Journal of Hematology & Thrombosis
Download PDF
Research Article
*Address for Correspondence: Agata Sobczyńska-Malefora, The Nutristasis Unit, Viapath, St. Thomas’ Hospital, London SE1 7EH, UK, Tel: +44 207 188 9543; Fax: +44 207 188 2726; E-mail: agata.malefora@viapath.co.uk
Citation: Sobczyńska-Malefora A, Critcher MS, Harrington DJ. The Application of Holotranscobalamin and Methylmalonic Acid in Hospital Patients and Total Vitamin B12 in Primary Care Patients to Assess Low Vitamin B12 Status. J Hematol Thromb 2015;1(2): 8.
Copyright © 2015 Sobczyńska-Malefora et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 1, Issue: 2
Submission: 09 October, 2015 | Accepted: 17 October, 2015 | Published: 21 October, 2015
Reviewed & Approved by: Dr. Raul H. Morales-Borges, Medical Director, American Red Cross in San Juan, Practices, Ashford Institute of Hematology & Oncology, USA
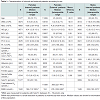
There were significant differences in most biochemical markers between internal and external patients for both genders, Table 1. The exception was serum folate for both, females and males (p = 0.99 and p = 0.27 respectively). In addition thyroid function test results were not significantly different between internal and external females (p = 0.20).
The incidence of elevated MMA from referrals for external patients
The Application of Holotranscobalamin and Methylmalonic Acid in Hospital Patients and Total Vitamin B12 in Primary Care Patients to Assess Low Vitamin B12 Status
Agata Sobczyńska-Malefora1*, Matthew S Critcher1,2 and Dominic J Harrington1
- 1The Nutristasis Unit, Viapath, St. Thomas’ Hospital, London, UK
- 2Nottingham Trent University, Nottingham, UK
*Address for Correspondence: Agata Sobczyńska-Malefora, The Nutristasis Unit, Viapath, St. Thomas’ Hospital, London SE1 7EH, UK, Tel: +44 207 188 9543; Fax: +44 207 188 2726; E-mail: agata.malefora@viapath.co.uk
Citation: Sobczyńska-Malefora A, Critcher MS, Harrington DJ. The Application of Holotranscobalamin and Methylmalonic Acid in Hospital Patients and Total Vitamin B12 in Primary Care Patients to Assess Low Vitamin B12 Status. J Hematol Thromb 2015;1(2): 8.
Copyright © 2015 Sobczyńska-Malefora et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 1, Issue: 2
Submission: 09 October, 2015 | Accepted: 17 October, 2015 | Published: 21 October, 2015
Reviewed & Approved by: Dr. Raul H. Morales-Borges, Medical Director, American Red Cross in San Juan, Practices, Ashford Institute of Hematology & Oncology, USA
Abstract
Background: Low vitamin B12 (B12) status is common in patient populations. The timely detection and correction of low B12 prevents megaloblastic anaemia and neurological impairment. However, prompt diagnosis is recognised as problematic especially when status is estimated using total B12 abundance in serum as the sole laboratory indicator. Emerging evidence indicates that holotranscobalamin (holoTC), the active fraction of B12, is a more reliable marker. Functional markers of B12 utilisation e.g. elevations in serum methylmalonic acid (MMA) concentration, can complement the assessment of B12 status.Methods: We assessed the prevalence of low vitamin B12 status using two different approaches. HoloTC was used for 9073 patients attending Guy’s & St. Thomas’ Hospital (‘internal patients’) supported by MMA if holoTC was between 25-70 pmol/L. The prevalence of low B12 status (serum B12 < 138 pmol/L) was also evaluated in 17875 primary care patients (‘external patients’). Markers associated with low vitamin B12 status were also investigated.
Results: The prevalence of low B12 status in internal and external patients was 14% (5.7% had a holoTC < 25 pmol/L and 8.3% had an elevated MMA) and 4.4% respectively. There was no difference in the prevalence of low serum folate between the two groups of patients. HoloTC and MMA correlated with markers associated with B12 deficiency as well as factors independent of B12 status.
Conclusions: Using the two different approaches the prevalence of low B12 status in internal patients was higher than for external patients. The incidence of elevated MMA in the small group of external patients was also high; suggesting that the application of total B12 alone may not identify all patients with low vitamin B12 status, and that MMA is a useful marker in confirming B12 status.
