Journal of Hematology & Thrombosis
Download PDF
Research Article
*Address for Correspondence: Genevieve Hale, Assistant Professor, Clinical Pharmacy Specialist-Cardiology, Nova Southeastern University College of Pharmacy, Palm Beach Campus, 11501 N. Military Trail Palm Beach Gardens, FL 33410, USA, Tel: (973)-766-2650; E-mail: Gmh418@yahoo.com
Citation: Hale G, Ebaugh S, Brenner M. A Comparative Evaluation of Adverse Drug Events and Incidence of Stroke amongst the Target Specific Oral Anticoagulants. J Hematol Thromb 2015;1(2): 6.
Copyright © 2015 Hale et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 1, Issue: 1
Submission: 28 July, 2015 | Accepted: 01 August, 2015 | Published: 06 August, 2015
Reviewed & Approved by: Dr. Neerja Vajpayee, Associate Professor, Department of Hematopathology, SUNY Upstate Medical University, USA
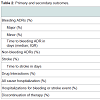
There were 20 bleeding ADRs identified amongst our patient population. Of the ADRs observed, 15.4% were attributed to dabigatran use, while 8.2% were associated with rivaroxaban use. Three major bleeding events were found amongst our patient population, two in the dabigatran group and one in the rivaroxaban group. A higher trend in the incidence (9.7%) of minor bleeding was identified more often (12.8 vs 7.1%, p = 0.205) and with less time to first occurrence (33 days vs 72.5 days, p = 0.193) in patients receiving dabigatran. One patient in the dabigatran group experienced a stroke three days after initiation of therapy for unclear reasons. This patient was subsequently switched to rivaroxaban therapy without further events. In the rivaroxaban group, a drug-drug interaction with concomitant prednisone treatment and was attributed to the only major bleeding event, GI bleeding, found in our study. Anticoagulation was subsequently discontinued and bleeding resolved. A significant difference in non-bleeding ADRs was found in favor of rivaroxaban (34.6% vs 7.1%, p < 0.001) (Table 2). This was mainly driven by a high incidence of dyspepsia (33.3%) and GI upset (18.0%) in the dabigatran group. Although all cause hospitalizations were not significantly different between groups (31.0% vs 21.5%, p = 0.168), hospitalizations for bleeding or stroke events showed a borderline significant trend in favor of rivaroxaban use (6.4% vs 1.0%, p = 0.089). Discontinuation rates were higher in the dabigatran group compared to the rivaroxaban group (24.4% vs 3.1%, p < 0.001) (Table 2). The median duration of any ADR occurrence was 27 days (IQR, 1-367 days) and 119 days (IQR, 3-379 days) for dabigatran and rivaroxaban groups, respectively.
A Comparative Evaluation of Adverse Drug Events and Incidence of Stroke amongst the Target Specific Oral Anticoagulants
Genevieve Hale1*, Sarah Ebaugh2 and MichaelBrenner3
- 1Clinical Pharmacy Specialist-Cardiology, Nova Southeastern University College of Pharmacy, Palm Beach Campus, Palm Beach Gardens, FL, USA
- 2Clinical Pharmacy Specialist-Anticoagulation, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA
- 3Clinical Pharmacy Specialist-Cardiology, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA
*Address for Correspondence: Genevieve Hale, Assistant Professor, Clinical Pharmacy Specialist-Cardiology, Nova Southeastern University College of Pharmacy, Palm Beach Campus, 11501 N. Military Trail Palm Beach Gardens, FL 33410, USA, Tel: (973)-766-2650; E-mail: Gmh418@yahoo.com
Citation: Hale G, Ebaugh S, Brenner M. A Comparative Evaluation of Adverse Drug Events and Incidence of Stroke amongst the Target Specific Oral Anticoagulants. J Hematol Thromb 2015;1(2): 6.
Copyright © 2015 Hale et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 1, Issue: 1
Submission: 28 July, 2015 | Accepted: 01 August, 2015 | Published: 06 August, 2015
Reviewed & Approved by: Dr. Neerja Vajpayee, Associate Professor, Department of Hematopathology, SUNY Upstate Medical University, USA
Abstract
Background: Dabigatran, rivaroxaban, and apixaban have similar bleeding and stroke risks compared to warfarin, but have never been evaluated against one another in the same respect.Objective: To evaluate the incidence of bleeding and stroke when using target specific oral anticoagulants (TSOAC).
