% vs. 0.47 - 1.12% lactic acid) during 30 days of storage at 10-15 °C due to inhibitory action of nisin on yoghurt cultures. Titratable acidity attained by nisin containing yoghurt up to 35 days of storage (0.86% lactic acid) was within the suggested limits of 0.85 - 0.90% lactic acid [41]. Progressive decline in volatile acidity during storage were noticed in yoghurt, irrespective of presence of nisin. Incorporation of nisin into yoghurt induced no change (P > 0.05) in volatile acidity up to 5 days, but subsequent storage up to 30 days showed higher content up to 30 days in nisin containing samples in contrast to those without nisin. Significantly higher (P < 0.05) proteolytic activity (0.02 - 0.05 vs. 0.01 - 0.02 mg tyrosine/ml) as well as diacetyl + acetoin content (17.0 - 51.0 vs. 10.0 - 29.2 ppm) were denoted in nisin containing yoghurt than those without nisin during 30 days of storage. Sarkar and Misra noted volatile acidity and diacetyl and acetoin content of probiotic yoghurt to reach the peak value (2.0 - 2.9 ml of 0.1 N NaOH/50 mg curd and 40.0 - 101.0 ppm, respectively) after 3 days, followed by a decline during subsequent storage [35]. A decline in concentrations of acetaldehyde but an increase in diacetyl and diacetyl and acetoin during storage of yoghurt have been reported [42,43]. Proteolytic activity demonstrated in nisin containing yoghurt at the end of 35 days (0.06 mg tyrosine/ml) was within the limits of 0.05 - 0.10 mg tyrosine/g required for good quality yoghurt [44]. Enhanced proteolytic activity in nisin containing yoghurt may be due to breakdown of nisin by nisinase enzyme producing S. thermophilus [45].
Effect of nisin on microbiological attributes of stirred yoghurt
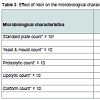
Results pertaining to effect of incorporation of nisin (25 RU/ml) on the microbiological attributes of stirred yoghurt have been proclaimed in Table 3. Total viable population (TVC) was significantly (P < 0.05) lower in nisin containing yoghurt than those without nisin (3 x 105 - 170 x 105 vs. 9 x 105 - 127 x 105 cfu/ml). It was further pointed out that there was a slight increase in TVC of nisin containing yoghurt up to 15 days (3 x 105 - 53 x 105 cfu/ml), followed by a gradual decline (53 x 105 - 15 x 105 cfu/ml) after 40 days. Reasons attributable to such behaviour of yoghurt cultures are synergistic action of nisin, other elaborated antimicrobial compounds and developed lactic acid in the product. Nisin is capable of binding the anionic phospholipids of the cell membrane which results in efflux of intracellular components and depolarisation of cytoplasmic membrane resulting in cessation of all biosynthetic processes [46,47]. No significant difference (P > 0.05) in counts of yeast and mould and proteolytic organisms in yoghurt up to 30 and 20 days, respectively were encountered due to application of nisin. Delves-Broughton has also mentioned non-effectiveness of nisin on yeasts and moulds. However, none of the yoghurt samples had lipolytic or coliforms [48].Effect of nisin on antibacterial properties of stirred yoghurt
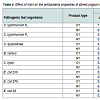
Exhibition of variable degree of antagonism against various pathogenic test organisms by yoghurt with or without nisin have been demonstrated, lower being observed in most of the samples containing nisin (Table 4). Reason attributable to reduced antagonism in nisin containing yoghurt is due to lower production of antimicrobial compounds resulting from inhibition of yoghurt cultures by nisin. During storage up to 40 days, a progressive increase in antagonistic activity of yoghurt against all test organisms, irrespective of presence of nisin were denoted, except against S. typhi for which a declining trend noted after 35 days. However, none of the yoghurt samples could exhibit antagonism against E. Coli 018. Maximum antibacterial activity was exhibited by nisin containing yoghurt against S. typhimurium P3 (10mm), followed by S. typhimurium P1 (9.5 mm), S. dysenteriae (8.0 mm) and E. Coli 03 after 40 days of storage. Inhibitory activity of yoghurt cultures against B. Cereus, S. typhimurium, S. Dysenteriae, Shigella spp. and E. Coli has been registered [39,49-51]. Mel’nikova and Koreleva denoted greater inhibitory activity of L. delbrueckii subsp. bulgaricus than S. Thermophilus [50].