Keywords
Holotranscobalamin; Vitamin B12; Methylmalonic acid; Serum folate; Anaemia; Macrocytosis; Mean corpuscular haemoglobin; Alkaline phosphataseAbbreviations
holoTC: Holotranscobalamin; holoHC: Holohaptocorrin; TC: Transcobalamin; MMA: Methylmalonic Acid; WBC: Leucocytes; RBC: Erythrocytes; Hb: Haemoglobin; MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Haemoglobin; PCV: Packed Cell Volume; PLT: Platelets; eGFR: Glomerular Filtration Rate; ALB: Albumin; TSH: Thyroid-Stimulating Hormone; ALP: Alkaline PhosphataseIntroduction
Vitamin B12 (cobalamin) deficiency is common, with significant and variable clinical sequelae [1,2]. Deficiency is particularly prevalent in the general ostensibly healthy elderly population affecting ~5% of those aged 65-74 years, increasing to >10% in those >75 years [3]. Risk factors for low B12 include restricted diets, impaired gastric and intestinal absorption, pancreatic insufficiency, congenital/inherited factors, excess alcohol consumption and some pharmaceutical drugs. Although it is typically the clinical presentation that is the primary factor when assessing the significance of laboratory generated test results, in the case of B12 this is problematic because of the variability and severity of symptoms.Macrocytosis is the most common reason that B12 status is investigated. However these pathological changes are a late haematological manifestation of an advanced low B12 status only. Importantly, it is not uncommon for neuropathy and neuropsychiatric changes as a consequence of low B12 status to occur in the absence of macrocytosis or anaemia [4]. Most commonly it is the measurement of B12 in serum using automated assays based on competitive-binding luminescence technologies that are used to evaluate B12 status. Through this approach the total abundance of B12 is estimated and compared to pre-established reference intervals.
It is not widely appreciated that, in the circulation, B12 is predominately bound to two proteins and that serum B12 assays are unable to discriminate between the different forms. Although ~80% of circulatory B12 is carried by haptocorrin (HC) - holohaptocorrin (holoHC), extra-hepatic cellular receptors for this form have not been identified. The circulatory concentration of holoHC declines slower than B12 carried by transcobalamin (TC) - holotranscobalamin (holoTC), in response to a negative B12 balance i.e. when metabolic requirements exceed absorption of the vitamin from dietary exposure. It is B12 carried by TC that is taken up via a receptor medicated process by cells. Moreover, the majority of newly absorbed B12 binds to TC [5], hence a decline in intake or absorption of B12 will lead to a decrease in holoTC before serum B12.
Emerging evidence indicates that a low concentration of holoTC is a moderately more reliable marker of impaired B12 status than a low concentration of serum vitamin B12 [6-8]. However, the lack of a consensus view for diagnostic cut offs for investigating B12 status is a barrier to the comparison of different markers of B12 status. There is also no consensus with regards to the clinical significance, or otherwise, of low B12 status (rather than overt deficiency states). Although holoTC may be the earliest marker for B12 depletion and several studies now support it being used as a first-line diagnostic test [7,9], there is still much to be learnt about the interpretation of holoTC in general patient populations. In particular, and in common with the widely used serum B12 assays, a wide indeterminate ‘grey area’ for holoTC result interpretation exists. For patients defined as having an indeterminate B12 status using holoTC, methylmalonic acid (MMA) is a useful second line test. An elevation in the serum concentration of MMA can indicate a disturbance in metabolic networks as a consequence of low B12 status because the enzyme methylmalonyl-CoA mutase requires adenosylcobalamin (metabolically active form of B12) as a cofactor for the conversation of methylmalonyl-CoA to succinyl-CoA. The excess methylmalonyl-CoA is hydrolyzed to MMA.
To highlight the current variance between different diagnostic approaches applied in the UK we report the prevalence of low B12 status in outpatients and inpatients attending Guy’s & St. Thomas’ Hospital (defined as ‘internal patients’) and primary care (‘external patients’) from the same geographic area (South London, UK) using example standard laboratory diagnostic procedures applied within a single laboratory: a diagnostic algorithm of holoTC supported by MMA, if holoTC is 25-70 pmol/L (defined as indeterminate for this study) for ‘internal patients’, and the more commonly applied approach of ‘stand alone’ serum B12 measurement for ‘external patients’. We also looked into markers associated with low vitamin B12 status and factors affecting these markers independently of vitamin B12 status.
