Journal of Environmental Studies
Download PDF
Research Article
*Address for Correspondence: Alexandra Istomina, Laboratory of Marine Ecotoxicology, V.I. Il’ichev Pacific Oceanological Institute of the Far Eastern Branch of the Russian Academy of Sciences, ul. Baltiyskaya 43, Vladivostok 690041, Russia, Tel: +7(4232) 31-25-92; E-mail: s-istomina1@mail.ru
Citation: Istomina A, Belcheva N, Slinko E, Chelomin V. Effect of Marine Environment Remediation on Oxidative Stress Indicators and Metals in the Digestive Gland of the Mussel Crenomytilus grayanus. J Environ Stud. 2016;2(2): 5.
Copyright © 2016 Istomina A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Environmental Studies | ISSN: 2471-4879 | Volume: 2, Issue: 2
Submission: 09 June, 2016 | Accepted: 28 June, 2016 | Published: 04 July, 2016
Reviewed & Approved by: Dr. Mark Meo, Department of Geography & Environmental Sustainability, University of Oklahoma, USA
The domestic solid waste landfill of Vladivostok was located on the shore of the Gornostai Bay in 1967-2010, which led to the pollution of contiguous waters. For example, according to a study by Shulkin [16], the mean content of heavy metals in sediment samples from Site 1 and contiguous waters collected at a distance of 50-100 m from the shore was 3739 μg/g dry wt for Zn, 2659 μg/g dry wt for Cu, and 2344 μg/g dry wt for Pb in 1996-1999. At the same time, the content of these metals in sediments of waters contiguous to Site 2 were 21 μg/g dry wt for Zn, 3 μg/g dry wt for Cu, and 10 μg/g dry wt for Pb [14]. The total concentration of organochlorine pesticides such as HCHs, DDT, and their metabolites) in bottom sediments of Site 1 was 5 times higher compared to that in Site 2 and amounted to 1.67 ng g-1 dry weight [15]. In 2008 and 2009 the concentration of oil products and phenols on the surface and in near-bottom layers of Site 1 exceeded the maximum permissible concentrations by 2- and 5-fold, respectively [17].
Among the studied antioxidants, only the SOD activity in the mussels from Site 1 was nearly 1.5 times higher than in the mussels from the clean area (Figure 3). The activity levels of the remaining antioxidant enzymes (CAT, GP and GR) and the levels of the low molecular weight antioxidant GSH did not differ significantly between the mussels from these regions (Figure 3).
Effect of Marine Environment Remediation on Oxidative Stress Indicators and Metals in the Digestive Gland of the Mussel Crenomytilus grayanus
Alexandra Istomina*, Nina Belcheva, Elena Slinko and Victor Chelomin
- Laboratory of Marine Ecotoxicology, V.I. Il`ichev Pacific Oceanological Institute of the Far Eastern Branch of the Russian Academy Sciences, Vladivostok 690041, Russia
*Address for Correspondence: Alexandra Istomina, Laboratory of Marine Ecotoxicology, V.I. Il’ichev Pacific Oceanological Institute of the Far Eastern Branch of the Russian Academy of Sciences, ul. Baltiyskaya 43, Vladivostok 690041, Russia, Tel: +7(4232) 31-25-92; E-mail: s-istomina1@mail.ru
Citation: Istomina A, Belcheva N, Slinko E, Chelomin V. Effect of Marine Environment Remediation on Oxidative Stress Indicators and Metals in the Digestive Gland of the Mussel Crenomytilus grayanus. J Environ Stud. 2016;2(2): 5.
