Journal of Veterinary Science & Medicine
Download PDF
Review Article
Overview on CommonPathological Changes and Diagnostic Methods of Caprine and Ovine Brucellosis
Morka Dandecha Bayu*
- Department of Veterinary Laboratory Technology, AmboUniversity, Ethiopia
*Address for Correspondence: Morka Dandecha Bayu, Department of Veterinary Laboratory Technology, Ambo University, Ambo, Ethiopia, Tel: +251 910309600; E-mail: morkadandech@gmail.com
Citation:Bayu MD. Overview on Common Pathological Changes and Diagnostic Methods of Caprine and Ovine Brucellosis. J Veter Sci Med. 2018;6(2):12.
Copyright: ©2018 Bayu MD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 6, Issue: 2
Submission: 05 September, 2018 | Accepted: 30 October, 2018 | Published: 05 November, 2018
Abstract
Brucellosis is one of the common bacterial zoonosis in the worldwide caused by organisms belong to the genus Brucella. Brucellosis in sheep and goats is an important animal disease which affects many regions where small ruminants are the predominant species of domestic animals. It is widely distributed around the world, which causes great economic losses in farm animals due to abortion, the slaughter of infected animals, birth of weak animals, decrease in milk production, and infertility. It is a disease of sexually matured animals and commonlytransmitted to other animals by direct or indirect contact with infected animals or discharges such as aborted fetuses, placental membranes or fluids. Brucella melitensis (biovars 1,2,3) is the main causative agent of caprine and ovine brucellosis. Sporadic cases caused by B. abortus have been observed, but cases of natural infection are rare in sheep and goats. Clinically, the disease is characterized by abortion, retainedplacenta, orchitis, epididymitis and, rarely, arthritis, with excretion of the organisms in uterine discharges and in milk. Brucella infected animals generally develop granulomatous inflammatory lesions which frequently are found in lymphoid tissues and organs such as reproductive organs, udder, supramammary lymph nodes and sometimes joints and synovial membranes. Diagnosis depends on the isolation of Brucella from abortion material, udder secretions or from tissues removed at postmortem. Rose Bengal plate test, complement fixation test, and ELISA have been used for the serological diagnosis of brucellosis in sheep and goats. Direct proof of Brucella infection requires isolation of bacteria with well-established methods or detection of bacterial genome by application of polymerase chain reaction. Humans become infected indirectly through contact with infected animals or by animal products consumption. Control of brucellosis in animals requires a correct diagnosis, culling of infected animals, and permanent monitoring of brucellosis-free herds.
Keywords
Brucella; Brucellosis; Brucella abortus; Brucella melitensis; Goat; Pathological changes; Diagnosis; Sheep
Abbreviations
c-ELISA: competitive Enzyme Linked Immunoassay; CFT: Complement Fixation Test; ELISA: Enzyme Linked Immunosorbent Assay; FAO: Food and Agriculture Organization; IBC: Institute of Biodiversity Conservation, IHC: Immunohistochemistry, i-ELISA: indirect Enzyme Linked Immunoassay, LMA: Livestock Marketing Authority, MRT: Milk Ring Test, OIE: Office International Des Epizooties, OPS: O-Polysaccharide, PCR: Polymerase Chain Reaction,PFE: Pastoralist Forum Ethiopia, RBPT: Rose Bengal Plate Test, RBT: Rose Bengal Test, RER: Rough Endoplasmic Reticulum, RID: Radial Immuno Diffussion, SAT: Serum Agglutination Test, SLPS: Smooth Lipopolysacharide, WHO: World Health Organization
Introduction
Small-ruminant brucellosis has been occur worldwide and is principally found in Mediterranean countries, the middle East, Africa, India, China, Mexico and parts of Latin America. The prevalence and incidence of brucellosis vary considerably between herds, areas and countries. The disease has been reported to occur in most countries in Africa including the sub-Saharan region [1].