Methods
We evaluated the results from all patients referred to the Nutristasis Unit between Jan-Jun 2013 for routine vitamin B12 assessment. Samples from internal patients (hospital wards and outpatient clinics), N = 9,073 at Guy’s & St. Thomas’ Hospital had holoTC measured as a first line test followed by MMA if the holoTC result was within the indeterminate range. Deficiency was defined as holoTC < 25 pmol/L, an indeterminate range for holoTC was set between 25-70 pmol/L [7,9]. 17875 samples from external patients (mainly local primary care providers based in the Southwark and Lambeth Boroughs of London), had vitamin B12 status assessed using the serum vitamin B12 test alone. Deficiency was defined as a serum vitamin B12 < 138 pmol/L (established by Abbott Diagnostics from 143 specimens from individuals with normal mean corpuscular volume, homocysteine and folate [10]). It is standard practice in our laboratory to suggest MMA analysis for vitamin B12 results between 110-147 pmol/L. Renal, liver, thyroid function profiles and full blood counts (FBC) results were also collected if available from the same sample or within one week of sample collection for vitamin B12 testing. All data was extracted from our Laboratory Information System (Pathnet, version Classic 306) using the Cerner Corporation Limited (US) variant of the industry standard query tool (SQL) as previously reported [9].Serum holoTC and total vitamin B12 were measured using the Architect 2000 Series analyser (Abbott Diagnostics). Serum MMA was measured in all samples with a holoTC concentration between 25-70 pmol/L, subject to an estimated glomerular filtration rate (eGFR) of ≥60 mL/min/1.73 m2. Methylmalonic acid was also measured if requested separately by the referring physician. A Gerstel Multi Purpose Sampler (MPS) coupled directly to liquid chromatography tandem mass spectrometry (MPS-LC-MS/MS) was used [11]. Intraand inter-assay CVs were < 10%. The MMA concentration >280 nmol/L was considered to be elevated (>360 nmol/L was applied to patients >65 years) [9,12-14]. We also applied our recently derived MMA cut-off for adults 19-64 years old of 452 nmol/L [15] (is being evaluated prior to introduction into diagnostic service) into analysis of the incidence of elevated MMA levels from serum B12 referrals.
Leucocytes (WBC), erythrocytes (RBC), haemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and platelets (PLT) were determined by Beckman Coulter analysers (LH750, LH500, DxH and Ac75) at the Haematology Department (Guy’s and St. Thomas’ Hospitals). Serum creatinine was analyzed on Modulars, Integras and c501 analysers by Roche. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula [16]. Ethnicity was not included in the calculations of eGFR. Serum folate and ferritin were analysed using the Architect System (Abbott Diagnostics). Liver function, thyroid function tests were performed using a Ross 8000 Series analyser with the tests performed on c702 and e602 modules respectively (Ross-Tech).
Statistical analyses were carried out using SPSS for Windows, version 22 (SPSS Inc, US). All the continuous variables were not normally distributed, and statistical analyses were carried out using nonparametric tests. Medians with interquartile ranges were used to display values from internal and external samples for females and males. The comparison of values according to sex between internal vs. external patients was carried out using the Mann-Whitney test. Mann-Whitney was also used to compare holoTC, vitamin B12 and serum folate between sexes. Spearman rank correlation coefficients were used to assess simple correlations between the markers. The chi-squared (χ2) test was used to compare the frequency of elevated MMA within the holoTC indeterminate range between patients ≤65 and >65 years old.Results were considered statistically significant if the observed two sided P value was < 0.05.
Results
Characteristics of internal and external patientsA total of 9,076 samples from internal patients were screened for B12 deficiency by holoTC. Of these MMA measurement was indicated by a holoTC 25-70 pmol/L for 3,290 (36.3%) of patients.
A total of 17875 samples were received from external patients and screened for B12 deficiency using the serum vitamin B12 assay. The referring physician directly requested MMA analysis for 255 external patients (1.4%).
