Journal of Veterinary Science & Medicine
Download PDF
Review Article
Characteristics and Identification Methods of Veterinary Important Mange Mites
Tewodros Alemneh¹* and Zewdu Seyoum²
1Woreta Town Office of Agriculture and Environmental Protection,
Woreta, S/Gondar Zone, Amhara Regional State, Ethiopia
2School of Veterinary Medicine and Animal Sciences, Department of
Veterinary Pathobiology, University of Gondar, Gondar, Ethiopia
*Address for correspondence: Alemneh T, Woreta Town Office of Agriculture and Environmental Protection, Woreta, Ethiopia, Email Id: tedyshow@gmail.com
Submission: 22 September 2023
Accepted: 18 December 2023
Published: 20 December 2023
Copyright: © 2023 Alemneh T, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords: Mite; Mange; Identification; Morphology; Serology; Molecular
Methods
Abstract
Mites are of among the serious skin parasites of both animals and humans
worldwide. The disease they cause is called mange or scabies. Mange mites
of medical importance are categorized into two major groups. One group is
the burrowing type that found deeply under the skin, and affects skin follicles
and sebaceous glands. This group consists of Demodex, Sarcoptes, etc.
The other group is no burrowing type and found on the surface of the skin.
Psoroptes and Chorioptes are the well-known non burrowing mites. Each mite
has its own unique morphological and behavioural characteristics. Hence for
effective therapy and prevention and control of the disease, identification and
characterization of mites is mandatory. Therefore, this mini-review highlights
mites’ characterization and identification using morphological, serological and
molecular methods.
Introduction
Mange (scabies) is a parasitic skin disease caused by microscopic
mites. Mites are obligate and permanent parasite belonging to the
order Acarines. Two different mange mites cause this skin disease in
animals. One lives just under the surface of the skin (e.g. psoroptic
mange), while the other resides in the hair follicles (e.g. demodectic
mange). In other words, mites causing mange of animals of veterinary
importance usually belong to two broad families: borrowing and nonborrowing.
The borrowing group consists of mainly Sarcoptidae and
Demodicidae while the non-borrowing groups are Psoroptidae and
Chorioptidae. Although both mites share similar characteristics,
there are also important differences. It is important not to confuse the
two types of mange because they have different causes, treatments,
and prognoses [1].
Similar in appearance to ticks but much smaller, mites have
bulbous, round, or pill-shaped bodies. Classified as arachnids, mites
have eight jointed legs. Their size varies by species, but most mites are
usually invisible to the naked eye. The largest mites measure about 6
mm long, while the smallest are about 0.1 mm. The colour of mites
varies greatly as well; most mites appear tan, brown, or reddishbrown,
but some species are bright red, blue, or green in colour [2].
Characteristics of Mange Mites
a. Sarcoptic Mange (Scabies):
Sarcoptesscabieivarbovis is a highly contagious disease spread by
direct contact between infested and naive animals or by contaminated
fomites. Lesions caused by this burrowing mite start on the head,
neck, and shoulders and can spread to other parts of the body. The
whole body may be involved in 6 weeks. Pruritus is intense, and
papules develop into crusts; the skin thickens and forms large folds.
S. scabieivarbovis can also be transmitted to people and result in a
transient, self-limiting dermatitis [3].b. Psoroptic Mange:
Psoroptic mange in animals is caused by infestation with Psoroptes
ovis. Current taxonomic and systematic classification of Psoroptes spp
indicates that P. ovis and P. cuniculi (ear canker in rabbits, ear mange
in sheep and goats) are strains or variants of the same species, with P.
ovis being found primarily on the backs and flanks of infested animals
and P. cuniculi in the ears. P. ovis is not zoonotic. P. ovis is a nonburrowing
mite that lives on the skin surface. All stages of the mite
are found on the host, and transmission is through direct contact of
infested and susceptible hosts. Transmission is also possible through
contact with contaminated environments or fomites, because P. ovis
can survive off the host for ≥2 weeks under the right conditions [3].c. Chorioptic Mange:
Chorioptic mange in cattle is caused by infestation with
Chorioptesbovis or C. texanus. Species of Chorioptes are not host
specific, and C. bovis can be found on domestic ruminants and horses
throughout the world. Chorioptic mange caused by infestation with
C. bovis is the most common type of mange in cattle in the USA. C.
texanus has been reported on cattle from Brazil, China, Germany,
Israel, Japan, Malaysia, South Korea, and USA. C. bovis and C. texanus
are not zoonotic. C. bovis live on the skin surface and do not burrow.
