Journal of Veterinary Science & Medicine
Download PDF
Research Article
Stable Isotope Ratios of Carbon, Nitrogen, Oxygen, and Mercury Concentrations in North Pacific Baleen Whales and the Comparison of Their Calves with Toothed Whale Calves
Endo T*, Terasaki M and Kimura O
School of Pharmaceutical Sciences, Health Sciences University of
Hokkaido, 1757 Ishikari-Tobetsu, Hokkaido 061-0293, Japan
*Address for correspondence:
Endo T, School of Pharmaceutical Sciences, Health Sciences University
of Hokkaido, 1757 Ishikari-Tobetsu, Hokkaido 061-0293, Japan;
E-mail: endotty531115@gmail.com
Submission: 20 May, 2022
Accepted: 24 June, 2022
Published: 11 July, 2022
Copyright: © 2022 Endo T, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
We quantified 13C, 15N,18O, and Hg concentrations in the muscle of calf
and immature humpback whales stranded along the coast of the North Pacific
Ocean around Hokkaido, Japan, and investigated those changes owing to
the lactation. Next, we compared these concentrations in stranded humpback
whale calves with those in stranded fin whale and North Pacific right whale
calves, and stranded calves from other species reported previously [1,2]. We
further compared those concentrations in stranded fin whales with those in
fin whales hunted from the North Atlantic and Antarctic Oceans. The δ13C
value in humpback whale calves increased with body length (7.0-8.7 m),
whereas the δ18O values tended to decrease. In contrast, a small δ15Nenriched
peak was found in middle-sized calves. Humpback whale calves
had trace Hg concentrations (≤0.05 μg/wet g), whereas these concentrations
exceeded 0.10 μg/wet g in immature humpback whales. These changes in
the δ13C, δ15N, and δ18O values and Hg concentrations in humpback whales
could reflect a feeding shift from milk to solid foods. The δ13C and δ15N levels
of calves, humpback and fin whales, and common minke whales reported
previously [1] were similar, slightly higher than those of North Pacific right
whales and significantly lower than those of killer whales [2]. These findings
suggest that the δ13C and δ15N values in the milk and weaning solid foods
of humpback, fin, and common minke whales are similar (opportunistic
fish eaters), slightly different from North Pacific right whales (zooplankton
eaters), and markedly different from killer whales (highest predator). Fin whales
stranded in the North Pacific Ocean could be distinguished from fin whales
hunted from the North Atlantic and Antarctic Oceans using δ13C, δ15N, and
δ18O values. The δ18O values, combined with the δ13C and δ15N values could
be an excellent proxy to discriminate fin whales from the three oceans.
Keywords
Humpback whale (Megaptera novaeangliae); Fin whale (Balaenoptera physalus); North Pacific right whale (Eubalaena japonica); Common minke whale (Balaenoptera acutorostrata); Killer whale (Orcinus orca); Dall’s porpoise (Phocoenoides dalli); Lactation
Introduction
Stable isotope analysis is a useful tool for obtaining information on
the feeding ecology, migration, and physiology of marine mammals
[3,4]. The stable isotope ratio of nitrogen (δ15N) increases as the
trophic level in a food chain increases, whereas the stable isotope ratio
of carbon (δ13C) is used to estimate the origin of base of food chain.
The stable isotope ratio of oxygen (δ18O), usually combined with
13C and δ15N, has been increasingly used to discriminate, verify, and
identify the habitat of marine mammals, as it reflects the geographic
and climatic conditions of habitats. The δ18O level in surface seawater
tends to be depleted at high latitude and low salinity [4-6]. Thus, the
δ18O values in the bones [7-9] and teeth [10-12] of marine mammals
reflect the latitude and salinity of their habitat.
The δ13C and δ15N values are particularly useful in evaluating mother-to-offspring nutrient transfer; nursing offspring of mammals
generally have higher δ15N levels, with similar or slight higher δ13C
levels to those of their mothers depending on the milk composition
and nursing period [3,4,6]. Many studies of marine mammals have
reported the elevated levels of δ15N in calf tissues such as bone,
tooth, muscle, blood, and skin; these seem to be associated with milk
consumption [1,4,8,9,13-17].
Elevated levels of δ18O in bones and teeth owing to breastfeeding
have been reported in prehistoric human new born and infants [18-20]. Ancient infants accumulated more 18O from ingesting milk
than drinking water, and the elevated δ18O values in teeth were
found in greater in teeth developed at a younger age of infants than
those developed at an older age [18]. However, to the best of our
knowledge, no studies have focused on changes in δ18O values of
marine mammals due to lactation.
Most baleen whales (mysticetes) have relatively brief lactation
periods (nursing and weaning: 5-7 months), whereas the lactation
periods in humpback whales (Megaptera novaeangliae) and North
Pacific right whales (Eubalaena japonica) may be slightly longer,
which is in contrast with the lactation periods in toothed whales,
dolphins, and porpoises (odontocetes), they have more extensive
lactation periods (1-3 years) [21]. Mysticetes feed lower biota in
the food chain (i.e., zooplankton and small fish), and typically have
less significant exposure to pollutants such as mercury (Hg) and
organochlorines, whereas as apex predators, most odontocetes are
exposed to high levels of pollutants by ingesting contaminated large
fish [22-24]. Positive correlations are generally observed between the
δ15N value and the Hg burden in marine animals as Hg is a typical
contaminant that accumulates through the marine food web [25]. A
sharp increase in the Hg burden is reported to be a weaning proxy for
piscivore [26].