Internal patients were older than external patients (p < 0.01; Table 1). 57% and 66% of internal and external patients were female respectively; hence analyses were performed according to sex.
There were significant differences in most biochemical markers between internal and external patients for both genders, Table 1. The exception was serum folate for both, females and males (p = 0.99 and p = 0.27 respectively). In addition thyroid function test results were not significantly different between internal and external females (p = 0.20).
For internal patients holoTC concentration was not sexdependent p = 0.288. However, in external patients, serum B12 concentrations were significantly higher in females than in males, p < 0.001. Females from both groups also had significantly higher serum folate than males, p < 0.001.
Frequency of results outside cut-offs with relevance to vitamin B12 deficiency
Vitamin B12 deficiency is often associated with increasing age, pancytopenia, low creatinine, low albumin (ALB), hypothyroidism, impaired renal function, deficiency of folate and iron. Table 2 shows the frequency of these markers and markers associated with these conditions which were outside our cut-offs.
The prevalence of patients >65 years was greater amongst internal patients (37%) than external patients (25%). Patients with a low WBC accounted for < 10% in both groups and sexes. However, a significant difference was observed in the frequency of one or more of a low ALB, Hb, PCV or RBC between internal and external patients, with a prevalence of ~50% for internal patients and ~25% for external patients. The prevalence of MCV >100 fl did not differ between internal and external patients. However MCH >32 pg was more prevalent in internal patients, for both females and males. In addition a high MCH was more prevalent than a high MCV for all patients. There were also more internal patients (>10%) than external patients (7%) with TSH >4.2 mlU/L. The prevalence of low B12 status among internal patients with TSH >4.2 mlU/L was 9% (holoTC < 25 pmol/L) and 10% had holoTC between 25-70 pmol/L range and an elevated MMA. Serum B12 < 138 pmol/L was seen in 6% of external patients with TSH >4.2 mlU/L.
Thrombocytopenia (PLT < 150 x 109/L) was more prevalent in males and in internal patients. The frequency of patients with low creatinine was < 10% for all groups. On the contrary, significant discrepancies between internal and external patients existed with regards to an elevated creatinine. Around ~40% of all internal subjects had an elevated creatinine/low GFR - implying impaired renal function compared to ~15% for the primary care population. No major differences in frequencies of low ferritin and folate between internal and external patients were observed.
The prevalence of folate deficiency (< 7 nmol/L) among internal patients was 17% compared to 5.7% of patients with low holoTC < 25 pmol/L and 8.3% with indeterminate holoTC and elevated MMA. Folate deficiency and total serum B12 below our cut-off of 138 pmol/L was found in 14% and 4.4% of external patients respectively. There was a significant correlation (Spearman’s test) between serum folate and holoTC (N = 6081; r = 0.166, p < 0.01) and serum folate and total B12 (N = 16039; r = 0.223, p < 0.01).
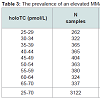
The prevalence of elevated MMA for internal patients with holoTC between 25 - 70 pmol/L
The overall incidence of an elevated MMA concentration (age related cut-offs applied), for patients with holoTC between 25-70 pmol/L was 24%. This represents 8.3% of the total internal patient population (Table 3). This is consistent with our previous study in which there was a decrease in the incidence of elevated MMA with an increasing holoTC concentration of 40% to 14% through the holoTC range of 25-50 pmol/L [9]. A significant difference (χ2 < 0.01) between the frequency of an elevation in MMA between patients ≤65 (22%) and >65 (28%) years old was observed.
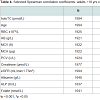
Selected Spearman rank correlation coefficients for holoTC and MMA with biochemical markers, for adult patients (>18 years) with holoTC 25-70 pmol/L and eGFR ≥60 mL/min/1.73 m2 are shown in Table 4. These analyses showed that holoTC in this range correlated positively with RBC, HB, PCV, creatinine, ALB and folate and negatively correlated with MMA, eGFR and ALP. Accordingly MMA correlated negatively with RBC and ALB and positively with ALP. In addition to the above there were also positive correlations of MMA with age, MCV, MCH, creatinine, folate and negative correlations with eGFR (Table 4).