Methods: Retrospective, observational study design. IRB approval was obtained. Inclusion criteria included anticoagulation clinic enrollment, age > 18 years, medication adherence. Exclusion criteria included prior use of TSOAC, renal/liver dysfunction, valvular disease. Oral administration of dabigatran 150 mg twice daily, rivaroxaban 20 mg daily and apixaban 5 mg twice daily were examined. Coprimary outcomes were the number of bleeding and stroke events with dabigatran and rivaroxaban use. Apixaban was excluded due to a small sample size. Secondary outcomes included the number of non-bleeding ADRs, drug-drug interactions, all cause hospitalizations, and time to occurrence of bleeding or non-bleeding ADRs and to occurrence of stroke events.
Results: A total of 176 patients were analyzed. A non-significant trend in favor of rivaroxaban was found for bleeding events (p = 0.134). One patient receiving dabigatran experienced a stroke. A higher, nonsignificant trend of hospitalizations due to bleeding or stroke was found in the dabigatran group (p = 0.089). A significantly greater incidence of non-bleeding ADRs was demonstrated in the dabigatran group (p < 0.001) as well as rate of discontinuation of therapy (p < 0.001).
Conclusions: Dabigatran and rivaroxaban have similar rates of stroke and bleeding, yet rivaroxaban is better tolerated due to less non-bleeding events, less rate of discontinuation, and trend towards a decrease in hospitalizations due to bleeding or stroke.
Keywords
Adverse drug events; Ambulatory care; Anticoagulation; Anticoagulants; Cardiology; StrokeIntroduction
Stroke is the fifth leading cause of death in the United States and the leading cause of long-term disability [1,2]. About every four minutes one American dies from a stroke [2]. Individuals with atrial fibrillation have a five-fold increase risk of stroke [3]. Since 1954 warfarin has been the mainstay of therapy for stroke prevention in nonvalvular atrial fibrillation [4]. Several trials have proven warfarin efficacious in primary and secondary stroke prevention; [5-8] however, frequent monitoring, unpredictable pharmacokinetics, and bleeding risks are some factors that hinder the use of this agent. Between 2010 and 2015 dabigatran, rivaroxaban, apixaban, and edoxaban, also known as target specific oral anticoagulants (TSOACs), gained FDA approved for the prevention of stroke in nonvalvular atrial fibrillation. These agents exhibit predictable pharmacokinetic properties and do not require routine monitoring, but also have a risk of bleeding.Efficacy of stroke prevention and the risk of bleeding in these agents have been well established in many clinical trials. Overall, the incidence of major bleeding in the general population has shown to be approximately 6% for dabigatran, 6% for rivaroxaban, and 2% for apixaban [9-12]. The incidence of stroke is similar for all three TSOACs at less than 2% per year [10-12]. The first major trial examining dabigatran, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), compared the incidence of stroke or systemic embolism, and major hemorrhage in dabigatran versus warfarin use. Dabigatran was found to be non-inferior to warfarin in regards to lower rates of stroke with similar rates of major hemorrhage [10]. Following RE-LY, the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) looked at a composite of stroke and systemic embolism for rivaroxaban versus warfarin treated patients. A composite of major and nonmajor clinically relevant bleeding events were also analyzed between groups. Rivaroxaban was found to be non-inferior to warfarin in the prevention of stroke and systemic embolism, and there was no significant difference regarding rates of bleeding [11]. Lastly, apixaban was compared to warfarin in the Apixaban for Reduction of Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, looking at stroke or systemic embolism as the primary efficacy endpoint, and major hemorrhage the primary safety endpoint. According to the investigators, apixaban was superior to warfarin in preventing stroke, caused less bleeding, and resulted in lower rates of mortality [12].
