Journal of Food Processing & Beverages
Download PDF
Research Article
*Address for Correspondence: Maria Paola Previdi, Stazione Sperimentale per l’Industria delle Conserve Alimentari, SSICA, Via Faustino Tanara, 31/A, 43121 Parma, Italy, Tel: +39 0521795263; Fax: +39 0521795218; E-mail: paola.previdi@ssica.it
Citation: Franceschini B, Manganelli E, Bloise C, Previdi MP. Tomato Products Spoilage by Alicyclobacillus Spp: Growing Ability of Spores Naturally Present as a Function of the Total Soluble Solids and Isolates Characterization. J Food Processing & Beverages. 2015;3(1): 6.
Copyright © 2015 Previdi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 3, Issue: 1
Submission: 08 September 2015 | Accepted: 31 October 2015 | Published: 07 November 2015
Spore presents in tomato paste were not able to growth in the source product.
Spores grew up to 9.5 Bx in the sample N.16; up to 8.5 Bx in the sample N.18; up to 10.5 Bx in the sample N.22.
These data highlight the variability among the different strains.The results were changed into a digital signal, and cluster analysis was performed using the UPGMA method by the FP Quest software to obtain a dendrogram (Figure 1).
According to the dendrogram, the DSM 2498 and DSM 3924 reference strains differ from all of the other strains tested (63% similarity). The similarity between SSICA 22b and SSICA 22g strains was very high (95.7%); while the relationship with the other tomato strains and the A. acidocaldarius reference strains was lower (71.8%). The comparison between SSICA18, SSICA16b and SSICA 16g showed a high similarity (84.6%), with a similarity of 90.9% between SSICA18 and SSICA16b.
Tomato Products Spoilage by Alicyclobacillus Spp: Growing Ability of Spores Naturally Present as a Function of the Total Soluble Solids and IsolatesCharacterization
Barbara Franceschini1, Elisabetta Manganelli1,Catia Bloise2 and Maria Paola Previdi1*
- 1Stazione Sperimentale per l’Industria delle Conserve Alimentari, SSICA, Via Faustino Tanara, 31/A, 43121 Parma, Italy
- 2Via Fornace Baistrocchi, 3, 42029 Sant’Ilario D’Enza RE, Italy
*Address for Correspondence: Maria Paola Previdi, Stazione Sperimentale per l’Industria delle Conserve Alimentari, SSICA, Via Faustino Tanara, 31/A, 43121 Parma, Italy, Tel: +39 0521795263; Fax: +39 0521795218; E-mail: paola.previdi@ssica.it
Citation: Franceschini B, Manganelli E, Bloise C, Previdi MP. Tomato Products Spoilage by Alicyclobacillus Spp: Growing Ability of Spores Naturally Present as a Function of the Total Soluble Solids and Isolates Characterization. J Food Processing & Beverages. 2015;3(1): 6.
Copyright © 2015 Previdi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 3, Issue: 1
Submission: 08 September 2015 | Accepted: 31 October 2015 | Published: 07 November 2015
Abstract
The aim of the present study was to investigate the ability of Alicyclobacillus spp. spores that are naturally present in tomato puree and paste to germinate and to grow in these products, according to the total soluble solids content (TSS-Bx).All these spores germinated and grew at 60 ºC up to 8.5 Bx; however, some were able to grow also at 10.0 and 10.5 Bx; high temperatures were essential for their growth. It was also noted that a few spores (5 spores/ml) were able to spoil the product if in suitable conditions (T = 60 ºC, pH ≤ 4.5 and in an aerobic condition).
The spores germinated and grown were isolated and the strains were compared with reference strains, characterized by means of physical and biochemical analysis, and typed by RAPD-PCR.
Dendrogram of biochemical characteristics and dendogram of the RAPD-PCR of the tomato strains were almost equal and allowed different groups of isolates to be distinguished. A. acidoterrestris and A. acidocaldarius collection strains differ from tomato strains tested.
The present paper highlights the heavy presence of Alicyclobacillus spores in tomato products and their ability to grow, the need to avoid prolonged stops of the product along the processing environment at high temperature, to perform a rapid cooling after the heat treatment and to store the product at temperatures below 30 ºC.