Ethiopia, located in Eastern Africa, is predominantly an agrarian country with over 85% of its population engaged in agricultural activity. The small ruminant population of Ethiopia is estimated to be nearly 23.33 million goats and 23.62 million sheep. These small ruminants and their milk/meat products represent an important export commodity, which significantly contributes to the national economy [2].
Sheep and goats are highly adaptable to broad range of environmental conditions. Moreover, low cost of production, requirement of little land and higher prolificacy made them attractive asset for development. Investment in sheep and goats avoid losses due to high inflation rates that are found in unstable economies ofmany underdeveloped countries like Ethiopia. This is because sheep and goats provide rapid cash turn over. There is also a growing export market for sheep and goats meat in the Middle Eastern Gulf states and some African countries. At optimum off take rates, Ethiopia can export 700,000 sheep and 2 million goats annually, and at the same time supply 1,078,000 sheep and 1,128,000 goats for the domestic market [3]. However, production from small ruminants does not realize its full potential, due to a number of technical and non-technical factors. First among the many factors which limit the economic returns from small ruminants is disease. One of such disease that hampers the productivity of small ruminants is brucellosis [4,5].
Brucellosis in small ruminants is mainly caused by Brucella melitensis and B. ovis. Brucella melitensis (biovars 1,2,3) is the main causative agent of caprine and ovine brucellosis and it is widespread in the country, highly pathogenic for humans causing one of the most serious zoonoses in the world [6,7].
Brucellosis is a zoonotic disease that leads to considerable morbidity. Also, it was characterized by abortion in females and epididymitis and orchitis in males. The economic and public health impact of brucellosis remains of concern in developing countries [8].
Currently ten Brucella species are recognized including the better known six classical species comprised of B. abortus (cattle, biovars 1-6, and 9), B. melitensis (goats, sheep, biovars 1-3), B. suis (pigs,reindeer and hares, biovars 1-5), Brucella ovis (sheep), Brucella canis (dogs) and Brucella neotomae (desert wood rats). More recently, new members to the genus include Brucella ceti and Brucella pinnipedialis (dolphins/porpoises and seals respectively), Brucella microti (voles) and Brucella inopinata (reservoir undetermined) was identified [9].
Brucellosis is commonly transmitted to susceptible animals by direct contact with infected animals or with an environment that has been contaminated with discharges from infected animals. There is a positive association between the number of animals kept in an area and the disease prevalence, which is attributed to increase contact among susceptible and infected animals [10,11].
In Ethiopia some investigators have established the endemicity of small ruminant brucellosis indifferent parts of the country and the available information on brucellosis clearly showed that the disease is endemic and wide spread with significant economic and public health importance. The consequences of brucellosis in small ruminants are infertility, a high mortality rate in lambs and kids and reduced milkproduction [12-14].
Diagnosis of brucellosis is the corner stone for any control and eradication program. It is made possible by direct demonstration of the causal organism using staining, culture, and PCR and indirectly by demonstration of antibodies using serological techniques [12].
The disease is existing as an acute or chronic with a diversity of clinical manifestations. Primary clinical manifestations of brucellosis are related to the reproductive tract. It is characterized by epizootic abortions, chronic endometritis, yellowish and sticky layers on the placenta, infertility, arthritis, orchitis and epididymitis in domestic nimals. Retention of placenta and metritis are common sequels to abortion [15]. Abortion and expulsion of the fetus will be the results of placentitis. Proliferation of Brucella in the uterus induces necrosis and destruction of the fetal and placental membranes resulting in death and then expulsion of the fetus. Brucella endotoxins may also play a role in inducing abortion [15].
Etiology of Caprine and Ovine brucellosis
Brucellosis is caused by bacteria of the genus Brucella. The genus Brucella consists of at least six species, designated on the basis of host preference, antigenic and biochemical characteristics as Brucella melitensis (goats and sheep), Brucella abortus (cattle), Brucella Suis (pigs), Brucella canis (dogs), Brucella ovis (sheep) and Brucella neotomae (wood rats). All members of the genus Brucella are closely related, and some microbiologists have proposed that this genus be reclassified into a single species (B. melitensis), which contains many biovars [12].