Copyright © 2016 Istomina A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Environmental Studies | ISSN: 2471-4879 | Volume: 2, Issue: 2
Submission: 09 June, 2016 | Accepted: 28 June, 2016 | Published: 04 July, 2016
Reviewed & Approved by: Dr. Mark Meo, Department of Geography & Environmental Sustainability, University of Oklahoma, USA
Abstract
The marine environment quality in Gornostai Bay of the Peter the Great Bay, East Sea/Sea of Japan (Russia’s Primorski Krai) was assessed based on oxidative stress biomarkers and the content of heavy metals in the digestive gland of the mussel Crenomytilus grayanus. Mussels were collected in Gornostai Bay in 2013, three years after the remediation of a domestic solid waste dump site located on the shore of the bay and in a relatively clean area at Reineke Island (Peter the Great Bay). The results were compared with the data from the studies performed on C. grayanus from the two locations in 1999 and 2011. It is concluded that the marine environment quality in Gornostai Bay is progressively (gradually) improving three years after the remediation of the domestic solid waste dump site compared to 1999 and 2011.Keywords
Heavy metals; Antioxidative defence; Oxidative damage; Mussels; Crenomytilus grayanusIntroduction
Aerobic species have evolved the capacity to use oxygen for the efficient release of energy. However, in vitro studies suggest that 1-3% of the oxygen molecules used are converted to reactive oxygen species (ROS), including both radical and non-radical species. Therefore, the ROS are continually produced as toxic bi-products of normal metabolism from various endogenous processes [1]. Key biological molecules, notably DNA, proteins, lipids, can all be adversely affected by ROS [2,3]. Furthermore, the reaction of ROS with these macromolecules generates additional ROS, setting in train a cascade of damage if left unchecked [4]. An antioxidant system (represented by low molecular weight free radical scavengers and specific antioxidant enzymes) is present in the body of all aerobes in order to retard the ROS generation processes and to protect the cellular components from oxidation and to repair the oxidized biomolecules [5].The accumulation of heavy metals, petroleum hydrocarbons and polychlorinated phenols in the tissues promotes the formation of ROS [1,6,7]. Molecular biomarkers of oxidative stress (oxidative degradation products of biological molecules and the levels of antioxidants) have been widely used in recent decades to identify the toxic effect of pollutants [8,9].
The aim of this study was to use biochemical indicators to assess changes in the marine environment quality after remediation of the anthropogenically polluted coastal zone. For this purpose, the content of heavy metals (Fe, Zn, Cu, Cd and Pb) and indicators of oxidative stress (activity of antioxidant enzymes and levels of reduced glutathione and lipid peroxidation products - diene conjugates, TBAreactive products) as well as total lipid content were determined in the digestive gland of Crenomytilus grayanus (Dunker 1853). Furthermore, studies focused both on the dynamics of release of heavy metals from digestive gland tissue of mussel and the dynamics of changes in biochemical indicators after remediation of the anthropogenically polluted coastal zone are of significant interest.
Therefore, a change in the marine environment quality in the Gornostai Bay in 2013 was evaluated based on the changes in contents of metals and biochemical indicators in the digestive gland of mussel by comparison of the obtained results with the data of studies conducted on C. grayanus from the same regions in 1999 [10,11] and 2011 [12].
Materials and Methods
Studied areasAquatic areas of the Gornostai Bay (Site 1) and Reineke Island (Site 2) (Figure 1), selected as the mussel sampling sites, are located at a distance of about 44 km from each other and virtually do not differ in their main hydrochemical (salinity, oxygen concentration) and hydrological (temperature, light intensity) parameters [13]. Coastal waters of Reineke Island were used as the reference site in the study of water pollution in the Peter the Great Gulf. It should be mentioned that there are no factories polluting the environment on the Shore of Reineke Island. Bottom sediments and the water column of this region are characterized by low content of heavy metals and chlorinated hydrocarbons (pesticides) [14-16].
Remediation of the landfill was completed by the end of 2010. Annual monitoring conducted by the Far Eastern Regional Hydrometeorological Research Institute (FERHRI) in the water area of the Peter the Great Gulf, 500-800 m off the shore, revealed a steady downward tendency in the content of metals and petroleum products in sediments of the Site 1 region by 2012 [18].
Sampling
Mussels were manually collected at 3-4 m depth at a distance of 100 m from both sites in July 2013. In each region 18 individuals were selected. Their age was determined using the prominent growth retardation rings [19]. The shell length of mussels from Site 1 was 10.36 ± 0.83 cm, their age amounted to 8-16 years; mussels from Site 2 were characterized by the shell length of 11.63 ± 0.75 cm and the age of 10-15 years. We suppose that the individuals from the two water areas were in the similar physiological state since C. grayanus is a long-living mollusk [20].
In order to determine the biochemical parameters, the digestive gland tissues from three individuals were combined into a single sample (total of 4 samples for each population), which was immediately frozen in liquid nitrogen and stored at -80 °C. To determine the metal content, the tissues from six individuals were divided into three replicates (two specimens per replicate) and dried until a constant weight was achieved at +75 °C.
Sample preparation
Digestive gland of mussels was homogenized (1:10, w/v) at 0 °C in 50 mM Tris-HCl buffer (pH 8.0), containing 0.1 mM of phenyl methyl sulphonyl fluoride (PMSF). A portion of the homogenate obtained was used to detect TBA-reactive products. The remainder of the homogenate was centrifuged for 20 min at 5,000 g and then for 40 min at 10,800 at 4 °C. The supernatant was used to determine the activity of antioxidant enzymes.