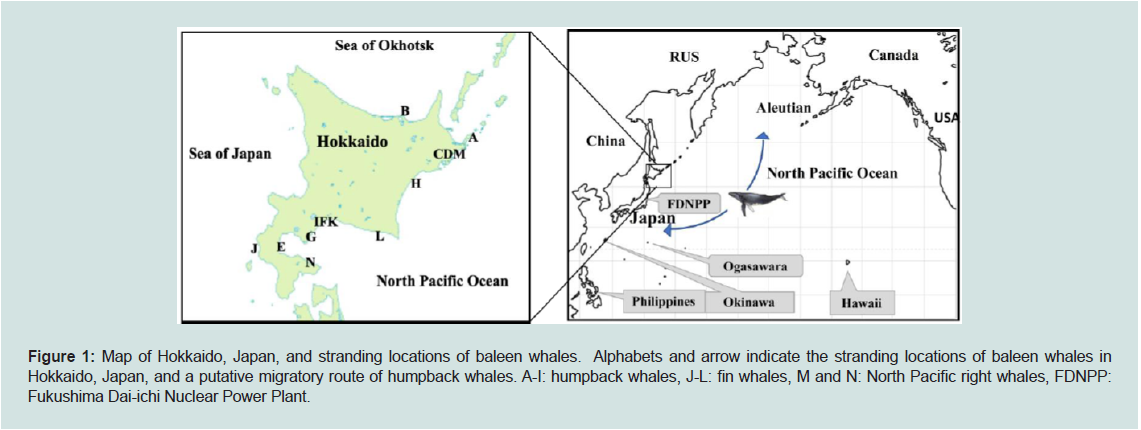
Hokkaido is the northern most large island in Japan, surrounded
by the North Pacific Ocean, Sea of Japan, and Sea of Okhotsk (Figure 1), with more than 50 cetaceans stranded annually along its coast
(SNH; http://www.kujira110.com/). Most stranded cetaceans are
odontocetes of dalli-type Dall’s porpoise (Phocoenoides dalli), harbor
porpoise (Phocoena phocoena), and Pacific white-sided dolphin
(Lagenorhynchus obliquidens), and a mysticete of common minke whale (Balaenoptera acutorostrata); other odontocete of killer
whale (Orcinus orca), and mysticetes of humpback whale, fin whale
(Balaenoptera physalus), and North Pacific right whale, are rarely
stranded [27,28]. Many calves of mysticetes strand in Hokkaido,
although the cause of strandings is unknown.
Among the baleen whales stranded along the coast of Hokkaido,
common minke whale is an opportunistic fish predator that
temporally and regionally adapts to prey type [29,30]. Humpback
and fin whales stranded in Hokkaido are also opportunistic fish
feeders, whereas the North Pacific right whale generally only feeds
on zooplankton [31,32]. Dietary overlap and resource partitioning
among sympatric baleen whales, humpback, fin and common minke
whales, in the North Atlantic Ocean have been investigated [33-35].
However, little information is available on the feeding ecology and
dietary overlap of humpback, fin, common minke, and North Pacific
right whales inhabiting in the North Pacific Ocean around Hokkaido.
Endo et al. reported a small δ15N-enriched peak in the muscle
of common minke whale calves stranded in Hokkaido during
the lactation period [1], which fitted to a quadratic function; the
increase in δ15N value before the peak may represent nursing,
whereas the following decrease may represent weaning. However,
the increase of δ15N value due to the lactation has not yet reported
in other mysteceteces, humpback, fin and North Pacific right whales
inhabiting in waters around Hokkaido. According to literature, a
brief weaning period results in a rapid decrease in δ15N values [36],
whereas a prolonged weaning period results in gradual decrease in
δ15N values [4,20]. Endo et al. [1] also reported a trace burden of Hg
in nursing common minke whales and the increase in the Hg burden
with growth related to the shift from nursing to weaning (consuming
solid foods). The degree of Hg burden in mysticetes is generally low,
but the Hg burden in opportunistic feeders of mysticetes could be
high in proportion to the amount of fish consumed [30].
Studies on lactation and mother-to-offspring nutrient transfer in
marine mammals have been increasingly conducted using pinnipeds
because they give birth and nurse pups on land or ice in accessible
areas; it is easy to observe them and collect paired sampling from
lactating mother and suckling pup, in comparison with large whales
[21,37,38]. For studies on pinnipeds, if samples from the mother are not available, foraging habitats and trophic position of mothers are
indirectly estimated from the δ13C and δ15N values of their suckling
pups [38], using the Δ15Npup-mother and Δ13Cpup-mother values. In contrast,
the δ15N and δ13C data of cetacean calves are scare and the Δ15Npupmother
and Δ13Cpup-mother data are even scare, due to difficulties of sample
collection. Available data for Δ15Ncalf-mother and Δ13Ccalf-mother values
calculated from killer whales [39] in addition to Δ15Ncalves-matures and
Δ13Ccalves-matures values calculated from common minke whales [1]
and Dall’s porpoises [36] are all small less than 2‰ for Δ15N values
and less than 1 for Δ13C values, respectively. Thus, the means of δ13C
and δ15N values in lactating mothers and mature animals seem to be
similar and slightly lower than those of their calves, respectively, as in
the case of pinniped [16,38,40].
In contrast to the fin whale inhabiting in the North Pacific and
North Atlantic Oceans (opportunistic fish eater), this species in the
Antarctic Ocean only feeds on zooplankton [41,42]. To the best of
our knowledge, a comparative study of the δ13C, δ15N and δ18O values,
and Hg concentrations in fin whales from the North Pacific, North
Atlantic, and Antarctic Oceans has not yet been conducted. As far as
I know discrimination of fin whales inhabiting the three oceans is not
possible by genetic analysis.
In the present study, we quantified the δ13C, δ15N, and δ18O values,
and Hg concentrations in muscle samples from humpback whale
calves and weaned immature animals stranded along the coast of the
North Pacific Ocean around Hokkaido. (1) We investigated changes
in the δ13C, δ15N, and δ18O values and Hg concentration in humpback
whales owing to the lactation, and compared these changes with
those reported previously in common minke whale calves [1]. (2)
Next, we compared the δ13C, δ15N, and δ18O values in calf muscle
samples from several cetaceans, humpback, fin and North Pacific
right whales stranded in Hokkaido (this study) and common minke
whales [1], killer whales [39] and Dall’s porpoises [36] stranded in
Hokkaido, and investigate whether the trophic position of mothers
(mature animals) could be indirectly estimated from the δ13C and
δ15N values of their calves. (3) Lastly, we compared quantified values
in the muscle samples of fin whale calves stranded in Hokkaido
(North Pacific Ocean) with those values in the red meat products of
fin whales hunted by whaling operations from the North Atlantic and
Antarctic Oceans, and investigated whether fin whales from the three oceans could be discriminated using the δ13C, δ15N, and δ18O values,
and Hg concentration.
Methods
Sampling of humpback, fin, and North Pacific right whales:
We collected muscle samples from humpback whales (n = 9),
fin whales (n = 3), and North Pacific right whales (n = 2) stranded
along the coast of Hokkaido, Japan, in 2012 and 2018 (Figure 1 and
Table 1). In addition, we collected liver samples from six humpback
whale individuals. Most samples were obtained from whales stranded
along the coast of the North Pacific Ocean around Hokkaido,
with samples obtained from one humpback whale from the Sea of
Okhotsk and one fin whale from the Sea of Japan, provided by the
Stranding Network of Hokkaido (SNH). Unfortunately, the sex of
some stranded individuals could not be determined owing to their
advanced decomposition (Table 1).