Life cycle stages include egg, larval, two nymphal, two female, and one
male stage. Eggs are deposited on the skin, and secretions from female
mites help secure eggs to the surface of their hosts. Eggs require 5-6
days to hatch, whereas each larval and nymphal stage requires 3-5
days for development. The entire life cycle may be completed in 21-
26 days and depends on temperature and humidity. Transmission
is by direct contact of infested and naive hosts. C. bovis can live off
their host for up to 3 weeks and can be transmitted to cattle through
contact with contaminated fomites and housing. Chorioptic mange
is less pathogenic than sarcoptic or psoroptic mange in cattle [3].d. Demodectic Mange (Follicular Mange):
Three species of Demodex are known to infest cattle: D. bovis, D.
ghanensis, and D. tauri. D. bovis is the most common and infests hair
follicles of cattle worldwide. D. ghanensis infests meibomian glands
of cattle from Ghana, and D. tauri has been recovered from hair
follicles and sebaceous glands of cattle from Czechoslovakia. Species
of Demodex are very host specific and typically occur either in hair
follicles or dermal glands, and they are not zoonotic. Demodexsppare
unique among parasitic mites, because they are elongated with short,
stumpy legs. Their distinct morphology is a presumed adaptation to
living in hair follicles and sebaceous glands of their hosts. All life cycle
stages are found on the host and include egg, larvae, two nymphs, and
adults. These mites feed on sebum, protoplasm, and epidermal debris.
Transmission of D. bovis occurs through close contact of infested and
naive hosts, with the transfer of mites from infested dams to neonates
being the primary route [3].Identification Methods
a. Morphological Identification Methods:
i. Sarcoptic mange
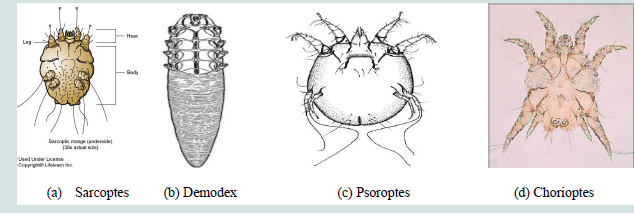
Sarcoptes is round in outline and up to 0.4mm in diameter
with prominent dorsal pegs and spines. And its most important
recognition characters are the numerous transverse ridges and
triangular scales on the dorsum, features possessed by no other mange
mite of domestic mammals. They have short legs and dorsally the legs
only just project beyond the edge of the body and the posterior two
pairs of legs do not extend beyond the body margin at all.Pulvillus
is originated on a stalk-like or unsegmentedpretarsus on 1st and 2nd
pairs of legs. The male is about 250 μm in length and is smaller than
mature female which is about 400 to 430 μm in length. The dorsal
surface of the body of Sarcoptesscabiei is covered with transverse
ridges but also bears a central patch of triangular scales. The dorsal
setae are strong and spine like. The anus is terminal and only slightly
dorsal [Figure 1] [4].ii. Demodectic mange
Demodex has an elongated tapering body (cigar shape), up
to 0.2mm long, with four pairs of stumpy legs anteriorly. Setae are
absent from the legs and body. The legs are located at the front of the
body so that the striated-opisthosoma forms at least half the body
length. The mouth parts consist of paired palps and chelicerae, and
an unpaired hypostome [4].
iii. Psoroptic mange
Psoroptes is a typically non burrowing mite, up to 0.75mm, oval
in shape, and with all the legs projecting beyond the body margin. Its
most important recognition features are the pointed (conical) mouth
parts, the rounded abdominal tubercles of the male and the three
jointed pedicles (pretarsi) bearing funnel-shaped suckers on most of
the legs (on 1st, 2nd and 4th pairs of legs) [Figure 1] [4].
iv. Chorioptic mange
The mouth parts are distinctly rounder, the abdominal tubercles
of the male are noticeably truncate and the pedicles are short and
unjointed (unsegmented), with cup-shaped sucker. Adult female
Chorioptes bovis are about 300 μm in length, considerably smaller
than Psoroptesovis [Figure 1] [4].
b. Serological Identification Methods:
Several serological assays for scabies have been developed over the
past decades. In 2007, Casais et al. [5] developed an enzyme-linked
immunosorbent assay (ELISA) based on identification of a 642 amino
acid polypeptide using a recombinant S. scabiei var. hominis library.