Biochemical analyses
Antioxidant enzymes were assayed at standard assay temperatures of 20 °C. Superoxide dismutase (SOD) (EC 1.15.1.1) activity was measured according to Paoletti et al. [21]. Catalase (CAT) (EC 1.11.1.6) activity was determined by the hydrogen peroxide decomposition rate. Glutathione reductase (GR) (EC 1.6.4.2) activity was measured by following the decrease in absorbance at 340 nm due to the oxidation of NADPH [6]. Glutathione peroxidase (GP) activities were measured in a coupled enzyme system where the formed oxidized glutathione is converted to its reduced form by GR [22]; cumenehydroperoxide was used as substrate (respectively for the sum of Se-dependent, EC 1.11.1.9, and Se-independent, EC 2.5.1.18, activities). The reduced glutathione (GSH) concentration was determined according to Moron et al. [23]. The TBA-reactive products (TBARS) concentration was determined via color reaction with TBA (2-thiobarbituric acid) [24]. Conjugated dienes (CoD) was determined spectrophotometrically [25]. The total lipid (TL) content was determined using the method of Amenta after extraction of lipids with a chloroform-methanol mixture (2:1, v/v) [26]. Protein concentration was determined using the modified Lowry method [27]. All measurements were performed using spectrophotometer Shimadzu UV-2550.
Metal analyses
Dried samples of the digestive gland were weighed and digested in Teflon bottles with nitric acid (16 M HNO3). Contents of metals Fe, Zn, Cu, Cd and Pb were analyzed using an atomic absorption spectrophotometer with flame atomization and deuterium background correction (Shimadzu AA-6800). Control of the analysis quality included measurement of the content of metals in the certified standard sample (ERM-CEZ78 mussel tissue). The standard sample analysis precision was above 90%.
Statistical analyses
Data were statistically compared using an unpaired t-test at the p < 0.05 significance level (STATISTICA; StatSoft, USA).
Results
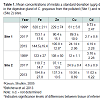
The contents of metals in the digestive gland of the mussels from the two water areas collected in 2013 (Table 1) showed that Fe, Cu and toxic Cd between the two populations of mussels. At the same time, the content of Pb in the digestive gland of mussels from Site 1 was 15 times higher. As seen from the results, the content of lipid peroxidation products CoD and TBARS in the tissues of the mussels from both biotopes did not differ significantly (Figure 2). However increase (by 45%) in TL content was observed in the mussels from Site 1.
Figure 2: Total lipids (TL, lipids/g tissue/100), conjugated dienes (CoD, μmol/mg lipids) and TBA-reactive products (TBARS, nmol/mg protein) levels in digestive gland of the mussel C. grayanus from the reference (Site 2) and polluted (Site 1) sites.* Indicates statistical differences (n = 4, p < 0.05).
Figure 3: Antioxidant levels in the digestive gland of the mussel C. grayanus from the reference (Site 2) and polluted (Site 1) sites: SOD: Superoxide Dismutase, U/mg; CAT: Catalase, μmol/min/mg; GP: Glutathione Peroxidase, nmol/min/mg*10; GR: Glutathione Reductase, nmol/min/mg; GSH: Glutathione, nmol/g wet weight/100.* Indicates statistical differences (n = 4, p < 0.05).