Of external patients, only 255 (1.4%) had MMA determined at the point of clinical referral, which included 10% of all external patients found to have a B12 < 138 pmol/L. Of these 255 patients, 83 (33%) had a B12 result of < 138 pmol/L. Correspondingly the prevalence of confirmed low B12 status by elevated MMA in this group was 53% (N = 44). The prevalence of elevated MMA in 172 patients with serum B12 >138 pmol/L was 36% (N = 62).
We subsequently re-analysed the diagnostic criteria for these patients using our recently derived MMA cut-off for adults 19-64 years old of 452 nmol/L [15]. In the 83 patients with B12 < 138 pmol/L 29 (35%) had an elevated MMA concentration including 17 (20%) with MMA >750 nmol/L, a value above which vitamin B12 deficiency is highly probable [17]. Folate deficiency was identified in 37% of these 83 patients. Low HB was present in 34%, and MCV and/or MCH were above the cut-offs in 44% of these patients.
In 172 patients (B12 result >138 pmol/L), 20% had an MMA >452 nmol/L and 6% >750 nmol/L. Low folate status was present in 19% of these patients, while low HB and MCV and/or MCH above the cutoffs in 28% and 27% patients respectively.
Discussion
We investigated the prevalence of low B12 status in hospital patients using a simple diagnostic algorithm of holoTC supported by MMA if the holoTC result was within the indeterminate range of 25-70 pmol/L, and the prevalence of low vitamin B12 in primary care patients using the more commonly applied approach of ‘stand alone’ serum B12 measurement. The cut offs of these two independent approaches were not intended to be directly comparable.The total prevalence of low vitamin B12 status in hospital patients was 14%. Of these 5.7% had a holoTC < 25 pmol/L and 8.3% had elevated MMA (with a holoTC between 25-70 pmol/L). The estimated prevalence of low B12 status based on the total B12 (< 138 pmol/L) measurement alone was 4.4%. Of the 839 external patients with an initial serum B12 of 110-147 pmol/L, only 10% went on to have MMA determined. Of those who were tested 54% had an MMA result consistent with low B12 status.
Our baseline characteristics demonstrated significant differences in biochemical and haematological markers between the groups, with a notable exception for serum folate. These differences may be attributed to age and renal status, since hospital patients were older and the prevalence of impaired renal function was higher in internal patients. These findings are not surprising since it is expected that a hospital in patient population has a higher proportion of patients with comorbidities potentially leading to biochemical and haematological abnormalities. A selection bias may account for the high proportion of internal patients with anaemia because anaemia is often the trigger for requesting folate and B12 status assessment. This approach is consistent with NICE guidelines for the management of chronic fatigue syndrome/myalgic encephalopathy which state that tests for folate and vitamin B12 status should not be carried out unless a FBC and MCV show a macrocytosis [18]. This is contradictory to the recent guidelines for the diagnosis and treatment of cobalamin and folate disorders, which state that elevated MCV is not a specific indicator of cobalamin deficiency, [1] and as already mentioned, a normal MCV does not to exclude a clinically significant vitamin B12 deficient state [4]. Moreover, B12 deficiency induced macrocytic anaemia, often coexists with iron deficiency induced microcytic anaemia [19], most likely because these nutrients share the ileum as a common site of absorption. Therefore, MCV in these cases is expected to be within the reference limits. Allied to this, a combined iron and vitamin B12 deficiency can also occur in people with the Helicobacter pylori infection. The pathophysiology by which Helicobacter pylori may lead to vitamin B12 and/or iron deficiency anaemia has not yet been fully understood. Some hypotheses include autoimmune processes [20] or changes in gastric pH. Of note is the finding that the prevalence of MCV >100 fl did not differ between internal and external patients. However MCH >32 pg was more prevalent in internal patients, for both females and males. Based on the correlations of MCH with iron deficiency, MCH has been suggested as a better marker of iron deficiency than MCV [21,22]. It is possible that MCH is also a superior marker of low vitamin B12 status than MCV, although limited evidence exists in the literature.
