Journal of Hematology & Thrombosis
Download PDF
Case Report
*Address for Correspondence: Nathan Visweshwar, MD, FRCPC, Division of Hematology, Morsani College of Medicine, 13330 USF Laurel Drive, Tampa, FL 33612, USA, Tel: 813-974-3725; E-mail: nviswesh@health.USF.edu
Citation: Visweshwar N, Patel A, Jaglal M, Laber D. Accidental and Surreptitious Intake of Oral Anticoagulants Including Older and Newer Agents - A Diagnostic Dilemma. J Hematol Thromb 2017;3(1): 3.
Copyright © 2017 Visweshwar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 3, Issue: 1
Submission: 27 December, 2016 | Accepted: 19 January, 2017 | Published: 26 January, 2017
Accidental and Surreptitious Intake of Oral Anticoagulants Including Older and Newer Agents - A Diagnostic Dilemma
Nathan Visweshwar1*, Ankita Patel2, Michael Jaglal2 and Damian Laber2
- 1Division of Hematology, Morsani College of Medicine, Tampa, FL 33612, USA
- 2Moffitt Cancer Center, Tampa, FL 33612, USA
*Address for Correspondence: Nathan Visweshwar, MD, FRCPC, Division of Hematology, Morsani College of Medicine, 13330 USF Laurel Drive, Tampa, FL 33612, USA, Tel: 813-974-3725; E-mail: nviswesh@health.USF.edu
Citation: Visweshwar N, Patel A, Jaglal M, Laber D. Accidental and Surreptitious Intake of Oral Anticoagulants Including Older and Newer Agents - A Diagnostic Dilemma. J Hematol Thromb 2017;3(1): 3.
Copyright © 2017 Visweshwar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 3, Issue: 1
Submission: 27 December, 2016 | Accepted: 19 January, 2017 | Published: 26 January, 2017
Abstract
The newer oral anticoagulants including dabigatran, rivaraxoban, apixaban and edoxaban are replacing warfarin in the management of atrial fibrillation and venous thromboembolism. Accidental and surreptitious overdose of warfarin has been reported, but so far there is no reported case of overdose involving NOACs. Overdose of warfarin prolongs coagulation tests including PT and APTT. This gives a clue as to the underlying cause of bleeding, but with the newer oral anticoagulants these tests are of no value. The diagnosis and management of bleeding with accidental and surreptitious intake of oral anticoagulants is very difficult and high index of suspicion is needed.Keywords
Newer oral anticoagulants; NOAC; Warfarin; Accidental; Surreptitious; BleedingIntroduction
Warfarin is being used as an oral anticoagulant for more than 50 years in patients with atrial fibrillation, preventing incidence of stroke in about two thirds of patients [1]. The most common drugrelated cause of hospitalization for adverse events among older adults in USA (accounting for 33% of such hospitalization), is warfarin toxicity [2]. It is estimated that 21,000 hospitalizations from 2000-2009 were from warfarin induced coagulopathy. Incidence of bleeding from the overdose of newer oral anticoagulants (NOACs) is not known. With warfarin, the hemorrhage is particularly high, when the INR exceeds 4. The NOACs have fixed dose and no monitoring is needed, according to the FDA guidelines. But even with NOACs like dabigatran, with proper monitoring and changing the dose as needed, major bleeds could be reduced by 30-40% in comparison with well controlled warfarin [3]. Accidental or surreptitious intake of warfarin when suspected is confirmed with decreased levels of II, VII, IX and X, which are vitamin K dependent coagulation factors [4]. With NOACs, increase of APTT with dabigatran and PT with factor Xa inhibitors is dependent on the sensitivity of the reagent used. Use of thrombin time may be of value in diagnosing dabigatran induced bleeding, as this agent is a direct thrombin inhibitor, but thrombin time is not a routine screening test in North America. We hereby report a case of overdose with oral anticoagulant warfarin and review the literature.Case Report
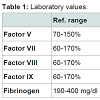