Figure 1: Map of Hokkaido, Japan, and stranding locations of baleen whales. Alphabets and arrow indicate the stranding locations of baleen whales in
Hokkaido, Japan, and a putative migratory route of humpback whales. A-I: humpback whales, J-L: fin whales, M and N: North Pacific right whales, FDNPP:
Fukushima Dai-ichi Nuclear Power Plant.
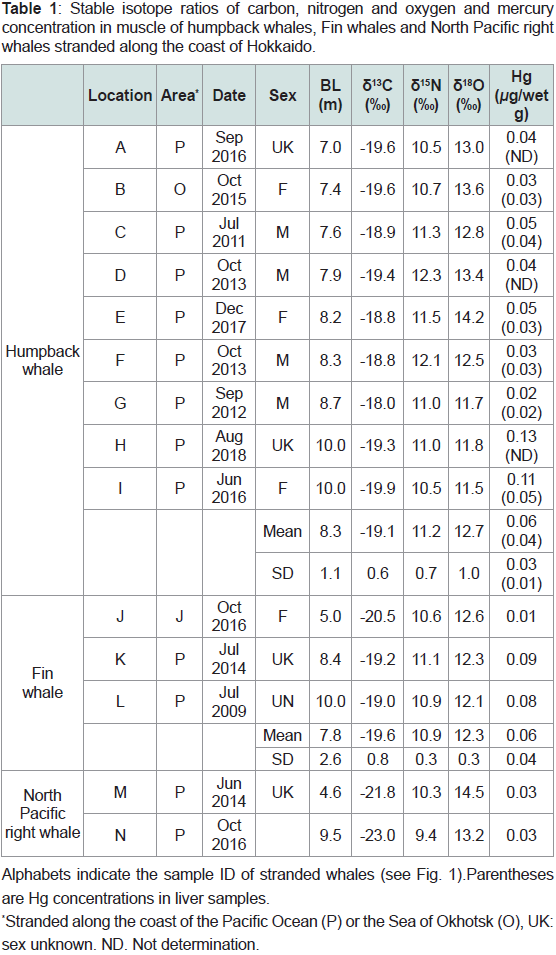
Table 1: Stable isotope ratios of carbon, nitrogen and oxygen and mercury
concentration in muscle of humpback whales, Fin whales and North Pacific right
whales stranded along the coast of Hokkaido.
The body length (BL) of humpback whale newborns, of calves at
the cessation of weaning, and at sexual maturity are 4.5-5.0, 8-9, and 11-12m, respectively [43]. Considering our data for δ13C values and
Hg concentrations (Figure 2), we categorized the humpback whale
of 10.0 m BL (sample ID, H, and G, Table 1) as weaned but not yet
mature animals. Thus, our humpback whale samples included seven
calves and two weaned immature animals (Table 1).
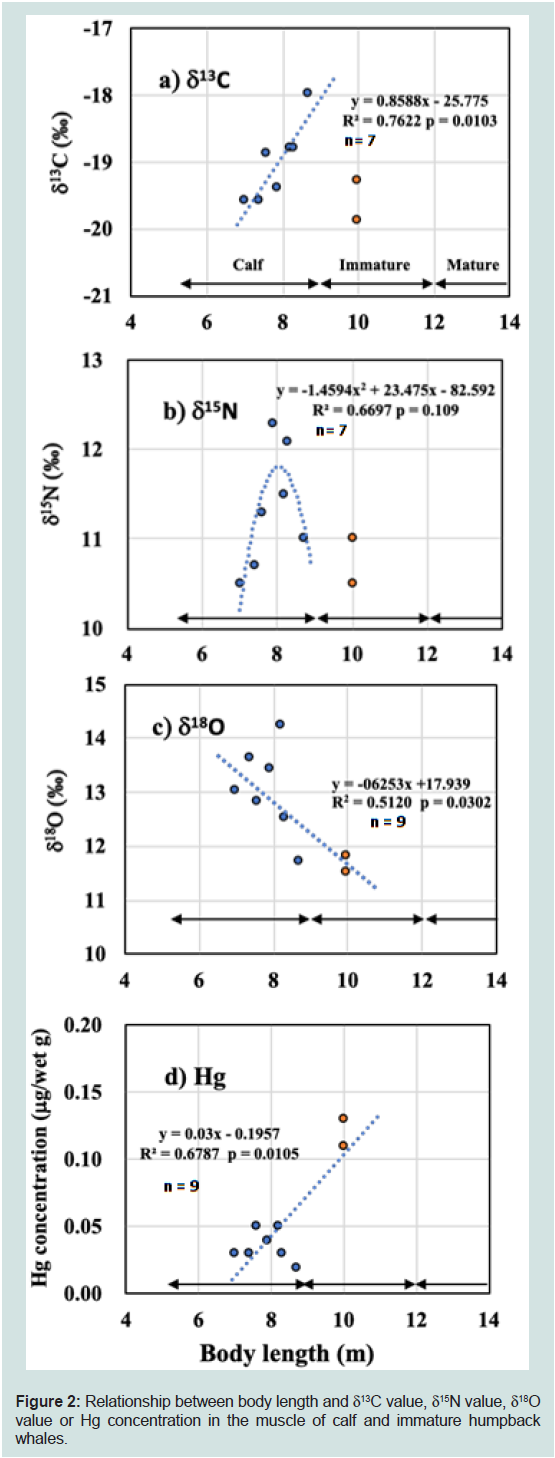
Figure 2: Relationship between body length and δC value, δ15N value, δ18O
value or Hg concentration in the muscle of calf and immature humpback
whales.
The BL of fin whale newborns, of calves at cessation of weaning,
and at sexual maturity are 6.0-6.5, ~11, and ~18 m, respectively
[44], and the largest fin whale fetus ever reported had a BL of 5.0 m
[45]. Thus, we categorized all fin whale samples (5.0, 8.4, and 10.0 m
BL; Table 1) as calves, and the smallest calf of 5.0 m BL might be a
premature animal.
The BL of North Pacific right whale newborns and weaned calves
are ~4.2 and ~10.3 m, respectively [46]. Thus, we considered the
two samples (4.6 and 9.5 m BL; Table 1) were from a newborn and
a weaning calf.