Keywords
Alicyclobacillus spp.; Tomato product; Soluble solid content; RAPD-PCRAbbreviations
TSS: Total Soluble Solid; Bx: Brix; RAPD-PCR: Random Amplification of Polymorphic DNA Polymerase Chain Reaction; UPGMA: Unweighted Pair Group Method with Arithmetic Mean; DSM: Deutsche Sammlung von MikroorganismenIntroduction
Alicyclobacillus species are a group of thermo-acidophilic, nonpathogenic, rod-shaped, endo-spore forming bacteria that are able to spoil fruit juices and nectars [1]. The most common characteristic of the spoilage is a medicinal, antiseptic offensive off-odour in commercial pasteurized products, owing to the production of badsmelling phenolic compounds, such as 2.6-dibromophenol and guaiacol [2-4].A. acidoterrestris proved to be the main responsible of the offodours in fruit juices and is regarded as a serious cause of spoilage for the fruit juice industry [1].
The literature records numerous new species of Alicyclobacillus, isolated both from fruit and from soil [5,6]; however, for these species, the ability to spoil products has not always been established. Furthermore, the search for metabolites is limited to determining the ability to produce guaiacol or other polyphenols [1].
In recent years, tomato juices and puree spoiled by Alicyclobacillus spp. has occurred [7].Spoilage was reported by consumers who smelled an off-flavour in the product. From a screening performed in 50 tomato-based products, Alicyclobacilli were found in 73.7% [7].
These strains resulted different from A. acidoterrestris and assimilable to A. acidocaldarius, since they grew at 65 ºC, did not produce guaiacol and the biochemical features were similar to A. acidocaldarius [8,9].
The heat resistance of the spores isolated from the tomato products was higher (D105 values range from 5.09 to 15.13 min according to the strains) than that reported in the literature for A. acidoterrestris (D90 values ranged from 5.95 to 23.10 min in different fruit juices) [5,8].
Fortunately, spoilage cases are few in number since these microorganisms are very demanding: they require the presence of oxygen and rather high temperatures to grow (greater than 30 ºC - 37 ºC).
The aim of this work is to assess the ability of Alicyclobacillus spores naturally present in tomato products to grow and to spoil according to the total soluble solids (TSS) content and to characterize the isolates by means of physical and biochemical analysis and to type them by RAPD-PCR.
Materials and Methods
Sampling and search for positive samplesTwenty-five tomato samples at different TSS contents from the tomato concentrating processing environment were collected: nineteen tomato puree (total soluble solids ranged from 6.1 to 8.5 Bx) and six tomato paste (total soluble solids ranged from 28 to 30 Bx). The pH measurements of the samples were carried out using Mettler Toledo pH meter (Schwerzenbach, Switzeland). The same quantity (10 g) of each samples were analysed for the presence of Alicyclobacillus spores. Tomato puree was heat-shocked in a water bath (FA90, Falc, Treviglio, Italy) at 80 ºC for 10 min. For tomato paste samples, a dilution 1:10 with sterile physiological salt solution before heat shocking was done. The number of spores was then determined by plate counting on Yeast Extract Soluble Starch Agar (YSG- pH 3.7) that was incubated at 44 ºC for 3-5 days and the suspect Alicyclobacillus colonies were confirmed according to the IFU Method N. 12 [10].
Growth ability of spores naturally present in tomato products
Thirteen samples of tomato puree found positive for Alicyclobacillus presence were incubated at 60 ºC under static conditions for four days to evaluate the indigenous spore’s ability to germinate and to grow. Tests were carried out in triplicate. The microbial concentrations were detected in the YSG agar.
Growth ability as a function of the TSS content
Three tomato paste samples found positive for Alicyclobacillus presence, diluted between 8.0 and 11 Bx, were used to evaluate the growth ability as a function of the TSS content. The Brix values tested are those normally related to tomato puree. The TSS content was measured using a pocket refractometer (Atago PAL-3 Tokyo, Japan). Tests were performed in triplicate: for each TSS value, jars containing 100 ml of the product were set; after a heat treatment in a water bath at 80 ºC for 10 min, the samples were incubated with stirring at 60 ºC in a Refrigerated Incubator Shaker (C24KC Edison, NJ, USA), both to facilitate the oxygenation of the products and to keep the TSS value uniform, avoiding a possible layering during the incubation. The microbial concentrations were detected in YSG agar at increasing times, up to a maximum of five days.