Discussion
Overall, the comparison of data on metals in the digestive gland of mussels collected in different years in Site 1 showed that the contents of all metals decreased by 2013 in comparison with 1999, e.g. the content of Fe reduced 4 times, Zn and Cd 2 times, Cu 2.5 times, and Pb 6.5 times (see Table 1). It should be noted that the contents of Fe, Zn, Cd and Pb decreased as soon as a half year after the remediation [12] in comparison with 1999; in this case, the content of Fe, Zn, Cd reached levels comparable to the levels of these metals in the digestive gland of mussels from Site 2. By 2013, the Cu content also reached the level typical of mussels from Site 2 (see Table 1). However, the Pb content in 2013 was not changed compared to that in 2011. Since the content of metals in the tissues of mussels is a sensitive indicator of the content of bioavailable forms of metals in the environment, the decrease in the content of metals in the digestive gland of mussels from Site 1 in 2011 and 2013 reflects a decrease in metal content in the marine environment. We suppose that the reduction in the content of metals in the digestive gland of mussels collected in Site 1 in 2011 and 2013 compared to that in 1999 is the result of remediation of the domestic solid waste dump site located on the Bay Shore. Nevertheless, the content of highly toxic metal - Pb - remains at a higher level in the mussels from Site 1 as compared to mussels from Site 2, probably due to the migration of previously accumulated toxins from the bottom sediments into the water [28] and reabsorption from the mussel valves. Certain metals, such as lead, can accumulate to a considerable extent in the shells of mollusks [29]. It has been shown that mussel Mytilus edulis can eliminate Pb from the tissues, but at very low rates [30,31]. Riget et al. investigated the loss of lead in whole soft tissues of mussel Mytilus edulis [32]. Thus Mussels transplanted from a polluted site to a clean one revealed that Pb depuration is not complete, and about a 50% of the Pb originally present in the mussel cannot be excreted even after 5 years of depuration.Lipid peroxidation products, CoD and TBARS, are widely used as markers of oxidative stress in aquatic organisms caused by pollution [9,33]. In 1999, the content of these products in mussels from Site 1 was 2 times higher compared to that in mussels from Site 2 [10]; while in 2011, only the TBARS content was 85% higher [12]. It should be noted that, in 2013, the CoD and TBARS content of the digestive gland of mussels did not differ significantly between specimens from the two water areas. At the same time, higher levels of TL in the digestive gland of mussels from Site 1 may indicate a continuing negative impact of the waste landfill. Accumulation of neutral lipids in the mollusk digestive gland and fish liver was demonstrated to be induced by the influence of toxic substances [34].
The antioxidant defense system maintains the organism integrity and chemical balance of its interior. Induction of antioxidant enzymes is known to be an important compensatory response to stress caused by environmental pollution [8,9]. Therefore it can be assumed that the increased SOD activity prevents lipid peroxidation in mussels from Site 1 and is a consequence of the mussels’ adaptation to chronicollution. We had obtained similar results in 2011 [12]. Thus, the digestive glands of the mussels also collected from Site 1 in July, showed an increase in the activity of only SOD; the other antioxidants (CAT, GP, GR and GSH) did not show significant differences when compared with the mussels from Site 2 [12].
In general, the comparative analysis of the data obtained in 1999 [10,11], 2011 [12], and 2013 (data of the present study) demonstrated that a reduction in the level of human-induced load on Site 1 due to remediation of the waste landfill is accompanied by a tendency towards decreasing the content of metals in mussel tissues and recovering a number of oxidative stress parameters to the background level. However, according to our data, elevated levels of SOD and TL in the digestive gland of the mussel from Site 1 indicate the continuing negative impact of pollutants, probably due to mobilization and migration of previously accumulated toxicants from sediments into water.
Acknowledgements
This work was supported by grants from the Russian Academy of Sciences (Programme for Basic Research No 1 on strategic areas of science to 2014 «Fundamental Problems of Mathematical Modeling») on the project «Simulation of currents field, transfer and transformation of pollutants and environmental hazards in the Far Eastern region of Russia».References
- Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue Med Vet 154: 427-430.
- Belcheva NN, Zakhartsev MV, Dovzhenko NV, Zhukovskaya AF, Kavun VY, et al. (2011) Anthropogenic pollution stimulates oxidative stress in soft tissues of mussel Crenomytilus grayanus (Dunker1853). Ocean Sci J 46: 85-94.
- Slobodskova VV, Zhukovskaya AF, Chelomin VP (2012) DNA damage in the gill cells of the marine scallop Mizuhopecten yessoensis during anoxic stress and aerobic recovery. Ocean Sci J 47: 95-100.
- Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12: 75-92.
- Halliwell B, Gutteridge JM (2007) Free radicals in biology and medicine, Fifth edition. Oxford University Press, Oxford, UK.
- Regoli F, Principato G (1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilis galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 31:143-164.
- Nicholson S, Lam PK (2005) Pollution monitoring in Southeast Asia using biomarkers in the mytilid mussel Perna viridis (Mytilidae: Bivalvia). Environ Int 31: 121-132.
- Funes V, Alhama J, Navas JI, Lopez-Barea J, Peinado J (2006) Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ Pollut 139: 214-223.
- Box A, Sureda A, Galgani F, Pons A, Deudero S (2007) Assessment of environmental pollution at Balearic Islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis. Comp Biochem Physiol C Toxicol Pharmacol 146: 531-539.