Interestingly, the disparities in most laboratory parameters under investigations between internal and external referrals did not translate into serum folate. Moreover the strongest correlations for serum folate were with serum B12 (r= 0.222, p < 0.01, N= 16,236) and holoTC (r= 0.195, p < 0.01, N= 7,600). These findings may indicate that the higher prevalence of anaemia in internal patients compared to external patients may not have been attributed to folate deficiency. The estimated prevalence of folate deficiency (< 7 nmol/L) in this study was between 12% and 18% for external female and internal male patients respectively. In comparison, the prevalence of folate deficiency using the same cut-off as in our study (7 nmol/L) was found to be 20% for males aged 67-74 years (23% for males >75 years) with a similar prevalence for females in a population-based study of older people living in Oxford, UK, N = 1562 [23]. Using a locally established cut-off of 8 nmol/L for serum folate, which was found to be optimal for the maintenance of ‘normal’ homocysteine levels < 14 μmol/L, Alfthan et al. estimated the prevalence of folate deficiency as 10.5% in a random Finnish population, N = 643 [24]. Both these studies and our present findings demonstrate that the prevalence of low serum folate concentrations is relatively high in patients and in the general population.
The reported prevalence of low B12 status, or overt B12 deficient states, is entirely dependent on the diagnostic criteria used. Estimates from 3% to 26% are reported in the literature [25,26]. In the Oxford study, the reported prevalence of individuals at high risk of vitamin B12 deficiency, defined as B12 < 150 pmol/L or borderline vitamin B12 (150-200 pmol/L), accompanied by elevated MMA (>0.35 μmol/L) or tHcy (>15.0 μmol/L), was 10% in the age group 65-74 and as much as 20% for over 74 year olds [23]. Using various statistical models, Bailey et al. found that 1% of adults in the United States are at high risk of vitamin B12 deficiency (serum B12 < 126 pmol/L) and 31% are within the intermediate range of 126-287 pmol/L [26]. The most commonly used cut-off for B12 deficiency is 148 pmol/L [27]. It has been estimated that this cut-offs misses 3-5% of patients with clinical deficiency, while the 200 pmol/L cut-off identifies all patients but produces more false-positive results [25]. Our cut-off of 138 pmol/L (Architect analyser, Abbott Diagnostics) is slightly lower to the one commonly used, but we do recommend confirmatory MMA analysis for results < 148 pmol/L. However, if we had applied this cut-off to our external patients in this study, the prevalence of low B12 status would have been 6% instead of the reported 4.4%.
Based on the current knowledge and variability between markers of B12 status, the assignment of the corresponding cut-offs for total B12 and holoTC is difficult. Hence the prevalence of low B12 status if we had used holoTC and MMA to screen the samples from external patients cannot be predicted. It has been estimated that holoTC consists of between 5-20% of total B12 [28]. In renal patients’ holoTC varied between 13% and 57% [29]. Our comparative data from another study (unpublished data) of patients with holoTC < 25 pmol/L only, indicated that holoTC consisted of 9.5% of the total B12 (N = 176; mean (SD): holoTC 18 (4.8), total B12 173 (153) pmol/L); whereas in 370 healthy subjects holoTC accounted for 24% of serum B12, mean (SD): holoTC 91 (59), total B12 380 (167) pmol/L.
From the MMA analysis, folate status and FBC indices in the small subset of external patients with B12 >138 pmol/L, which showed that 36% of these patients had elevated MMA, we can extrapolate this data to hypothesise that a proportion of external patients are likely to have had elevated MMA.
Our current strategy for B12 assessment of internal patients is similar to that of Hermann & Obeid [7]. In their grey zone of holoTC between 23-75 pmol/L (the range extending from the 90% diagnostic sensitivity to the 90% diagnostic specificity), elevated MMA (>300 nmol/L) was found in 18% of cases [7]. We found that 24% of patients had elevated MMA in the holoTC range of 25-70 pmol/L (Table 3).Other strategies used for B12 assessment include the use of four biochemical markers: total B12, holoTC, MMA and tHcy combined. The combined biochemical indicator ‘w’ (wellness parameter) can be additionally adjusted for age [30]. Although the use of the ‘w’ indicator may more reliably assess vitamin B12 status of an individual by categorising it into excellent, normal, transitional, deficient and pernicious anaemia states, the four markers for B12 status may not always be available in all hospital settings. However, a recent modified approach allows for the calculation of a combined indicator of B12 (cB12) status when some biomarkers are not available [31]. The use of cB12 is currently being evaluated in research and diagnostic settings.