Brucellosis in sheep and goats is primarily caused by Brucella melitensis biovars 1,2 or 3 rarely by B. abortus and B. ovis. B. melitensis is the most pathogenic of all the Brucella species and morphologically indistinguishable from B. abortus but can be identified using serological methods [16]. Smooth cultures are usually exhibited and these are more pathogenic for laboratory animals than the rough mutant cultures. B. melitensis was first isolated by Bruce in 1887 from the spleens of soldiers dying of Mediterranean fever on the island of Malta bruce called it Micrococas melitensis. Ram epididymitis is caused by B. ovis which is morphologically similar to the other members of the genus, except that it stains blue with modified Koster’s stain, in contrast to the other Brucella spp. which stain pink. Its cultures exist only in rough colonial phase, do not agglutinate with mono specificantisera for A and M surface antigens, but are agglutinated by antisera for the rough surface antigen [17].
Epidemiology
Geographical distributions
Brucellosis occurs worldwide in domestic and game animals. It creates a serious economic problem for the intensive and extensive animal production systems. Brucellosis has been eradicated from most industrialized countries, such as in Finland, Norway, Sweden Denmark, Netherlands, Belgium, Switzerland, Germany, Australia, Hungary, the former Czechoslovakia, Rumania, Bulgaria and some others (Figure 1) [18,19].
Figure 1: Mode of transmission of brucellosis [18].
In other parts of the world the rates of brucellosis caused by Brucella spp. vary greatly from one country to another, between regions and between herds within a country. Caprine and ovine brucellosis is a disease of economic importance in the Mediterranean region, especially along its northern and eastern shores, Middle East region and other parts of the world such as Africa, Latin America,especially Mexico, Peru and northern Argentina where the incidence is very high and the disease is known to be enzootic [20]. Even highly developed countries like USA and France have so far not been able to eradicate brucellosis completely. In Latin America alone, official estimates put the annual losses from brucellosis at approximately US$ 600 million [15,19].
In Africa, a high incidence (above 30% of herds) was described for a ring of countries situated in the wet and dry savanna areas and tropical rain forest zone of West, Central and East Africa. The occurrence of brucellosis in sub-Saharan countries (either prevalence or incidence) is not well documented and reports submitted to the WHO are largely confined to serological surveys, and mainlyconducted for cattle and less for goats and sheep [21,22]. Referred to a great variation in prevalence in sub-Saharan Africa (ranging from 4.8 to 41%) in pastoral systems.
In Ethiopia, there is paucity of published data on the status of small ruminant brucellosis. Among few reports, from Tigray and tested sera from 2000 sheep and goats in pastoral regions of Ethiopia and documented 1.9% (n = 38) positive using RBPT and 9.7% (n = 193) positive by i-ELISA [23,24]. Tekelye et al. reported prevalence proportions of 1.5% in sheep and 1.3% in goats in the central highlands [7]. Yibeltal recorded prevalence proportions of 15% in sheep and 16.5% in goats in the Afar region and 1.6% in sheep and 1.7% in goats in the Somali region [25]. Another cross-sectional study conducted on 1,568 serum samples from sheep and goats in the pastoral region of Afar revealed 9.4% positive using RBPT and 4.8% positive by CFT [26]. In Jijiga, screened 730 serum samples (430 sheep and 300 goats) and the result revealed 1.64 and 1.51% positivity using RBPT and CFT, respectively [27]. Mengistu examined a total of 3964 small ruminants (2905 sheep and 1059 goats) in Southern Ethiopia and reported an overall seroprevalence of 1.6% in sheep and 3.2% in goats after serial testing using RBPT and CFT [11, 28 tested a total of 2409 sheep in the eastern part of Amhara Region and found out a seroprevalence of 4.89% after serial testing using RBPT and CFT [28-30].