The pharmacological profile of the TSOACs offers an explanation to the results found in the landmark studies. The goal of target specific oral anticoagulation is to inhibit fibrin (clot) formation. Dabigatran is a direct thrombin inhibitor, while rivaroxaban and apixaban are direct factor Xa inhibitors. This process aims to prevent cerebrovascular events including stroke and transient ischemic attacks (TIA). Pharmacokinetic and pharmacodynamic properties differ between TSOACs. Regarding protein binding, the factor Xa inhibitors are nearly 90% bound, however, dabigatran is only 35% bound [13-15]. This becomes important in elderly patients where the half-life of factor Xa inhibitors can be prolonged. In comparison to warfarin, the TSOACs have a much shorter half-life, which is a disadvantage in patients who are non-adherent with their medication regimen. An advantage is their fast onset of action, and a more predictable pharmacokinetic/dynamic profile that eliminates the need for routine monitoring, including the international normalized ratio (INR). Each agent is metabolized through P-glycoproteins; however, rivaroxaban and apixaban are mainly metabolized by the CYP 3A4 isoenzymes [13-15]. Nevertheless, presently the amount of drug interactions have been shown to be similar between TSOACs, and significantly less compared to warfarin. All agents are renally excreted requiring dose adjustments in moderate or severe kidney dysfunction [13-15].
As aforementioned, dabigatran, rivaroxaban, and apixaban have been identified as having a bleeding risk similar to warfarin, and similar or superior risk of stroke. However, these agents have not been compared to one another with respect to adverse drug reactions (ADRs), such as bleeding, and compared to one another with respect to the rate of stroke. This study aims to examine the rate of bleeding and stroke events in patients receiving TSOAC therapy.
Methods
Study designThis single center, retrospective, observational study evaluated the incidence of bleeding and stroke amongst dabigatran, rivaroxaban and apixaban therapy. Approval for this investigation was obtained by the institutional review board (IRB) at the VA Ann Arbor Healthcare System (VAAAHS). A chart review was conducted through patients’ medical electronic record at the VAAAHS main site in Ann Arbor, Michigan from January 2011 until January 2014. Inclusion criteria included patient enrollment in the anticoaglution clinics at the VAAAHS including the main site and community-based outpatient clinics (CBOC) in Toledo, Flint, and Jackson, Michigan. Patients included were newly started on a TSOAC for prevention of stroke in the setting of atrial fibrillation/flutter, aged greater than 18 years, and had a history of good medication adherence (based on refill records).The anticoagulation clinics provide 30-day supplies of TSOAC with refills. Non-adherence was defined as greater than 45 days between any fill of medication occurring more frequently than once. Exclusion criteria included prior use of a TSOAC before January 2011, creatinine clearance less than 30 mL/min, significant liver disease (e.g. acute clinical hepatitis, chronic active hepatitis, cirrhosis, liver function test elevations greater than 3 times the upper limit of normal), active endocarditis or valvular disease, hypersensitivity to dabigatran, rivaroxaban, or apixaban, pregnancy, history of medication non adherence, or receiving care for anticoagulation outside of the VAAAHS. Of note, edoxaban was not FDA approved during the study period; therefore, was not included in our investigation. Patients were separated into dabigatran, rivaroxaban, and apixaban groups.
Data collection
Data was obtained from the Computerized Patient Record System (CPRS) and included the following demographic information: age, race, gender, weight, creatinine clearance, agent used, dose of agent used, and indication, date of medication start, CHA2DS2VASc score, aspirin and proton pump inhibitor (PPI) use. Baseline and followup information included: date of medication discontinuation, ADR, date of ADR, intervention associated with ADR, date of stroke, date of hospital admission, date of hospital discharge, reason for hospital admission, date of drug interaction, reaction of drug interaction, offending agent associated with drug interaction, creatinine clearance using the Cockgroft-Gault equation incorporating ideal body weight, hemoglobin level, hematocrit level, aspartate transferase (AST) level, alanine transaminase (ALT) level. Follow up data was collected at 3, 6, and 12 months post-therapy initiation. Major bleeding was defined as bleeding events involving blood transfusion or surgical intervention. Minor bleeding encompassed all other bleeding events.