We report a 62-year-old Caucasian female working in attorney’s office presenting with gross vaginal and rectal bleeding. Her previous history is significant for morbid obesity for which she has had Rouxen- Y gastric bypass surgery in 2007. Her past medical history also includes hypothyroidism, hypertension and GERD but no history of muco-cutaneous bleeding. Patient’s dental extraction and the gastric bypass surgery in the past were uneventful. In March 2014 when patient developed vaginal and rectal bleeding, the PT was 80 sec and APTT 65 sec. The INR was 7. She had further screening work up (see Table 1). She was given fresh frozen plasma and gynecological and gastroenterological workup were requested. Gynecological work up and the upper and lower GI endoscopies were reported unremarkable. In February 2016 patient was readmitted again with vaginal and rectal bleeding (in between she has had inpatient care at other hospitals in the State of Florida). This time the gastroenterologist who did the sigmoidoscopy noted crusted blood in the mucosal fold (prior to any treatment with blood products). Her INR was 12. Further workup including factor assays were requested (correlation studies in Table 1). Patient was treated with fresh frozen plasma and PCC. The toxicology screen came back positive for warfarin. On further questioning patient did not admit taking warfarin and there was no other member in the household taking this agent. Patient was referred to the psychiatrist for further management.Discussion
Accidental or surreptitious ingestion of oral anticoagulants is very difficult to diagnose. In elderly patients dementia, low body mass index, altered body composition of fat and muscle, renal impairment and concurrent presence of multiple comorbidities and secondary drug interactions from polypharmacy predispose to accidental overdose of oral anticoagulants [5]. Surreptitious intake of oral anticoagulants is suspected when patient has multiple sites of mucosal bleeding with no proven anatomic defect, has access to health care, use of multiple healthcare facilities, evading questions, providing inappropriate history, lengthy hospital stay each time with extensive diagnostic work up and willingness to undergo extensive invasive procedures [6]. Expensive laborious coagulation test results reveal discrepant results. With surreptitious ingestion of oral anticoagulants, it is very difficult to get detailed personal or family history because of the evasiveness in answering questions. During the hospital stay, the patient invariably has no contact with friends or family members.With warfarin overdose, PT and APTT are prolonged, with very low vitamin K dependent coagulation factors II, VII, IX, and X. Toxicology screen for warfarin confirms the diagnosis. But patients who ingest other vitamin K antagonists including brodifacoum (new rodenticide) the warfarin assay is negative [7]. One may need to resort to fresh frozen plasma to control the bleeding from this agent to prevent life-threatening complications. Management of intracranial hemorrhage is especially very difficult as it has a mortality rate of about 60% [8].
For decades, warfarin had been the only anticoagulant to manage venous thromboembolism. Recently newer oral anticoagulants (NOACs) including direct Thrombin Inhibitor (dabigatran) and Factor IXa Inhibitors (rivaraxaban, apixaban and edaxaban) have been approved by FDA. They were initially approved for prophylaxis during orthopedic surgery, but later approved in patients with nonvalvular atrial fibrillation and for generalized prophylaxis and treatment of VTE. Excepting dabigatran (idarucicumab), other NOACs have no antidote, but these are undergoing phase III trials. NOACs are also contraindicated in severe renal failure, but apixaban can be used at a modified dose in moderate renal failure. These agents are not yet approved for patients with lupus anticoagulant syndrome and in patients with cancer. These agents were compared head to head with warfarin (ReLy/Rocket trials) and were found to be safe, with less of intracranial bleed. But these agents have marginally increased GI bleeding, because unlike warfarin, these agents are locally active in the GI tract and in patients with inflammatory bowel disease and AV malformation causes GI bleeding with NOACs, drug-drug interactions were less than warfarin, but need to be considered. Thus, co administration dabigatran with atorvastatin and dronedarone raised the levels of the NOAC by 18% and 70%, respectively. Diltiazem increases plasma levels of apixaban by 40%, another NOAC. Data from the Ljubljana Registry showed interruption or discontinuation of NOAC increased thromboembolic risk more than 20-fold, perhaps related to the short half-life of these agents [9].