We purchased the red meat products of fin whales caught off
Japan Scientific Research Whaling from the Antarctic Ocean in 2000
and 2006 at retail outlets in Japan (Table 2). We also purchased the
red meat products of fin whales from retail outlets in Japan in 2012
and 2013, which were caught off Iceland from the North Atlantic
Ocean for human consumption (Table 2).
Table 2: Stable isotope ratios of carbon, nitrogen and oxygen and mercury
concentration in red meat products of fin whales caught off North Atlantic Ocean
and Antarctic Ocean sold in Japan.
All stranded whale and red meat product samples were stored at
-20°C until chemical analysis.
Chemical analyses:
Before the 13C, 15N, and 18O analyses, the lipids in the muscle
samples and red meat products were removed by chloroform/
methanol extraction [47]. Lipid extraction was repeated three or
more times until the color of the extraction solvent became clear.The analyses of 13C and 15N in the muscle and red meat product
samples were performed using an IRMS (Delta S, Finnigan MAT,
Bremen, Germany and EA1108, Fisons, Roano, Milan, Italy), as described previously [1,48]. The analyses of 18O in the muscle and
red meat product samples were also performed using an IRMS
(Delta V PLUS, Thermo Fisher Scientific, Tokyo, Japan), as described
previously [2,39]. CERKU-1, -2, and -5, certified by the Kyoto
University and Institute of Biogeosciences, Japan [49], were used as
the working standards for δ13C and δ15N. NBS127, and benzoic acids
(A and B) certified by Indiana University (IN, USA), were used as
the working standards for δ18O. The replicate errors of the working
standards for δ13C, δ15N, and δ18O were within 0.2%, 0.3%, and 0.4%,
respectively, and the R2 values of their calibration curves were greater
than 0.99.
Isotope ratios are in the standard delta (δ) notation relative to the
internal standard of Vienna Pee Dee Belemnite (δ13C), atmospheric
nitrogen (δ15N), and the standard mean ocean water (δ18O) using the
following equation:
δ (‰) = [(Rsample/Rstandard) -1] × 1000
The total Hg concentrations in the muscle, liver and red meat
product samples were quantified using a flameless atomic absorption
spectrophotometer (HG-310; Hiranuma Sangyo Co. Ltd., Ibaraki,
Japan). As reported previously [1], approximately 0.5 g of sample
was digested in a mixture of HNO3, H2SO4, and HClO4. DOLT-2
(National Research Council of Canada) was used as the analytical
quality control for Hg, and the recovery of Hg was 94 ± 3% (n = 5).
The Hg concentrations in the muscle, liver and red meat product
samples were expressed on a wet weight basis, and the determination
limit of Hg was approximately 0.01 μg/wet g.
Statistical analyses:
The values of δ13C, δ15N, δ18O, and Hg concentration presented
for each sample are the means of at least two measurements. Data
are shown as mean ± S.D, and the level of significance chosen was
p< 0.05.We investigated whether the relationship between BL and
isotope data (δ13C, δ15N, and δ18Ο values) or Hg concentration could be
fitted to a linear, Quadratic, or exponential function using JMP (SAS
Institute Japan Ltd., version 14.3, Tokyo, Japan). The 95% confidence
ellipses were also calculated using JMP. Significant difference among
multi groups was tested using Tukey-Kramer’s method.
Results
δ13C, δ15N, and δ18O values and mercury concentration in humpback whale:
Table 1 shows the 13C, δ15N, and δ18O values in muscle samples
and the Hg concentrations in muscle and liver samples of humpback
whales. The average δ13C, δ15N, and δ18O values for all humpback
whales (sample ID; A-
I, n = 9) were -19.1 ± 0.6‰, 11.2 ± 0.7‰, and
12.7 ± 1.0‰, respectively, and those for only humpback calves (C-
I, n
= 7) were -19.0 ± 0.6 ‰, 11.4 ± 0.7 ‰, and 13.0 ± 0.8 ‰, respectively.
The average Hg concentrations in muscle and liver samples were 0.06
± 0.04μg/wet g (n = 9) and 0.03 ± 0.01 μg/wet g (n =6), respectively.Figure 2a,b,c and d show the relationships between the BL
and the isotopic or Hg data of humpback whales. The δ13C values for
calves (n = 7) increased linearly with increases in BL (F5 = 16.03, R2 =
0.7622, p = 0.0103, Figure 2a). The δ13C values for the two immature whales of 10.0m BL were -19.3‰ and -19.9‰, respectively, lower
than that of the largest calf of 8.7 m BL (-18.0‰).
The δ15N values for calves peaked in animals with ~8 m BL, as
the highest and the next highest δ15N values were found in the calves
with BL of 7.9 m (12.3‰) and 8.3 m (12.1‰). The δ15N values for
humpback whale calves were fitted to a quadratic equation, although
this was not significant (F5= 4.055, R2 = 0.6697, p = 0.109, Figure 2b);
the peak δ15N value calculated from this equation was 11.8‰ at 8.0
m BL. The lowest δ15N value (10.5‰) was found in the smallest calf
(7.0 m BL) and the weaned immature animal (10.0 m BL). Among all
humpback whales, the difference of δ15N value between the maximum
(12.3‰) and minimum (10.5‰) was 1.8‰, and the difference of δ13C
value between maximum and minimum was 1.9‰ (Table 1).
For all humpback whales, δ18O values decreased linearly with
increases in BL (F7 = 7.344, R2 = 0.5120, p = 0.0302, Figure 2c): The
δ18O values in the seven calves did not fit to a quadratic function (F5=
1.909, R2 = 0.4880, p = 0.262) although the two highest δ18O values
were found in the middle-sized calves (14.2 ‰ at 8.2 m BL and 13.6 ‰
at 7.4 m BL). There was no correlation between δ18O and δ15N values
of calves (n = 7, p = 0.865) and all humpback (n = 9, p = 0.386).
Among all humpback whales, the difference of δ18O values between
the maximum (14.2‰) and minimum (11.5‰) of all humpback
whales was 2.7‰ (Table 1)
Trace amounts of Hg were found in the muscle samples of
humpback calves (0.02-0.05 μg/wet g), whereas the Hg concentrations
of two immature humpback whales were slightly greater than 0.10 μg/
wet g (Table 1) (Figure 2). The Hg concentrations increased linearly
as BL increased in all humpback whales (F7= 13.46, R2 = 0.6787, p =
0.0105, Figure 2d). The Hg concentrations in humpback whale liver
samples (0.03 ± 0.01 μg/wet g, n = 6) were similar to or slightly lower
than those in humpback whale muscle samples (0.06 ± 0.04 μg/wet g,
n = 9) (Table 1).