Study outcomes
The co-primary outcomes of this study were the number of bleeding events associated with dabigatran and rivaroxaban use, and the number of stroke events observed in patients receiving dabigatran or rivaroxaban therapy. Apixaban therapy was excluded due to a small sample size of patients on this agent. Major bleeding was defined as bleeding events involving blood transfusions or surgical intervention. Minor bleeding encompassed all other bleeding events. The secondary outcomes were the number of non-bleeding ADRs, the number of drug-drug interactions, all cause hospitalizations, time to occurrence of bleeding or non-bleeding ADRs, and time to occurrence of stroke events.
Statistical analysis
The power analysis to compare major bleeding and stroke events between the dabigatran and rivaroxban groups was based on a planned analysis using a 2x2 table and Pearson’s chi-square test. An alpha level of 0.05 was utilized. An incidence rate of 6% was assumed for major bleeding, and an incidence rate of 1.5% was assumed for stroke events. In order to detect a minimum of 14.5% difference in bleeding rates and a minimum of 11% difference in stroke events between dabigatran and rivaroxaban groups, a total sample size of 176 patients was needed in order to achieve 80% power. Due to a limited sample for apixaban, as this agent was not on the VAAAHS formulary at the time of data collection, this group was excluded from the power analysis.
Descriptive statistics were used for demographic/baseline information. Independent sample T-test and one-way ANOVA were used to compare baseline characteristics between the dabigatran and rivaroxaban groups. Chi-square and Fisher’s exact tests were used to compare, as appropriate, the number of bleeds, strokes, nonbleeding ADRs, drug-drug interactions, and hospitalizations between dabigatran and rivaroxaban groups. Student t-tests or Mann-Whitney U tests were used to analyze time to occurrence of bleeding or nonbleeding ADR and time to stroke for patients that received dabigatran and rivaroxaban. Descriptive statistics and subgroup analyses using Scheffe’s post-hoc comparison test and one-way ANOVA were conducted to evaluate patients in the apixaban group. A p-value of < 0.05 denoted statistical significance. Assistance from the Veterans Affairs Health Services Research and Development (HSRD) service was provided during analysis of collected data.
Results
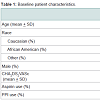
Primary and secondary outcomesOne hundred seventy-six patients were included in our study. Baseline characteristics were similar between groups (Table 1). Of these patients, 99.4% were male, 74.4% were Caucasian, the mean age was 68.43 + 8.77, and the mean CHA2DS2VASc score was 3.40 ± 1.55, signifying a high stroke risk. Overall, 78 received dabigatran therapy and 98 received rivaroxaban therapy for stroke prevention. Of note, 43.8% of patients used aspirin and 52.8% used PPI therapy concomitantly with antithrombotic therapy. The use of aspirin and PPI agents were assessed to investigate their affects on bleeding risk.
There were 20 bleeding ADRs identified amongst our patient population. Of the ADRs observed, 15.4% were attributed to dabigatran use, while 8.2% were associated with rivaroxaban use. Three major bleeding events were found amongst our patient population, two in the dabigatran group and one in the rivaroxaban group. A higher trend in the incidence (9.7%) of minor bleeding was identified more often (12.8 vs 7.1%, p = 0.205) and with less time to first occurrence (33 days vs 72.5 days, p = 0.193) in patients receiving dabigatran. One patient in the dabigatran group experienced a stroke three days after initiation of therapy for unclear reasons. This patient was subsequently switched to rivaroxaban therapy without further events. In the rivaroxaban group, a drug-drug interaction with concomitant prednisone treatment and was attributed to the only major bleeding event, GI bleeding, found in our study. Anticoagulation was subsequently discontinued and bleeding resolved. A significant difference in non-bleeding ADRs was found in favor of rivaroxaban (34.6% vs 7.1%, p < 0.001) (Table 2). This was mainly driven by a high incidence of dyspepsia (33.3%) and GI upset (18.0%) in the dabigatran group. Although all cause hospitalizations were not significantly different between groups (31.0% vs 21.5%, p = 0.168), hospitalizations for bleeding or stroke events showed a borderline significant trend in favor of rivaroxaban use (6.4% vs 1.0%, p = 0.089). Discontinuation rates were higher in the dabigatran group compared to the rivaroxaban group (24.4% vs 3.1%, p < 0.001) (Table 2). The median duration of any ADR occurrence was 27 days (IQR, 1-367 days) and 119 days (IQR, 3-379 days) for dabigatran and rivaroxaban groups, respectively.