Conclusion
Due to least post-acidification (0.69% lactic acid after 120 h of storage at 15 ± 1 °C) and highest volatile acid production (2.3 ml 0.1 N NaOH/50 mg curd after 6 h of incubation at 42 ± 1 °C), 3% Yoghurt YH-3 and nisin (25 RU/ml) incorporation time after 5 h of incubation were recommended for the manufacture of stirred yoghurt containing nisin. Inclusion of B. bifidum NDRI during the manufacture of yoghurt was not suggested. Stirred yoghurt containing 25 RU/ml nisin retained desirable technological, microbiological as well as antibacterial characteristics during 40 days of storage at 10 - 15 °C. Application of nisin for shelf-life extension of stirred yoghurt made from cow milk is suggested. Though the difference in quality of curd obtained employing cow milk and full cream milk is beyond the scope of this research, however no significant is expected as the average fat content of cow milk (3.50%) produced in West Bengal, India is similar with those in full cream milk (3.50%).Greater inclination of health conscious consumers towards yoghurt owing to its diverse nutritional and therapeutic has been noted. Manufactures could not satisfy their consumers due to few technical problems such as high post acidification, thereby reducing the viability of starter cultures and antibacterial activity towards pathogens or undesirable flora. Application of nisin in yoghurt will overcome all the technological problems and extend market reach.
References
- Sarkar S, Misra AK (2002) Yoghurt: nutritional and therapeutic significance. Indian J Microbiol 42: 275-287.
- Kowalska A, Jachnowicz AZ, Babuchowski A (2000) Yoghurt market in the United Kingdom. Nat Sci 6: 131-141.
- Sivak C (2000) Growth culture. Dairy Field 183: 1, 24-33.
- Sarkar S (2006) Shelf-life extension of cultured milk products. Nutr Food Sci 36: 24-31.
- Zottola EA, Yessi TL, Ajao DB, Roberts RF (1994) Utilization of cheddar cheese containing nisin as an antimicrobial agent in other foods. Int J Food Microbiol 24: 227-238.
- Lipinska E (1973) Use of nisin-producing lactic streptococci in cheese making. Int Dairy J 73: l-37.
- Lipinska E (1977) Nisin and its applications. In: Woodbine M (Ed), Antibiotics and antibiosis in agriculture, Butterworths, London, pp 103-130.
- Kramer NE, Hasper HE, van den Bogaard PT, Morath S, de Kruijff B, et al. (2008) Increased D-alanylation of lipoteichoic acid and a thickened septum are main determinants in the nisin resist¬ance mechanism of Lactococcus lactis. Microbiology 154: 1755-1762.
- Wiedemann I, Benz R, Sahl HG (2004) Lipid II-mediated pore formation by the peptide antibiotic nisin: a black lipid membrane study. J Bacteriol 186: 3259-3261.
- Punyauppa-path S, Phumkhachorn P, Rattanachaikunsopon P (2015) Nisin: production and mechanism of antimicrobial action. Int J Cur Res Rev 7: 47-53.
- Fowler GG, Gasson MJ (1991) Antibiotics - nisin. In: Russel NJ, Goulds GW (Eds) Food preservatives. Blackie and Sons, Glasgow, United Kindom, pp. 135-152.
- Gupta RK, Prasad DN (1989) Incorporation of nisin in stirred yoghurt. II. Effect on biochemical activities during storage. Cult Dairy Prod J 24: 9-10.
- Gupta RK, Prasad DN (1989) Incorporation of nisin in stirred yoghurt. III. Quantitative estimation of residual nisin. Cult Dairy Prod J 24: 11.
- Gupta RK, Prasad N, Prasad DN (1989) Use of nisin in dairy industry. Indian Dairyman 41: 229-233.
- Roberts RF, Zottola EA (1993) Shelf-life of pasteurized process cheese spreads made from cheddar cheese manufactured with a nisin-producing starter culture. J Dairy Sci 76: 1829-1836.
- Williams DJ, Tatini SR (1990) Behaviour of listeria monocytogenes in associative growth with nisin producing starter cultures. J Dairy Sci 73: 87.
- Jarvis B, Mahoney RR (1969) Inactivation of nisin by alpha-chymotrypsin. J Dairy Sci 52: 1448-1449.
- Claypool L, Hainemann B, Voris L, Stumbo, CR (1966) Residence time of nisin in the oral cavity following consumption of chocolate milk containing nisin. J Dairy Sci 49: 314-316.
- European Food Safety Authority (2006) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to: the use of nisin (E 234) as a food additive, Question number EFSA-Q-2005-031, Eur Food Saf Authority J 314: 1-16.
- U.S. Food and Drug Administration (2001) Agency Response Letter GRAS Notice No. GRN 000065, CFSAN/Office of Premarket Approval.
- Hurst A (1981) Nisin. Adv Appl Microbiol 27: 85-123.
- Scientific Committee on Food (1992) Opinions of the Scientific Committee for Food 26th Series, Commission of the European Communities, Luxembourg.
- Misra AK, Kuila RK (1991) The selection of bifidobacteria for the manufacture of fermented milks. Aust J Dairy Technol 46: 24-26.