After insertion of the encoding complementary DNA in Escherichia
coli, antiserum was raised and used in Western blots of serum from
chamois with scabies. The technique proved to be highly sensitive
(100%) and specific (97%) in identifying infected animals. In 2010,
Walton et al [6] developed a quantitative immunoglobulin E (IgE)
inhibition assay for human use that identified Ig E immunoreactivity
of scabies mite antigens based on recombinant-produced S. scabiei
cysteine or serine proteases and apolipoproteins. These antigens
are located in the mite cuticle or gastrointestinal tract. They found
significant IgE levels in patients with scabies compared with normal
control subjects and, by using inhibition ELISA, no or minimal crossreactivity
with house dust mite antigens was observed. With the
apolipoproteinSsag 1.2, the sensitivity of the IgE assay was 88% and
specificity was 100%. They tested patients with different clinical forms
of scabies and found that greater IgE reactivity was seen for mite
apolipoproteins with serum from patients with the crusted forms
compared with normal scabies.In 2015, Arlian et al. [7] prepared aqueous extracts from S.
scabiei var. canis as well as from common house dust mites. The
antigen was then tested using sera from 91 patients and screened
for IgA, IgE, IgG, IgM and IgD antibodies to S. scabiei. However,
antibodies were found to cross react with house dust mites, including
Dermatophagoidesfarinae, Dermatophagoidespteronyssinus and
Euroglyphusmaynei. Later, a further assay was developed with a
recombinant S. scabiei actin-associated cofilin protein from rabbit
scabies which is present in the splanchnic area, but not the exoskeleton,
and shares 90% identity with D. farinaecofilin. One of the serological
assays developed using this antigen of S.scabiei was an indirect ELISA
that was tested using rabbit serum from rabbits infected with scabies
and uninfected controls as well as some with Psoroptescuniculi and
Cysticercosispisiformis infestations. This method showed 83.33%
sensitivity and 87.9% specificity [8].
Figure 1: Morphological features of the four important Mange mites: (a) Sarcoptes (b) Demodex (c) Psoroptes and (d) Chorioptes. Source: Abriham [4].
A dissociation-enhanced lanthanide fluorescent immunoassay
(DELFIA) was also developed based on a recombinant Sar s 14.3
major scabies antigen. No cross-reactivity was noticed using the
house dust mite homologue Der p 14, and in a study of infested and
control human subjects it proved to be a highly sensitive (100%) and
specific (93.75%) method for scabies diagnosis in clinical settings [8].
Another cloned protein that has been investigated is a S. scabiei
tyrosine kinase (SsPTK), which is mainly located in the mouth part
region of the scabies mite. It was evaluated in a rabbit model and
was found to have a sensitivity of 95.2% and a specificity of 94.1%; it
was also able to detect infection early in its course after 1 week.Other
potential protein targets that have been assessed in experimental
scabies models are triosephosphateisomerase, calmodulin and
chitinase. In summary, detection of targeted scabies antibodies using
immunoassays has shown promise, but the antigens targeted by these
tests have not been adopted for use in immunologically based antigen
detection assays [8].
c. Molecular Identification Methods:
Scabies can also be identified by molecular methods that select
different targets, including microsatellites, ITS-2 ribosomal DNA
(rDNA), mitochondrial 12S/16S rRNA and S. scabiei myosin
heavy chain genes. Additionally, an attempt was made recently to
characterize the parasite proteins in order to establish the diagnosis
using matrix-assisted laser desorption ionization–time of flight mass
spectrometry (MALDI-TOF MS). This work has now generated
details of many new molecular targets for subsequent proteomic
work [8].v. DNA Isolation:
S. scabiei DNA can be extracted using several different protocols.
This can be accomplished directly from human samples such as skin
biopsiesand swab specimens. DNA can be extracted with commercial
kits such as the Qiagen Tissue Kit (Qiagen, Hilden, Germany),
QIAamp DNA minikit (Qiagen), Nucleospin Tissue kit (Macherey-
Nagel, Düren, Germany)and silica magnetic NucliSENSeasyMAG kit
(bioMérieux, Marcy-l’Étoile, France) [8].vi. Conventional Polymerase Chain Reaction (PCR):
In 2001, Bezold et al. were able to identify S. scabiei in skin
samples of a patient using primer sequences from highly conserved
regions of S. scabiei microsatellite 15 (Sarms15). Later, in 2015,
a further S. scabiei–specific conventional PCR was developed.