δ13C, δ15N, and δ18O values and Hg concentration in fin and North Pacific right whale muscles:
As BL increased, the δ13C values of fin whale calves (Table 1,
n=3) tended to increase (-20.5, -19.2, and -19.0‰), whereas the δ18O
values tended to decrease (12.6, 12.3, and 12.1‰). In contrast, the
δ15N values of calves did not change with BL (10.6, 11.1, and 10.9‰).
Trace Hg was found in the smallest calf (0.01 μg/wet g), but the Hg
concentrations in the larger calves were 0.08 and 0.09 μg/wet g.We only obtained two samples of North Pacific right whale calves
(Table 1). The δ13C, δ15N, and δ18O values of the large calf (weaning
calf) were lower than those of the small calf (newborn), respectively
(Table 1). The δ13C values of right whale calves (-21.8 and -23.0‰)
were markedly lower than those of humpback and fin whale calves.
Similarly, the δ15N values of right whale calves (10.3 and 9.4‰) were
lower than those of humpback and fin whale calves, whereas the δ18O
values of right whale calves (14.5 and 13.2‰) were higher than those
of fin whale calves. Trace level of Hg was found in the two right whale
calves (0.03 μg/wet g).
Isotopic discrimination of calves from sex cetacean species stranded in Hokkaido using δ13C and δ15N values:
We investigated whether calves from humpback, fin, and North Pacific right whale (mysticetes, Table 1), and previously reported
calves from common minke whale (mysticete, [1]), killer whale
(odontocete, [39]) and Dall’s porpoise (odontocete, [36]) stranded in
Hokkaido, couldbe discriminated using their δ13C, and δ15N values.
The respective δ13C, and δ15N values previously reported were -19.2 ±
0.5‰ and 12.6 ± 0.8‰(n = 12) in common minke whale calves [1],
-16.8 ± 0.10‰, and 18.1± 0.06 ‰ (n = 3) in killer whale calves [39],
and-19.5 ± 0.62‰ and 14.6 ± 0.21‰ (n=7) in Dall’s porpoise calves
[36]. The δ15N values of calves were in the following order (F5,30=
74.51, p< 0.01): killer whale >Dall’s porpoise >common minke whale
= humpback whale = fin whale ≥North Pacific right whale. On the
other hand, the order of δ13C values was in the following order (F5,30 =
23.65, p< 0.01): killer whale >Dall’s porpoise = common minke whale
= humpback whale = fin whale ≥North Pacific right whale (Figure 3).
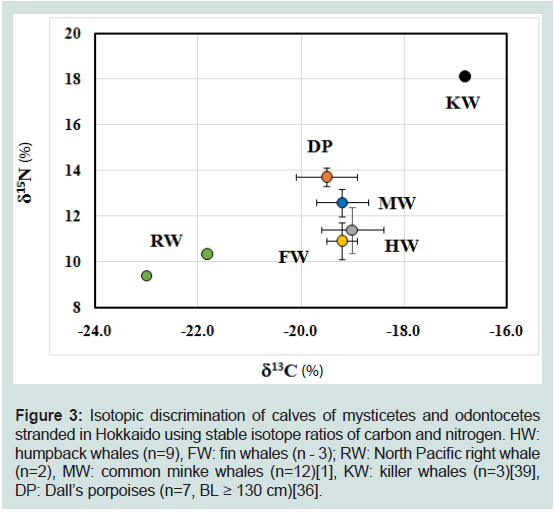
Figure 3: Isotopic discrimination of calves of mysticetes and odontocetes
stranded in Hokkaido using stable isotope ratios of carbon and nitrogen. HW:
humpback whales (n=9), FW: fin whales (n - 3); RW: North Pacific right whale
(n=2), MW: common minke whales (n=12)[1], KW: killer whales (n=3)[39],
DP: Dall’s porpoises (n=7, BL ≥ 130 cm)[36].
Figure 3 depicts the dual-isotope plot of calves from six species.
The δ13C and δ15N values of killer whale calves were the highest among
six species (p< 0.05), whereas those values of North Pacific right
whale calves were the lowest. The dual-isotope plot could apparently
discriminated killer whale calves and North Pacific right whale calves
from other whale calves. The δ15N value of Dall’s porpoise calves
was the next highest, and slightly but significantly higher than that
of common minke, humpback, and fin whale calves (p< 0.05) and
markedly higher than North Pacific right whale calves (n =2), whereas
the δ13C value was intermediate between killer and North Pacific right
whale calves, and similar to that of common minke, humpback and
fin whale calves.