- Dovzhenko NV, Belcheva NN, Chelomin VP (2002) Biochemical indicators of oxidative stress as markers (indicators) of anthropogenic pollution of aquatic ecosystems. IV Conf of Young Scientists on Oceanologic Studies, Institute of Pacific Ocean Studies of the Far-East Branch (IPOS FEB) of RAS, Vladivostok, November 27-30, Summary of presentations Vladivostok: IPOS FEB of RAS, pp. 291-296.
- Kavun VY, Shulkin VM (2005) Change in the microelemental composition of organs and tissues of the Crenomytilus grayanus mussel at acclimatization in the biotope chronically polluted with heavy metals. Biology of the Sea 31: 123-128.
- Belcheva NN, Istomina AA, Kudryashova YuV, Chelomin VP (2013) Marine environment assessment based on oxidative stress indicators and heavy metal content in tissues of the mussel Crenomytilus grayanus (Dunker 1853) (Bivalvia: Mytilidae). Russ J Mar Biol 39: 281-286.
- Podorvanova NF, Ivashinnikova TS, Petrenko VS, Khomichuk LS (1989) The main hydrochemical features of Peter the Great Gulf (the East Sea). Far East Branch of the Russian Academy of Sciences, Vladivostok, pp. 201.
- Tkalin AV, Lishavskaya TS, Shulkin VM (1998) Radionuclides and trace metals in mussels and bottom sediments around Vladivostok, Russia. Mar Pollut Bull 36: 551-554.
- Tkalin AV, Samsonov DP, Lishavskaya TS, Chernik GV (2000) New data on organochlorine distributions in the marine environment near Vladivostok. Mar Pollut Bull 40: 879-881.
- Shulkin VM (2004) Heavy metals in marine shallow-water ecosystems. Dalnauka, Vladivostok, pp. 279.
- Yutsuk AV (2010) Monitoring the pollution of the aquatic area of Ussuriysky Bay in the solid waste landfill impact zone. Proc of the All-Russia Scientific Conference “Ecological issues of the marine shelf”, Vladivostok, pp. 201-203.
- Sevastyanov AV, Lishavskaya TS, Chatkina TV (2014) Monitoring of self-purification of seawater and bottom sediment of Ussuriysky Bay in the area of the former municipal dumping site (Gornostai Bay). In: Trudy DVNIGMI (Transactions of Far Eastern Regional Hydrometeorological Research Institute), Dalnauka Press, Vladivostok, 155: 160-175.
- Alimov AF, L’vova AA, Makarova GE, Soldatova IN (1990) Growth and age. In: Shkorbatov GL (Ed). Methods to study bivalves, Leningrad: Proc Zoo Inst Acad Sci USSR, pp. 121-140.
- Scarlato OA (1981) Bivalved mollusks of the temperate waters of the north-western part of the Pacific Ocean. Leningrad, Science, pp. 480.
- Paoletti F, Aldinuccio D, Mocali A, Carparrini AA (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154: 536-541.
- Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71: 952-958.
- Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582: 67-78.
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Fleischer S and Packer L (Ed). Methods in Enzymology 52, Academic Press, New York, USA, pp. 302-310.
- Corongiu FP, Banni S (1994) Detection of conjugated dienes by second derivative ultraviolet spectrophotometry. In: Packer L (Ed). Methods in enzymology 233, Academic Press, New York, USA, pp. 303-310.
- Amenta JS (1964) A rapid chemical method for quantification of lipids separated by thin-layer chromatography. J Lipid Res 5: 270-272.
- Markwell MA, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87: 206-210.
- Rivera-Duarte I, Flegal AR (1994) Benthic lead fluxes in San Francisco Bay, California, USA. Geochim Cosmochim Acta 58: 3307-3313.
- Sturesson U (1976) Lead enrichment in shells of Mytilus edulis. AMBIO 5: 253-256.
- Schulz-Baldes M (1974) Lead uptake from sea water and food, and lead loss in the common mussel Mytilus edulis. Mar Biol 25: 177-193.
- Sánchez-Marín P, Bellas J, Mubiana VK, Lorenzo JI, Blust R, et al. (2011) Pb uptake by the marine mussel Mytilus sp. Interactions with dissolved organic matter. Aquat Toxicol 102: 48-57.
- Riget F, Johansen P, Asmund G (1997) Uptake and release of lead and zinc by blue mussels. Experience from transplantation experiments in Greenland. Mar Pollut Bull 34: 805-815.
- Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101: 13-30.
- Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C Toxicol Pharmacol 146: 281-300.