This PCR was based on the mitochondrial cytochrome c oxidase
subunit 1 (cox1) gene of S. scabiei, in which coding regions
were selected that had no homology to any known sequences of
potentially cross-reactive mites, including Demodexfolliculorum,
Demodexbrevis, Dermatophagoidespteronyssinus, Dermatophagoides
farina, Dermanyssusgallinae, Tyrophagusputrescentiae and
Cheyletusmalaccensis. PCR primers scabF1 and scabR2 appeared to be
specific for S. scabiei and generated a 250 bp product. The sensitivity
and specificity were 100%. The assay detected all 17 microscopypositive
patients and confirmed the diagnosis in an additional 12
patients. All these additional patients had compatible skin lesions and
responded to antiscabetics [8].In further work, another conventional PCR was developed by
Angelone-Alasaadet al [9] in 2015 for the rapid diagnosis of scabies.
Based on mitochondrial 16S rDNA, a primer set was developed that
generated a PCR product of 135 bp with S. scabiei. This was used for
the diagnosis of animal scabies, using skin samples taken from six
different animal species with confirmed sarcoptic mange (scabies)
[8].
Recently Delaunay et aldeveloped a PCR assay for identification
based on the ITS-2 region that showed somewhat lower sensitivity
to previously developed PCR assays in human scabies using a
different sampling technique of swabbing the skin over the wrist
and interdigital spaces of infested patients. Of 87 patients with
dermatoscopically confirmed scabies, 33 had positive scabies PCRs,
with a sensitivity of 37.9% and a negative predictive value of 61.7%.
The authors pointed out the potential use of this as a screening
method in scabies outbreaks [8].
vii. Real-time Polymerase Chain Reaction (RT-PCR):
To discontinue the use of gel electrophoresis and to generate
results more quickly for some causative agents, real-time PCR is
appropriate. In 2015, a novel quantitative PCR (qPCR) targeting
a 121-bp fragment of the cox1 gene of S. scabiei was designed and
evaluated using samples collected from human skin at different body
sites before and after medical treatment. The newly developed qPCR
could also be used to monitor treatment response, as the number of
S. scabiei DNA copies was higher before the treatment and decreased
after initiating treatment, becoming undetectable at days 14, 21 and
28 after the start of treatment [8].As a further approach to rapid detection, a TaqMan real-time
PCR assay was developed in 2015 by Angelone-Alasaadet al [9].
They used the assay as a diagnostic method for sarcoptic mange in
different animal species. In this assay, a specific probe for S. scabiei
was developed using amplification of 135 bp from mitochondrial 16S
rDNA. The technique was highly sensitive and no cross-reactivity
was observed. It was also more sensitive than endpoint PCR, as a
minimum amount of Sarcoptes genomic DNA of 10 pg/μLwas needed
compared with 80 pg/μL for the conventional assay [8].
In 2020, Bae et al. [10] developed an in-house reverse transcription
PCR assay based on the cox1 gene of S. scabiei, a 196 fragment using
primers cox1F and cox1R with a cox1P2 probe. The authors used the
IACS criteria as their case validation method. A total of 47 patients
were tested; 33 had a suspected diagnosis of scabies, 10 had unrelated
disease and 4 were healthy individuals. Of the 33 suspected cases, 22
had microscopy-proven scabies, 2 had clinically diagnosed scabies,
6 had suspected scabies and 3 were negative. Samples were obtained
by scraping lesional skin. The assay showed a sensitivity of 86% in
confirmed scabies cases, 83% in confirmed but clinically diagnosed
scabies and 80% in clinically suspected scabies and 100% specificity.
The results also matched the declining certainty of the diagnosis
based on the IACS clinical criteria [8].
viii. Isothermal Amplification Techniques:
The downside of using PCR-based identification tools is that there
is still the need to use thermocyclers to amplify the DNA. In the past
few years, isothermal amplification techniques have been developed
to overcome this shortcoming. These include loop-mediated
isothermal amplification (LAMP), rolling circle amplification (RCA)
and multiple displacement amplification (MDA). These techniques
are available to identify wide varieties of microorganisms. A LAMP
assay has been designed based on the ITS-2 gene for the identification
of S. scabiei and has been found to be promising after evaluation
of skin scrapings from infected animals with sarcoptic mange that
showed 100% sensitivity and 92.3% specificity [8].