Comparison of δ13C, δ15N, and δ18O values and Hg concentration in fin whale from the North Pacific, North Atlantic, and Antarctic Oceans:
We compared the δ13C, δ15N, and δ18O values and the Hg
concentration in the muscle samples off in whale calves stranded in
Hokkaido (North Pacific Ocean, Table 1) with those in the red meat
products of fin whales hunted from the North Atlantic and Antarctic
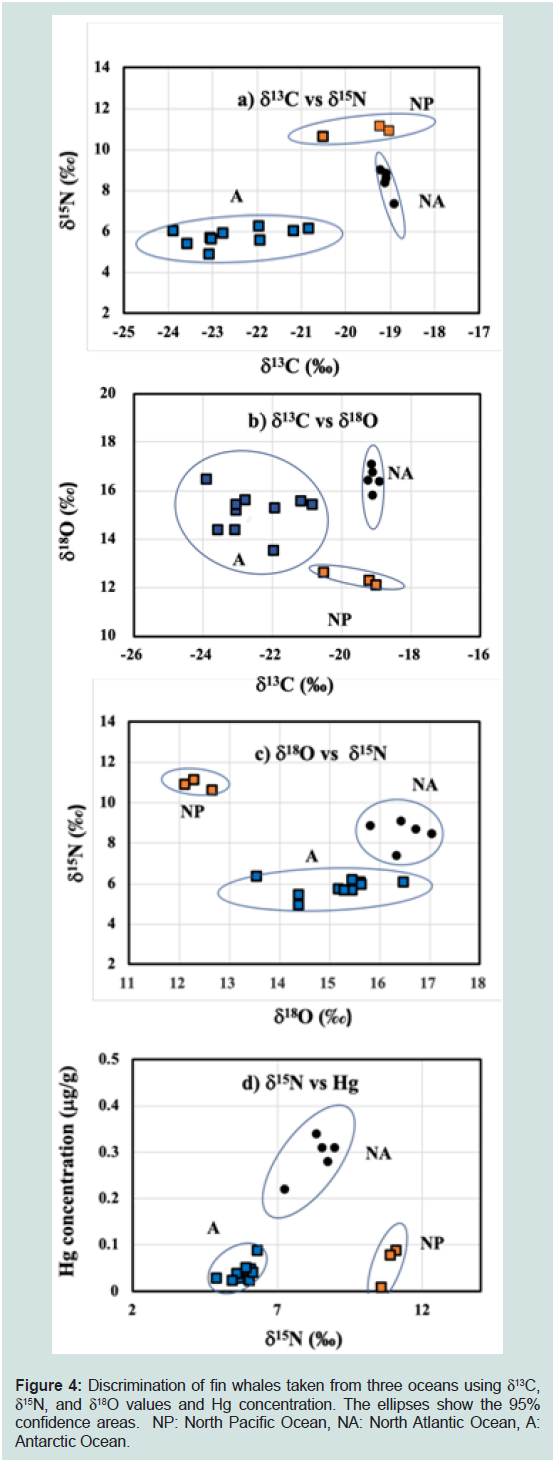
Oceans (Table 2). Figures 4a, b, c, and d show biplots of δ13C vs. δ15N,
δ13C vs. δ18O, δ18O vs. δ15N, δ15N vs. Hg, respectively. As the red meat products of fin whale hunted from the North Atlantic and the Antarctic
Oceans were purchased from retail outlets, their BL were unknown.
In contrast, all fin whales stranded in the North Pacific Ocean were
calves.The δ13C and δ15N values, and Hg concentration in fin whales from
the North Atlantic Ocean were higher than those from the Antarctic
Ocean, respectively, whereas the δ18O value was lower (Table 2). All
four biplots (Figure 4a,b,c and d) clearly discriminated fin whales
from the North Pacific, North Atlantic, and Antarctic Oceans. The
δ13C and δ18O values of fin whales from the Antarctic Ocean varied
more than those from the North Pacific and North Atlantic Oceans
(Figure 4b), whereas the variation in δ13C value was small in fin
whales from the North Atlantic Ocean (Figure 4a).
Discussion
Changes in δ15N, δ13C, and δ18O values and Hg concentration in calves of humpback whale by lactation:
We found a small δ15N-enriched peak in the muscle sample of
humpback whale calf at ~8 m BL, which may be due to the nursing
and weaning of δ15N-enriched milk (Figure 2b). Endo et al. previously
reported a similar δ15N-enriched peak in the muscle samples of
common minke whale calves [1], which fitted to a quadratic function
(p< 0.05); the increase in δ15N value before the peak may represent
nursing, whereas the decrease in this value after the peak likely
represents weaning. However, in this study, the curve fitting of δ15N
peak in humpback whale calves (Figure 2b) was not statistically
significant (p = 0.109) because of the small sample size (n = 7) and
biased sample (no newborns, few nursing animals). It is also expected
that the weaning period of humpback whale may be short compared
with the nursing period. Further investigation with a larger sample
size is needed to confirm the δ15N-enriched peak of humpback whale
calves.In all humpback whales (seven calves and two weaned immature
animals), δ18O values decreased with growth (p = 0.0301; Figure
2c), and the possibility of a δ18O-enriched peak, due to nursing and
weaning of calves, was not statistically supported (n = 7, p = 0.262).
In addition, there was no correlation between δ18O and δ15N values
of humpback whale calves (n = 7, p = 0.865). The δ18O values likely
decreased by weaning because the 18O concentration in milk is higher
than that in solid foods [18,19,50]. To the best of our knowledge, this
is the first study showing a decrease in δ18O values in the muscle of
weaning and immature whales. In this study, the difference between
the maximum and minimum values of δ15N and that of δ18O were
1.8‰ and 2.7‰, respectively (Table 1). These differences are
consistent with the small increases in δ15N value [3,4,17] and δ18O
value [20] due to lactation.
In contrast, δ13C values of humpback whale calves increased
with increases in BL; however, the δ13C values of weaned immature
humpback whale (-19.3 and -19.9‰) were markedly lower than
that of the largest calf (8.7 m BL; -18.0‰; Figure 2a). Thus, the δ13Cenriched
peak could be assumed between the largest calf of 8.7 m BL
and the weaned immature animals of 10 m BL, and this putative peak
may be associated with the feeding shift from milk to solid foods at
least in part. Endo et al. [1] previously reported no particular change
in the δ13C values in muscle samples of common minke whale because of the variability in δ13C values in calves and the decreasing trend of
δ13C values in mature animals. Thus, the change in δ13C values in the
small number of humpback whales (Figure 2a) does not contradict
with that in common minke whales previousy reported [1]. Although
δ13C values may be highly variable, δ13C-enriched peaks are visible
in the ontogenetic changes of vibrissae and skull bones in South
American fur seals (Arctocephalus australis) [15], and a decreasing
trend of δ13C values is observed in the bone from weaning Northern
fur seals (Callorhinusursinus) [14]. The fat (lipids) concentration in
humpback whale milk may increase during mid-lactation [21], but
change in δ13C level in cetacean milk during lactation has not yet been
reported. Changes in δ13C level as well as δ15N and δ18O levels and
the fat concentration in humpback whale milk during lactation are
needed to investigate.
The different patterns of δ13C, δ15N, and δ18O values found in
humpback whale calves (Figure 2a,b and c) could be ascribed to
their different origins: the δ15N-enriched peak could be derived
from the nursing and weaning of δ15N-enriched proteins in milk
[4,17], whereas the high δ18O values in calves could be derived from
δ18O-enriched water, proteins, and other milk components [18,20]. Furthermore, the increase in the δ13C values of calves and its
putative peak may be derived from the feeding shift from milk to
solid foods at least in part. In addition to their different origins, the
different turnover rates of components such as protein, water, and
lipids may explain the different patterns of δ15N, δ18O, and δ13C values
in calf muscle samples (Figure 2a,b and c). This study quantified
the δ13C, δ15N, and δ18O values in the decomposed muscle samples
obtained from stranded whales. Payo-Paya et al. reported that the
decomposition of marine mammal muscles does not affect δ13C and
δ15N values quantified [51]; however, this effect has not yet been
investigated for δ18O value and requires consideration.