FDA does not mandate routine testing, while patients are receiving NOACs, but several experts have suggested the need for monitoring in certain clinical situations to assess drug interactions and to determine compliance [10]. Dabigatran levels were increased by 18% and 70%, when co administered with atorvastatin and dronedarone, respectively [11]. Diltiazem increased 40% of plasma levels of apixaban [12]. Routine coagulation tests such as PT and APTT are not ideal to measure NOACs, as the reagents used vary in sensitivity to thrombin and factor Xa inhibitors [13,14]. Direct thrombin inhibitors are sensitive to thrombin time and factor Xa inhibitors to factor Xa assay [15,16]. NOACs acting through factor Xa inhibition can be monitored by modified chromogenic Xa assays. Dabigatran can be monitored by using modified thrombin time. The reagents used for coagulation tests have varying threshold to each of these agents. Thrombin time is markedly prolonged with dabigatran, but unfortunately thrombin time is not routinely used in North America for coagulation screening. NOACs also interfere with coagulation factor assays. Dabigatron interferes with factor VIII and factor IX assay [17]. Factor Xa inhibitors including rivaroxaban and apixaban interfere with factor II, VIII and factor X assays [18]. Once there is a strong suspicion of accidental/surreptitious overdose of NOACs, specific testing for the appropriate agent in a referral center using the manufacturer’s companion diagnostic assay for each agent and mass spectrometry, is the only way to confirm the plasma level of NOACs.
Conclusion
As age is not a contraindication for use of anticoagulant therapy (incidence of atrial fibrillation increases with age), increasing numbers of elderly patients are receiving anticoagulant therapy than ever before. NOACs are preferred in this age group. Incidence of accidental overdose and bleeding complications in the elderly and very elderly (above 85 years of age), with long term use of NOACs is not known, but caution is needed in this vulnerable population.References
- Hart RG, Pearce LA, Aguilar MI (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146: 857-867.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL (2011) Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 365: 2002-2012.
- Cohen D (2014) Concerns over data in key dabigatran trial. BMJ 349: g4747.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, et al. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(6 Suppl): 160s-198s.
- Kundu A, Sardar P, Chatterjee S, Aronow WS, Owan T, et al. (2016) Minimizing the risk of bleeding with NOACs in the elderly. Drugs Aging 33: 491-500.
- Asher R (1951) Munchausen's syndrome. Lancet 1: 339-341.
- Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, et al. (1990) Surreptitious ingestion of a long-acting vitamin K antagonist/rodenticide, brodifacoum: clinical and metabolic studies of three cases. Blood 76: 2555-2559.
- Rådberg JA, Olsson JE, Rådberg CT (1991) Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke 22: 571-576.
- Vene N, Mavri A, Gubenšek M, Tratar G, Vižintin Cuderman T, et al. (2016) Risk of thromboembolic events in patients with non-valvular atrial fibrillation after dabigatran or rivaroxaban discontinuation - Data from the Ljubljana Registry. PLoS One 11: e0156943.
- Kitchen S, Gray E, Mackie I, Baglin T, Makris M, et al. (2014) Measurement of non-coumarin anticoagulants and their effects on tests of Haemostasis: Guidance from the British Committee for Standards in Haematology. Br J Haematol 166: 830-841.
- Stangier J, Rathgen K, Stähle H, Reseski K, Körnicke T, et al. (2009) Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs 9: 59-68.
- Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, et al. (2016) Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur Heart J.
- Hillarp A, Gustafsson KM, Faxälv L, Strandberg K, Baghaei F, et al. (2014) Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost 12: 1545-1553.
- Lindahl TL, Baghaei F, Blixter IF, Gustafsson KM, Stigendal L, et al. (2011) Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost 105: 371-378.
- Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, et al. (2012) Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 107: 379-387.
- Stangier J, Feuring M (2012) Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 23: 138-143.
- Funk DM (2012) Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012: 460-465.
- Tichelaar V, de Jong H, Nijland H, Kluin-Nelemans H, Meijer K, et al. (2011) Interference of rivaroxaban in one-stage and chromogenic factor VIII:C assays. Thromb Haemost 106: 990-992.