Microorganisms
Among samples in which Alicyclobacillus spores were present (positive samples), five different strains (signed SSICA 16g, SSICA 16b, SSICA 22b, SSICA 22g and SSICA 18) were collected and characterized. The strains were isolated onto YSG agar, stored at 4 ºC and maintained by weekly transfer as working stock cultures. Four reference strains of A. acidocaldarius subsp. acidocaldarius (DSM 446 and DSM 452) and A. acidoterrestris (DSM 2498 and DSM 3924) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and were used to make a comparison with the tomato isolated strains.
Physiological characterization
The growth ability of the five isolates and of the four reference strains at different temperatures was tested in the YSG broth: 0.1 ml of each fresh microbial culture was inoculated in test tubes and incubated at 25 ºC +/- 1, 30 ºC +/- 1, 37 ºC +/- 1, 44 ºC +/- 1, 50 ºC +/- 1, 65 ºC +/- 1 and 70 ºC+/- 1 for seven days. Tests were performed in triplicate [10].
Biochemical characterization
A biochemical characterization of the strains was performed by means of a miniaturized system API 50 CH- bioMérieux gallery, according to the technique proposed by Deinhard et al. The API tests were incubated at 50 ºC for 48 h [11].The results were analysed by using the InfoQuest™FP software version 4.5 (Bio-Rad Hercules CA, USA); Dice’s similarity coefficients were calculated and the strains were grouped to obtain a Dendrogram by using the UPGMA method.
Molecular characterization
The template DNA was prepared as described by Garcia, and the total chromosomal DNA was extracted from 1 ml YSG broth cultures of the isolated strains [12].
The isolates were typed by RAPD-PCR using the primers BA10 (5’-AACGCGCAAC-3’), F61 (5’-CCTGTGATGGGC-3’), F64 (5’-GCCGCGCCAGTA-3’) [13-15] and the M13 minisatellite core sequence (5’-GAGGGTGGCGGTTCT-3’) [16,17].
The PCR reactions were performed in a 25 μl volume reaction containing 12.5 μl iQ™ supermix (Bio-Rad Hercules CA, USA), 2 μM of each primer and 60 ng of DNA. The cycling conditions were initial denaturation at 94 ºC for 2 min, 40 cycles of denaturation at 94 ºC for 60 s, annealing at 45 ºC for 20 s, elongation at 72 ºC for 120 s and a final elongation step at 72 ºC for 10 min.
The DNA was amplified with GeneAmp 9700 PCR Applied Biosystems. The amplification products were resolved by electrophoresis on a 1% agarose gel (Readyagarose™Wide-mini Gel, Bio-Rad Hercules CA, USA); at 90 Volts for 95 min in TBE buffer (1 M Tris, 10 mM EDTA, 0.9 M boric acid, pH 8.3). The 100 bp Molecular Ruler (Bio-Rad Hercules CA, USA) was used as a DNA molecular weight marker. Photographs of the gels were scanned with the GEL DOC 2000 (Bio-Rad Hercules CA, USA) and analysed with the Quantity One® 1-D analysis software (Bio-Rad Hercules CA). The conversion, normalization and further processing of the stained gel images were performed by the InfoQuest™FP software version 4.5 (Bio-Rad Hercules CA, USA). Dice’s similarity coefficients were calculated; the strains were grouped to obtain a Dendrogram by using the UPGMA method.
Results
Growth ability of spores naturally present in tomato productsThe analysis of twenty-five tomato samples from the concentrating processing environment showed the presence of Alicyclobacillus spores in sixteen samples (64%): thirteen tomato purees and three tomato paste finished products.
A further test was carried out to verify the growth ability of these spores in their native products.