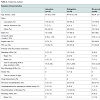
Sixteen patients received apixaban therapy for stroke prevention. Similar to dabigatran and rivaroxaban, the majority of patients were Caucasian (72.4%) and male (100%). Aspirin and PPI use were also similar between groups (31.3% and 62.5%, respectively). However, the mean age of apixaban patients was significantly older, 81.06 + 6.87 (p < 0.001), with a higher mean CHA2DS2VASc score of 4.69 + 1.66 (p = 0.002) compared to those receiving dabigatran and rivaroxaban therapy (Table 3).
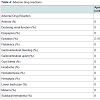
Only two minor bleeding events, both epistaxis, occurred in patients receiving apixaban with a rate to first event similar to dabigatran and rivaroxaban groups (18 days, p = 0.457). These events both resulted in hospitalization and discontinuation of therapy. There were no identified non-bleeding ADRs, which was significant compared to dabigatran and rivaroxaban groups (p < 0.001). No stroke events occurred (Table 4). The median time from apixaban administration to any ADR was 30 days (IQR, 6-311).
Only two minor bleeding events, both epistaxis, occurred in patients receiving apixaban with a rate to first event similar to dabigatran and rivaroxaban groups (18 days, p = 0.457). These events both resulted in hospitalization and discontinuation of therapy. There were no identified non-bleeding ADRs, which was significant compared to dabigatran and rivaroxaban groups (p < 0.001). No stroke events occurred (Table 4). The median time from apixaban administration to any ADR was 30 days (IQR, 6-311).
Discussion
To our knowledge, this study is the first investigation to compare the incidence of bleeding and stroke events with dabigatran and rivaroxaban use in nonvalvular atrial fibrillation directly. The body of literature comparing TSOACs is scarce in large-scale controlled trials with available indirect comparisons and reviews [16-19]. By reviewing the results of the RE-LY, ROCKET-AF, and ARISTOTLE trials, our findings are parallel with those highlighted in these clinical trials, showing a low rate of stroke events and major bleeding [10-12]. Current guidelines discussing stroke prevention in nonvalvular atrial fibrillation endorse the use of all TSOACs, with the exception of edoxaban. The AHA/ASA secondary stroke prevention guidelines recommend apixaban (Class I; Level of Evidence A) and dabigatran (Class I; Level of Evidence B) as indicated therapy for prevention of recurrent stroke in patients with nonvalvular atrial fibrillation. Rivaroxaban is considered a reasonable option as well (Class IIa; Level of Evidence B) [20]. Similarly, the 2014 AHA/ACC/HRS atrial fibrillation guidelines recommend apixaban, dabigatran, or rivaroxaban use in patients with nonvalvular atrial fibrillation and a high stroke risk. (Class I; Level of Evidence B). Patients with a moderate stroke risk can also choose to use TSOACs (Class IIb; Level of Evidence C) [21].This investigation had several important findings regarding the safety and efficacy of TSOAC use in nonvalvular atrial fibrillation. However, there are several limitations that need to be taken into consideration, such as small sample size, lack of female and non-Caucasian patients, bringing into question the external validity and generalizability outside of the veteran population. Additionally, possible selection bias by prescribing patterns or information bias may have occurred.