Most baleen whales migrate seasonally, staying in low-latitude
breading grounds in winter and moving to high-latitude feeding
grounds where food is more abundant in summer. In the western
North Pacific Ocean, female humpback whale gives birth mainly
in November around Okinawa (Ryukyu) and Ogasawara regions,
and believed to migrate with her calf to the Aleutian region (see
Figure 1) [52-54]. However, details of where humpback whale calves
stranded in Hokkaido during June and December were born and
where they were migrating to are unknown. The available migratory
information is the detection of considerable contamination of 134Cs
and 137Cs in humpback whale calf stranded in Hokkaido, July 2011
(sample ID, C; Table 1), shortly after the disruption of Fukushima
Dai-ichi Nuclear Power Plant (FDNPP) in May 2011 (Figure 1) [27].
The contaminations of δ34Cs and 137Cs are direct evidences that this
humpback whale calf migrated through the rapidly and temporarily
contaminated sea area off FDNPP shortly after the disruption. No
information is available about δ13C, δ15N, and δ18O values in muscle
of humpback whales stranded along the coast of the western North
Pacific Ocean around Hokkaido. The δ13C and δ15N values in the
skin of humpback whales inhabiting in waters around Okinawa,
Ogasawara and Philippines (see Figure 1) were reported to be -18.3 ±
0.06‰ and 12.1 ± 0.13‰, respectively [54].
The Hg concentrations in cetacean milk are trace; for instance,
0.003 ± 0.002 μg/wet g for striped dolphins (Stenella coeruleoalba,
[55]) and 0.22 ng/mL for franciscana dolphins (Pontoporia blainvillei, [56]). In agreement, the Hg concentrations in the muscle of humpback
whale calves were trace (0.02-0.05 μg/wet g, n = 7), and those in two
immature humpback whales slightly above 0.10 μg/wet g, reflecting
the feeding shift from milk to solid foods. The Hg concentrations
in the muscle of calves and immature animals of humpback whales
(Table 1) and are compatible levels of those of calves and mature
animals of common minke whales (0.031 ± 0.024 and 0.133 ± 0.035
μg/wet g, respectively [1] , as humpback and common minke whales
are opportunistic fish-eaters [29,31]. No previous studies have
assessed Hg concentrations in humpback whale tissues. To the best
of our knowledge, we are the first to report Hg concentrations in the
muscle and liver of humpback whale calves (Table 1).
As fin and North Pacific right whales stranding along the
Hokkaido coast are rare, the number of samples of fin whales (n =
3) and right whales (n = 2) collected in 2012 and 2018 were limited.
However, the δ13C values off in whale calves tended to increase in BL,
whereas the δ18O values tended to decrease, and the δ13C, δ15N, and
δ18O values in small right whale calf were higher than those in large
right whale calf, respectively (Table 1). These changes in δ13C, δ15N,
and δ18O values found in fin and North Pacific right whale calves are
not inconsistent with those found in humpback whale calves (Figure 2a,b and c).
Isotopic discrimination of calves from sex cetacean species stranded in Hokkaido using δ13C and δ15N values:
To our best knowledge, this is the first study to compare the δ13C
and δ15N values of calves from several mystecetes and odontocetes.
The δ13C and δ15N values of killer whale calves were the highest, which
reflect the fact that mature killer whales occupy the highest position
in the marine food chain [57]. The δ15N value of Dall’s porpoise
calves (odontocete) was the next highest and higher than that of
mystecetes of common minke, humpback, fin and North Pacific
right whale calves, whereas the δ13C value was similar to those whale
calves. The distribution of calves from six species is similar to the
distribution inferred from trophic positions of their mature animals
(Figure 3). Thus, we could indirectly estimate the values of the δ13C
and δ15N values of lactating mothers and mature animals from those
values of their calves. To confirm the universality of small values of
Δ15Ncalves-mothers and Δ15Ncalves-matures (less than 2‰) and Δ13Ccalves-mothers
and Δ13Ccalves-matures (less than 1‰) calculated from killer whales [39],
common minke whale [1], and Dall’s porpoises [36], further analyses
of these values of humpback, fin, North Pacific right whales, etc. are
needed.The δ13C and δ15N values of North Pacific right whale calves
were the lowest although the sample number was limited (n = 2),
which may be associated with the fact that mature right whales are
zooplankton feeders, and their trophic level seems to be lower than
that of opportunistic fish feeders of mysticetes and odontocetes. As
the δ15N, δ13C, and δ18O values of milk and plankton may vary by
season and region, further samples of right whale calf, in addition to
fin and humpback whale calves, are necessary to enable the statistical
discrimination of those species of calves. There are no other reports
on δ13C, δ15N and δ18O values in the muscle sample of North Pacific
right whales. Comparable δ13C and δ15N values have been reported
in the bone of southern right whales (Eubalaena australis) (-20.4 ±
3.1‰ and 9.3 ± 2.3‰, respectively [8].
The δ13C values of humpback, fin, and common minke whale
calves stranded in Hokkaido were of similar ranges (-19.0 ± 0.6‰,
-19.6 ± 0.8‰, and -19.2 ± 0.5‰, respectively), with the δ15N values
of common minke whale calves (12.6 ± 0.6 ‰) being slightly higher
than those of humpback whale calves (11.4 ± 0.7‰) and fin whale
calves (10.9 ± 0.3‰). Similar δ13C levels and little difference of δ15N
values in humpback, fin, and minke whale calve simply similar δ13C
levels in their milks and weaning solid foods. Gavrilchuk et al. [35]
reported similar δ13C levels in the skin of mature baleen whales from
the Northwest Atlantic Ocean (fin whales, -18.6 ± 0.4‰; common
minke whales, -18.6 ± 0.4‰; humpback whales, -18.7 ± 0.4‰), with
humpback whales having the highest δ15N values (14.3 ± 0.6‰),
followed by common minke whales (13.0 ± 1.4‰) and fin whales
(12.2 ± 1.3‰). They suggest the dietary overlap of prey species among
those mysticete species and the consumption of different portions of
shared prey.