Germination and growth of spores present in all the thirteen puree samples occurred up to 7.5 (sample N.3), 8.0 (samples N.1, N.5, N.12, N.24, N.8, N.25) and 8.5 Bx (N.15), according to the samples, but regardless of the initial spore concentration that ranged between 0.7 and 3.41 log cfu/ml (Table 1).
Spoilage was enhanced by the formation, on the product surface, of a white halo clearly contrasting with red pigmentation; the spoiled samples also showed an off-flavour.
In previous studies [8,9], the growth ability of Alicyclobacillus spp. in tomato juices and puree had been evaluated using spores obtained in vitro in cultural medium, inoculated at high concentration (103 spores/g).
It is important to underline that this study was conducted using only spores naturally present in tomato products, regardless of their age and concentration and not spores obtained in vitro, not to affect their behavior in any way.
Growth ability as a function of the TSS content
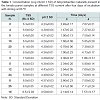
The growth ability as a function of the TSS content was evaluated on the three positive tomato paste samples diluted between 8.0 and 11 Bx (Table 2).
Even though the tests were performed using only three tomato sauce samples, the ability to grow as a function of the TSS content appears to be closely related to the strain present.
These results are very important, since in literature there are no data related to Alicyclobacillus spp. different from A. acidoterrestris growth ability in tomato products.
Regarding the fruit products, it has been reported that a Brix close to 10 allows A. acidoterrestris growth, with an upper limit of 18-20 Bx [1,18]. These values are higher than what found in tomato with native spores.
The characterization of the isolates
In the three positive tomato paste samples, used for determining the growth ability, five different colonies (strains) signed SSICA 16g, SSICA 16b, SSICA 22b, SSICA 22g and SSICA18 were isolated.
Physiological characterization: The DSM 2498 and DSM 3924 (A. acidoterrestris) strains did not grow at 65 ºC but grew at 50 ºC, 30 ºC and 25 ºC. DSM 452 and DSM 446 (A. acidocaldarius subsp. acidocaldarius) strains did not grow at 25 ºC and 30 ºC, but grew at 65 ºC and 70 ºC as is already widely documented [1,5].
The indigenous strains isolated in the tomato products proved to have thermophiles properties like A. acidocaldarius: they grew at 65 ºC and 70 ºC and did not grow at 30 ºC.
Biochemical characterization: The biochemical characteristics of all of the isolates were compared with those of the reference strains. Results are shown in (Table 3). The strains native to tomato products exhibited some differences from each other and from the reference strains. These strains were not able to metabolize D-Ribose, L-Rhamnose, D-Lactose as DSM 452 did, but not as DSM 446 did; they also did not resulted able to use Inositol, D-Melibiose and Glycogen, while DSM 446 and DSM 452 did. Three of them used D-Turanose. The strains SSICA 22b and SSICA 22g, isolated from the same sample N.22, did not use Glycerol and Mannitol, which were metabolized by all other strains. The colonies of these two strains were morphologically different in the Petri dishes; however, they biochemically differ only by the fact that strain SSICA 22g was able to metabolize Melezitose, which was not used by all of the other strains. Strain SSICA 16b did not grow in the presence of D-Xylose and D-Maltose. Strain SSICA18 was not able to utilize D-Maltose and D-Trehalose. Only the A. acidoterrestris strains DSM 2498 and DSM 3924 were able to metabolize Erythritol and D-Adonitol.
Molecular characterization: RAPD-PCR was used for a differentiation amongst the species and to generate species-specific banding patterns. The use of four different primers allowed a separation of the different electrophoretic profiles for each of the tested strains. The dendrogram (Figure 2) was almost equal to that obtained with the biochemical characteristics: SSICA 22b and SSICA 22g strains showed a high similarity (96.4%), belonging to a distinct cluster, different from the other tomato strains and from the A. acidocaldarius reference strains (75.4%). The comparison between SSICA 18, SSICA16b and SSICA 16g strains and the A. acidocaldarius DSM 452 and DSM 446 strains showed a 77.4% similarity, like the biochemical dendrogram similarity (75.8%). The A. acidoterrestris reference strains differ from all of the other strains tested (66.7% similarity).