Based on the strong and well known correlations of MMA with age and creatinine which were also confirmed in this study, any approach to B12 assessment should take these factors into account when interpreting results [9,12. Other factors such as sex, thyroid function, drug use or bacterial infection also need to be included when interpreting biochemical results related to B12 status. Interestingly MMA correlation analysis for adult patients with holoTC values between 25-70 pmol/L and eGFR ≥60 mL/min/1.73 m2 in addition to significant correlations age, renal function and FBC markers revealed correlations with ALP. A number of studies have previously reported associations of vitamin B12 deficiency with ALP and bone metabolism [32-3. The exact mechanisms by which B12 affects bone metabolism are not yet understood. Hypotheses include through the elevated homocysteine, MMA and cytokine concentrations or a direct effect on bone [33,38].
In conclusion, the prevalence of low B12 status in hospital patients, assessed with holoTC as a first line test and confirmatory MMA where applicable, was higher than for external patients who were evaluated using the total B12 test. The incidence of elevated MMA in a small group of external patients, referred by physicians for additional testing, following total serum B12 measurement was also high (~44%). These finding suggests that the application of total B12 alone is likely to miss patients with low vitamin B12 status and that MMA is a useful marker in confirming B12 status. In addition to these, the difference in prevalence, besides the methodologies used and cut-offs applied, can be attributed to the fact that hospital patients were older and had a higher prevalence of anaemia and renal impairment.
Sub-analysis of adult patients with eGFR ≥60 whose MMA was measured confirmed the positive correlations of MMA with markers related to B12 deficiency e.g. MCV, MCH, ALP as well as with other variables known to elevate MMA e.g. age, creatinine independently of vitamin B12 status. The latter needs to be incorporated into diagnostic algorithms for B12 status assessment.
Acknowledgements
We would like thank Mr Bernie Witchlow of Laboratory Informatics, Viapath for extracting the data for this study from the Laboratory Information System.References
- Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology (2014) Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 166: 496-513.
- Hunt A, Harrington D, Robinson S (2014) Vitamin B12 deficiency. BMJ 349: g5226.
- Clarke R, Grimley Evans J, Schneede J, Nexo E, Bates C, et al. (2004) Vitamin B12 and folate deficiency in later life. Age Ageing 33: 34-41.
- Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, et al. (1988) Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or m acrocytosis. N Eng J Med 318: 1720-1728.
- Arendt JF, Nexo E (2013) Unexpected high plasma cobalamin: proposal for a diagnostic strategy. Clin Chem Lab Med 51: 489-496.
- Heil SG, de Jonge R, de Rotte MC, van Wijnen M, Heiner-Fokkema RM, et al. (2012) Screening for metabolic vitamin B12 deficiency by holotranscobalamin in patients suspected of vitamin B12 deficiency: a multicentre study. Ann Clin Biochem 49: 184-189.
- Herrmann W, Obeid R (2013) Utility and limitations of biochemical markers of vitamin B12 deficiency. Eur J Clin Invest 43: 231-237.
- Obeid R, Herrmann W (2007) Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid. Clin Chem Lab Med 45: 1746-1750.
- Sobczynska-Malefora A, Gorska R, Pelisser M, Ruwona P, Witchlow B, et al. (2014) An audit of holotranscobalamin ("Active" B12) and methylmalonic acid assays for the assessment of vitamin B12 status: application in a mixed patient population. Clin Biochem 47: 82-86.
- Abbott Diagnostics (2010) B12, Architect System; REF 7K61; 49-3244/R6. Abbott Diagnostics.
- Gorska R, Harrington DJ, Roberts P (2014) Development of a fully automated method for determination of methylmalonic acid in human serum/plasma by MPS-LC-MS/MS. Clin Chem Lab Med 52: eA311.
- Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon CA, et al (2009) Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem 55: 2198-2206.
- Erdogan E, Nelson GJ, Rockwood AL, Frank EL (2010) Evaluation of reference intervals for methylmalonic acid in plasma/serum and urine. Clin Chim Acta 411: 1827-1829.
- Lloyd-Wright Z, Hvas AM, Moller J, Sanders TA, Nexo E (2003) Holotranscobalamin as an indicator of dietary vitamin B12 deficiency. Clin Chem 49: 2076-2078.