Discrimination of fin whale from three oceans using δ13C, δ15N, and δ18O values and Hg concentration:
Fin whales from three oceans were discriminated by the biplots
using the δ13C, δ15N, and δ18O values, and Hg concentration (Figure 4a,b,c and d). As fin whale samples from three oceans are not strictly
comparable because the fin whale samples from North Pacific Ocean
were calves and only three (n = 3) and the BL of fin whales from the
North Atlantic and Antarctic Oceans was unknown. However, the
target of commercial whaling is mature whales prohibition of hunting
for immature and lactating females, and we believe that the δ15N and
δ13C values of fin whale calves are only slightly higher and similar
to those values of mature whales as in the case of Δ15Ncalves-matures
and Δ13Ccalves-matures found common minke whales and other marine
mammals. Thus, the discrimination of fin whales from three oceans,
at least between the North Atlantic and Antarctic Oceans, maybe
possible because of: 1) the low trophic position of fin whales from
the Antarctic Ocean (low δ15N and Hg concentration), 2) lower 13C
concentration in Antarctic Ocean seawater [58], and 3) high δ18O
concentration in Antarctic Ocean seawater owing to geographical
conditions [5,12]. We previously reported the δ13C, δ15N, and δ18O
values and Hg concentration in the red meat products of common
minke whales from the North Pacific Ocean and those of Antarctic
minke whales (Balaenoptera bonaerensis) from the Antarctic Ocean
(zooplankton feeder) [1]. In agreement with the present results, the
δ13C and δ15N values and the Hg concentration in minke whales from
the North Pacific Ocean are apparently higher than those of minke
whales from the Antarctic Ocean, respectively (-18.4 ± 0.7‰vs.-24.6
± 0.4‰, 12.0 ± 1.7‰ vs. 6.2 ± 0.4‰, and 0.091 ± 0.065 μg/wet g vs.
0.027 ± 0.021 μg/wet g), whereas the δ18O value is lower (12.0 ± 1.2‰
vs. 14.6 ± 0.7‰).The variability in the δ13C and δ18O values found in
fin whales from the Antarctic Ocean were larger than those from the
North Pacific and North Atlantic Oceans (Figure 4a,b,c and d), which
may suggest a wide migration range for fin whales in the Antarctic
Ocean [59].The Hg concentration in the smallest fin whale calf stranded in
Hokkaido was trace (0.01 μg/wet g), whereas the Hg concentration
in the weaning calves were 0.08 and 0.09 μg/wet g (Table 1), which
may reflect the consumption of solid foods. However, so far we know,
the Hg concentrations in the mature fin whale in the North Pacific Ocean (opportunistic fish eater) have not yet been reported. The Hg
concentration in the red meat products of fin whales from the Atlantic
Ocean (opportunistic fish eater) has been reported to be 0.150 (0.08-
0.350) μg/wet g [60], which is higher than those in the muscle of
fin whale calves in the present study (Table 1). In contrast, the Hg
concentration in fin whale from the Antarctic Ocean (zooplankton
feeder) was only 0.044 ± 0.019 μg/wet g ()Table 2), reflecting the low
trophic level. The Hg concentrations in newborn and weaning North
Pacific right whales were only 0.03 μg/wet g (Table 2 ). A similar Hg
concentration was reported in the muscle and kidney samples of
southern right whales (zooplankton feeder; 0.04 μg/wet g [61]).
Comparison of δ18O values in the muscle, bone, and tooth samples of marine mammals:
Mammalian calcified tissue, such as enamel, dentine, and bone,
are all mineral/organic compounds; the mineral component in
these tissues is hydroxylapatite (Ca10(PO4)6OH2), often referred to as
bioapatite, whereas the organic component is mostly collagen [62].
Studies of feeding ecology and migration using δ18O values have
preferentially investigated mammal bone and tooth samples and
rarely investigated muscle samples. The δ18O levels in bones and teeth
are markedly higher than those in muscle. More specifically, the 18O
values in the teeth of cetaceans were 22.8-32.6‰ [10], and those in
the bones of right whale and fin whale were 29.5 ± 1.2‰ and 29.8 ±
0.4‰ [8,9], respectively, whereas in this study, the δ18O levels in the
muscle samples were 11.5-14.5‰ and those in the red meat samples
were 13.5-17.1‰ (Table 1 and 2). Furthermore, the δ18O levels in
the red meat products of six baleen whale species were 9.7-16.7‰
[1]. The difference in δ18O levels between muscle sample and tooth
and bone samples may be ascribed to different origins (bioapatite vs.
protein). In contrast, the δ13C and δ15N levels in bone samples [8,9],
which are mostly collagen, are compatible with those in the present
muscle samples and those from previous studies [2,39] (Table 1 and 2 ).Muscle is metabolically more active than bone and tooth. As
such, we believe that muscle samples may be suitable for investigating
the relatively rapid changes in δ13C, δ15N, and δ18O values (e.g. brief
lactation period of mysticetes), whereas bone and tooth samples
maybe suitable for obtaining information on slow changes over a
long period. Williams et al. found small enrichments of the δ15N and
δ18O levels in prehistoric human infants’ bones, and estimated that
breastfeeding had ceased between 3 and 4 years [19].`
Conclusion
A small δ15N-enriched peak, likely related to nursing and weaning,
was found in the muscle of middle-sized humpback whale calf. In
contrast, the patterns of δ13C and δ18O changes were different from
that of δ15N change and no correlation was found among δ15N, δ13C
and δ18O values. The different patterns of δ13C, δ15N, and δ18O changes
may be because of different origins of nutrients and turnover rates.
The Hg concentrations in the muscle of immature humpback
whales were higher than those of calves. These changes in Hg
concentrations as well as δ13C and δ18O values likely reflect the feeding
shift from milk to solid foods.
The δ13C and δ15N values of calf muscles from six species were as follows: killer whale >Dall’s porpoise >common minke whale =
humpback whale = fin whale ≥North Pacific right whale. This order
of δ15N values in calves is similar to the expected order of their mature
animals: The δ13C and δ15N values of lactating mothers and mature
animals seem to be estimated from those of calves.
The δ18O value, combined with the δ13C and δ15N values, and Hg
concentration, discriminated fin whales from the North Pacific, North
Atlantic, and Antarctic Oceans. The δ18O value could be an excellent
proxy to discriminate fin whales inhabiting these three oceans.
Acknowledgment
We would like to thank the Stranding Network Hokkaido (SNH)
for providing samples and information on the stranded cetaceans
investigated in this study.