Conclusions
Alicyclobacillus spores were widespread in the tomato products investigated.These naturally contaminating spores were able to germinate and to spoil the tomato puree up to 10.5 Bx at 60 ºC. The spoilage sign was an off-flavour in the product.It was noted that a few spores (5 cfu/ml) were able to spoil the product in eligible conditions (temperature, pH and oxygen).Therefore in order to reduce the risk of spoilage, are of pivotal importance to avoid prolonged stops along the processing environment at high temperatures. It is also essential to perform a rapid cooling after the heat treatment and to store the product at temperatures below 30 ºC.References
- Yokota A, Fujii T, Goto K (2007) Alicyclobacillus thermophilic acidophilic bacilli. Springer, Japan.
- Brown KL (1995) Challenges in aseptics: Alicyclobacillus acidoterrestris spoilage in aseptically packed fruit juices. Proc. of Int. Symp. "Advances in aseptic processing and packaging technologies". Copenaghen, 11-12.
- Borlinghaus A, Engel R (1997) Alicyclobacillus incidence in commercial Apple Juice Concentrate (AJC) supplies - method development and validation. Fruit Process 7: 262-266.
- Pettipher GL, Osmundson ME, Murphy JM (1997) Methods for the detection and enumeration of Alicyclobacillus acidoterrestris and investigation of growth and production of taint in fruit juice-containing drinks. Lett Appl Microbiol 24: 185-189.
- Smit Y, Cameron M, Venter P, Witthuhn RC (2011) Alicyclobacillus spoilage and isolation--a review. Food Microbiol 28: 331-349.
- Steyn C, Cameron M, Witthuhn R (2011) Occurrence of Alicyclobacillus in the fruit processing environment--a review. Int J Food Microbiol 147: 1-11.
- Previdi M, Franceschini B, Manganelli E (2009) Evaluations of the behaviour of Alicyclobacillus spp. in acid products. Industria Conserve 84: 179-182.
- Lottici C, Previdi MP, Bolzoni L (2007) Characterization and study of Alicyclobacillus isolated from tomato products [vegetable and fruit products; biological contamination]. Industria Conserve 81: 251-267.
- Previdi MP, Franceschini B, Rapacciuolo M, Trifirò A (2010) Search for spoilage markers of Alicyclobacillus species. Industria Conserve 85: 1-21.
- IFU, International Federation of Fruit Juice Producers (2007) Methods of detection of taint producing Alicyclobacillus in fruit juices: IFU Method No. 12. In: Microbiological methods Paris, pp. 1-11.
- Deinhard G, Blanz P, Poralla K, Altan E (1987) Bacillus acidoterrestris spp. a new thermotolerant acidophile isolated from different solis-system. Syst Appl Microbiol 10: 47-53.
- García González LA, Rodrigo Tapia JP, Sánchez Lazo P, Ramos S, Suárez Nieto C (2004) DNA extraction using chelex resin for the oncogenic amplification analysis in nead and neck tumours. Acta Otorrinolaringol Esp 55: 139-144.
- Yamazaki K, Okubo T, Inoue N, Shinano H (1997) Randomly amplified polymorphic DNA (RAPD) for rapid identification of the spoilage bacterium Alicyclobacillus acidoterrestris. Biosci Biotechnol Biochem 61: 1016-1019.
- Groenewald WH, Gouws PA, Witthuhn RC (2009) Isolation, identification and typification of Alicyclobacillus acidoterrestris and Alicyclobacillus acidocaldarius strain from archard soil and the fruit processing environment in South Africa. Food Microbiol 26: 71-76.
- McKnight IC, Eiroa MN, Sant’Ana AS, Massaguer PR (2010) Alicyclobacillus acidoterrestris in pasteurized exotic Brazilian fruit juices: Isolation, genotypic characterization and heat resistance. Food Microbiol 27: 1016-1022.
- Hury B, Hall J (1989) Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol 171: 2528-2532.
- Giraffa G, Rossetti L (2004) Monitoring of the bacterial composition of diary starter cultures by RAPD-PCR. FEMS Microbiol Lett 237: 133-138.
- Splittstoesser D, Churey J, Lee C (1994) Growth characteristics of acidiuric sporeforming bacilli isolated from fruit juices. J Food Protect 57: 1080-1083.