Our findings suggest that stroke prevention and bleeding events associated with dabigatran and rivaroxaban use does not differ between agents. Furthermore, drug interactions, all cause hospitalizations, and the time to bleeding or stroke events is not significantly different. On the other hand, we found that the incidence of non-bleeding ADRs, especially dyspepsia and GI upset, related to dabigatran use is significantly greater compared to rivaroxaban therapy. This led to a significantly higher rate of discontinuation in dabigatran therapy. This reinforces the need for practitioners to assess tolerability when choosing a TSOAC to prescribe to patients with nonvalvular atrial fibrillation, particularly in individuals with a history of gastrointestinal reflux disease or other gastrointestinal conditions. Of note, a decline in renal function and hematuria were also found more often patients receiving dabigatran therapy. Renal atheroembolic disease is a rare complication seen with warfarin, heparin, and thrombolytic use; however, acute renal failure associated with dabigatran is sparse in the current body of literature [22-25]. As dabigatran is mostly excreted in the urine, the renal clearance is approximately 80% of the total clearance after administration [13]. Pharmacokinetic modeling has demonstrated that the anticoagulation activity and half-life of dabigatran is increased in patients with renal impairment [13]. This increases the risk of exposure to dabigatran, increasing the risk of bleeding. An unexpected higher trend in hospitalizations due to bleeding or stroke was identified in patients receiving dabigatran therapy. Additionally, a greater number of all cause hospitalizations were also found in the dabigatran group. We can speculate with a larger sample size this finding may have shown true significance.
Our subgroup analysis results suggest that apixaban is also a well tolerated agent with a similar rate of bleeding or stroke compared to dabigatran or rivaroxaban. However, given the very small sample size of this group, it is hard to extrapolate this information. As mentioned previously, the data is limited regarding the efficacy and safety between these agents head to head for stroke prevention in nonvalvular atrial fibrillation. Further investigation with a prospective, randomized trial, including apixaban, would be valuable to further evaluate the incidence of stroke and bleeding rates between TSOACs.
Conclusion
Our investigation has demonstrated that in patients with nonvalvular atrial fibrillation the rates of stroke and bleeding when receiving dabigatran or rivaroxaban therapy did not differ. However, rivaroxaban may be a better tolerated agent due to less non-bleeding ADRs, rate of discontinuation of therapy, and trend towards a decrease in hospitalizations due to bleeding or stroke.References
- CDC (2014) Leading causes of death. Centers for Disease Control and Prevention.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. (2014) Heart disease and stroke statistics-- 2014 update: a report from the American Heart Association. Circulation 129: e28-e292.
- Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 22: 983-988.
- (1991) Stroke prevention in atrial fibrillation study: Final results. Circulation 84: 527-539.
- (2011) Coumadin® [package insert] Princeton, NJ: Bristol-Myers Squibb Company.
- (1994) Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke prevention in atrial fibrillation II study. Lancet 343: 687-691.
- (1996) Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomized clinical trial. Lancet 348: 633-638.
- ACTIVE Writing Group of the ACTIVE Investigators, Connolly S, Pogue J, Hart R, Pfeffer M, et al. (2006) Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with Irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet 367: 1903-1912.
- (2014) Lexi-DrugsTM. Lexi-Comp, Inc.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139-1151.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883-891.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981-992.
- (2013) Pradaxa® [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.
- (2011) Xarelto® [package insert]. Leverkusen, Germany: Janssen Pharmaceuticals, Inc.
- (2012) Eliquis® [package insert]. Princeton, NJ, New York, NY: Bristol-Myers Squibb Company, Pfizer, Inc.
- Mantha S, Ansell J (2012) An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost 108: 476-484.
- Lip GY, Larsen TB, Skjoth F, Rasmussen LH (2012) Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 60: 738-746.
- Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, et al. (2012) Comparison of efficacy and safety of dabigatran, rivaroxaban, and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol 31: 330-339.
- Harenberg J, Marx S, Wehling M (2012) Head-to-head or indirect comparisons of novel oral anticoagulants in atrial fibrillation: what’s next? Thromb Haemost 108: 407-409.
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, et al. (2014) AHA/ASA guideline: Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 45: 2160-2236.
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary. A report of the American college of cardiology/American heart association task force on practice guidelines and the Heart rhythm society. J Am Coll Cardiol 64: 2246-2280.
- Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31-41.
- Winter MA, Guhr KN, Berg GM (2012) Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy 32: 604-612.
- Brown DL, Masselink AJ, Lalla CD (2013) Functional range of creatinine clearance for renal drug dosing: a practical solution to the controversy of which weight to use in the Crockcroft-Gault equation. Ann Pharmacother 47: 1039-1044.
- Shafi ST, Negrete H, Roy P, Julius CJ, Sarac E (2013) A case of dabigatran-associated acute renal failure. WMJ 112: 173-175.