- Gupta RK, Prasad DN (1988) Incorporation of nisin in stirred yoghurt. I. Effect on lactic and non-lactic organisms during storage, Cult Dairy Prod J 23: 17-18.
- Bureau of Indian Standards (1960) Methods of test for dairy industry. Part -I Rapid Examination of Milk, IS: 1479 Manak Bhavan, New Delhi, India.
- King N (1948) A modification of Voges-Proskauer test for rapid colorimetric determination of acetyl methyl carbinol+diacetyl in butter cultures. Dairy Ind 8: 860-864.
- Hempeniens WL, Liska BJ (1968) Method for determining volatile acids in cultured dairy products. J Dairy Sci.
- Hull ME (1947) Studies on milk protein II. Colorimetric determination of the partial hydrolysis of the proteins in milk. J Dairy Sci 30: 881-884.
- BS 4285 (1968) Methods of microbiological examination for dairy purposes, London.
- Snedecor GW, Cochran WG (1967) Statistical methods (6th edn), Oxford and IBH Publishers, Calcutta, India.
- Shahani KM (1962) Inhibitory effect of nisin upon various organisms. J Dairy Sci 45: 827-832.
- Kebary KM, Kamaly KM (1991) Susceptibility of starter and non-starter organisms to nisin in stirred yoghurt during storage. Egypt J Dairy Sci 19: 157-167.
- Bossi MG, Giraffa G, Carminati D, Neviani E (1988) Action of nisin on lactic acid bacteria: conductance measurements. Scienza Tecnica Lattiero Casearia 39: 249-261.
- Fowler GG, McCann B (1971) The growing use of nisin in the dairy industry. Aust J Dairy Technol 26: 44.
- Sarkar S, Misra AK (2006) Probiotic acidophilus milk for infants and children. Nutr Food Sci 36: 349-356.
- Misra AK, Kuila RK (1994) Use of bifidobacterium bifidum for the manufacture of bio-yoghurt and fruit bloyoghurt. Indian J Dairy Sci 47: 1-192.
- Srinivas H, Prabha R, Shankar PA (1997) Characteristics of cultured milks, yoghurt and probiotic yoghurts prepared from pre-refrigerated milks. J Food Sci Technol 34: 162-164.
- Sarkar S, Misra AK (2001) Characteristics of dietetic yoghurt. Indian J Dairy BioSci 12: 76-79.
- Sarkar S, Misra AK (1998) Selection of starter cultures for the manufacture of probiotic yoghurt. Egypt J Dairy Sci 26: 295-307.
- Kisza J, Zbikowski Z, Kolenda H (1978) Biochemical and rheological properties of yoghurt with added Bifidobacterium bifidum. XX Int Dairy Congr E: 545-546.
- Foster EM, Nelson FE, Speck ML, Doetsch RN, Olson JC (1958) Dairy microbiology. MacMillan & Co Ltd, London.
- Hruskar M, Vaheie N, Ritz M (1995) Aroma profiles and sensory evaluation of yoghurt during storage. Mljekarstvo: J Dairy Prod Process Improv 45: 175-90.
- Bozanic R, Tratnik LJ, Hruskar M (2005) Influence of culture activity on aroma compounds in yoghurts produced from goat’s and cow’s milk. Acta Aliment 32.
- Asperger H (1973) Acceptability of analytical methods for the assessment of yoghurt quality. Ost Milchwiss 28: 125.
- Boone P (1966) Mode of action and application of nisin. Food Manuf 41: 49-51.
- Bonev BB, Breukink E, Swiezewska E, De Kruijff B, Watts A (2004) Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J 18: 1862-1869.
- Kramer NE, Smid EJ, Kok J, de Kruijff B, Kuipers OP, et al. (2004) Resistance of Gram-positive bacteria to nisin is not determined by lipid II levels. FEMS Microbiol Lett 239: 157-161.
- Delves-Broughton J (1990) Nisin and its application as a food preservative. Int J Dairy Technol 43: 73-76
- Abd-El-Hady HM (1998) Viability of Bacillus cereus and Staphylococcus aureus in chocolate milk and fruit yoghurt stored at 4 degrees C and room temperature. Eighth Scientific Congress, Faculty of Veterinary Medicine, Assiut University, 15-17 November, pp. 13-21.
- Mel’nikova EV, Koreleva NS (1974) Capacity of a Lbm. bulgaricum and Str. thermophilus starter to produce antibiotic substances. Trudy, Vsesoyuznyi Nauchno issledovatel' skii Institut Molochnoi Promyshlennosti 33: 92-97, 130
- Aslim B, Beyatli Y (2000) The inhibition effect of yoghurt starter culture metabolites. Turkish J Biol 24.