- Sobczynska-Malefora A, Harrington DJ, Voong K, Shearer MJ (2014) Plasma and red cell reference intervals of 5-methyltetrahydrofolate of healthy adults in whom biochemical functional deficiencies of folate and vitamin B12 had been excluded. Adv Hematol 2014: 1-7.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, et al. (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461-470.
- Bor MV, Nexo E (2011) Vitamin B12. In: Herrmann W, Obeid R (eds.). Vitamins in the prevention of human diseases, Walter de Gruyter & Co. KG, Berlin/New York, pp. 187-199.
- National Collaborating Centre for Primary Care (UK) (2007) Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): Diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) in adults and children [Internet]. National Institute for Health and Clinical Excellence: Guidance.
- Remacha AF, Sarda MP, Canals C, Queralto JM, Zapico E, et al. (2013) Combined cobalamin and iron deficiency anemia: a diagnostic approach using a model based on age and homocysteine assessment. Ann Hematol 92: 527-531.
- Osborne D, Sobczynska-Malefora A (2015) Autoimmune mechanisms in pernicious anaemia & thyroid disease. Autoimmun Rev 14: 763-768.
- Francis J, Sheridan D, Samanta A, Nichol F (2005) Iron deficiency anaemia in chronic inflammatory rheumatic diseases: low mean cell haemoglobin is a better marker than low mean cell volume. Ann Rheum Dis 64: 787-788.
- Jolobe OM (2012) Potential interchangeability of erythrocyte hemoglobin content and mean corpuscular hemoglobin for identifying iron deficiency anemia: comment on the article by van Santen et al. Arthritis Rheumatol 64: 4162.
- Clarke R, Refsum H, Birks J, Evans JG, Johnston C, et al. (2003) Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr 77: 1241-1247.
- Alfthan G, Laurinen MS, Valsta LM, Pastinen T, Aro A (2003) Folate intake, plasma folate and homocysteine status in a random Finnish population. Eur J Clin Nutr 57: 81-88.
- Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, et al. (2011) Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 94: 313S-321S.
- Bailey RL, Durazo-Arvizu RA, Carmel R, Green R, Pfeiffer CM, et al. (2013) Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in NHANES. Am J Clin Nutr 98: 460-467.
- Carmel R (2011) Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 94: 348S-358S.
- Nexo E, Christensen AL, Hvas AM, Petersen TE, Fedosov SN (2002) Quantification of holo-transcobalamin, a marker of vitamin B12 deficiency. Clin Chem 48: 561-562.
- Obeid R, Kuhlmann M, Kirsch CM, Herrmann W (2005) Cellular uptake of vitamin B12 in patients with chronic renal failure. Nephron Clin Pract 99: c42-c48.
- Fedosov SN (2013) Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: the age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta 422: 47-53.
- Fedosov SN, Brito A, Miller JW, Green R, Allen LH (2015) Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med 53: 1215-1225.
- Herrmann W, Obeid R, Schorr H, Hubner U, Geisel J, et al. (2009) Enhanced bone metabolism in vegetarians--the role of vitamin B12 deficiency. Clin Chem Lab Med 47: 1381-1387.
- Shahab-Ferdows S, Anaya-Loyola MA, Vergara-Castaneda H, Rosado JL, Keyes WR, et al. (2012) Vitamin B-12 supplementation of rural Mexican women changes biochemical vitamin B-12 status indicators but does not affect hematology or a bone turnover marker. J Nutr 142: 1881-1887.
- Vaes BL, Lute C, Blom HJ, Bravenboer N, de Vries TJ, et al. (2009) Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif Tissue Int 84: 413-422.
- Herrmann M, Schmidt J, Umanskaya N, Colaianni G, Al Marrawi F, et al. (2007) Stimulation of osteoclast activity by low B-vitamin concentrations. Bone 41: 584-591.
- Carmel R, Lau KH, Baylink DJ, Saxena S, Singer FR (1988) Cobalamin and osteoblast-specific proteins. N Engl J Med 319: 70-75.
- Kim GS, Kim CH, Park JY, Lee KU, Park CS (1996) Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism 45: 1443-1446.
- Scalabrino G, Nicolini G, Buccellato FR, Peracchi M, Tredici G, et al. (1999) Epidermal growth factor as a local mediator of the neurotrophic action of vitamin B(12) (cobalamin) in the rat central nervous system. FASEB J 13: 2083-2090.