The presence of this disease has been reported in the Southern Nations, Nationalities and Peoples Regional State and pastoral areas of Borana. However, in general, the status of small-ruminant brucellosis in Ethiopia is not well studied. Yibeltal observed that the prevalence of small-ruminant brucellosis was much higher in the Afar region [25], where farmers practice the communal use of grazing land, than in the Somali region, where clan-based flock/herd segregation is common.
Source of infection and mode of transmission
Generally, transmission occurs in the same way in sheep and goats as in cattle, materials excreted from the female genital tract forming the main supply of organisms for transmission to other animals and man. The primary route of dissemination of Brucella is the placenta, fetal fluids and vaginal discharges expelled by infected ewes after abortion or full-term parturition [16]. Very large numbers of organisms are shed at the time of parturition or abortion. In goats, excretion of the organisms from the vagina is prolonged and copious (2 to 3 months generally) and 3 weeks after abortion or full-term parturition in sheep [31].
Sources of infection include aborted fetuses, fetal membranes, vaginal discharges and milk from infected cows [32]. Primary clinical manifestations of brucellosis among livestock are related to the reproductive tract [33]. The modes of infection are direct or indirect. Animals become infected directly by infected aerosols or by uptake of infected material. Another mode of infection is grazing pastures where infected animals mix with brucellosis-free animals or get in touch with contaminated materials. B. ovis is introduced into flocks through introduction of infected rams. Infection spreads from the infected rams to ewes through coitus. The incidence of B. ovis is increase with increasing age of rams. Ewes can carry this organism in the vagina for at least two months and act as mechanical vectors. Some ewes become infected and shed B. ovis in vaginal discharges and milk. B. ovis may be transmitted from ewes to rams during mating. However, this is believed to be only by mechanical means when ewes which harbor the microorganisms, are mated in succession by different rams during the same heat period (Figure 2) [34,35].
Figure 2: Hyperplasia of the epididymal epithelium with severe vacuolation and infiltration of inflammatory cells (left) and Sperm granuloma, with several multinucleated giant macrophages (right) [34].
Similar to B. abortus infection in cattle, B. melitensis can be transmitted from the dams to lambs or kids (vertical transmission). A small proportion of lambs or kids can be infected in uterus, but the majority of infections are probably acquired by consumption of colostrum or milk. Lambs and kids remain fully susceptible when they reach sexual maturity [36].
Clinical Signs
Acute brucellosis
The main clinical manifestations of brucellosis in sheep and goats are reproductive failure, i.e. abortion and birth of weak offspring. This disease is mainly characterized by abortion, with the development of yellowish, sticky layers on the placenta in females. Abortion generally occurs during the last 2 months of pregnancy and is followed by retention of fetal membranes. Goats may show systemic signs such as fever, diarrhoea and weight loss which may be followed by mastitis, lameness and hygroma. In the male, localization in the testis, epididymis and accessory sex organs is common, and bacteria may be shed in the semen. This may result in acute orchitis and epididymitis and later in infertility. The clinical features of B. melitensis infection in sheep and goats vary according to the biovar involved [15,37].
Chronic brucellosis
Animals generally abort once, although reinvasion of the uterus occurs in subsequent pregnancies and Brucella organisms are shed with the membranes and fluids. Non-pregnant animals exposed to small numbers of organisms may develop self-limiting, immunizing infections or they may become latent carriers. Persistent infection of the mammary glands and supramammary lymph nodes is common in goats with constant or intermittent shedding of the organisms in the milk, while the self-limiting nature of the disease in sheep, which is seldom accompanied by prolonged excretion of the bacteria, has been observed. The inflammatory changes in the infected mammary gland reduce milk production [38]. B. melitensis infection causes disease only in adult (sexually mature) females and males. Young animals may be infected but do not show any clinical sign and generally show only a weak and transient serological response.
However, susceptibility increases after sexual maturity and especially with pregnancy. B. ovis can cause epididymitis, orchitis and impaired fertility in rams. The most consistent clinical sign of epididymitis is evidenced by swelling of the tail, more often than the head of the epididymis. Epididymitis may be unilateral or bilateral, acute or chronic [16,39]. In general brucellosis can cause significant loss of productivity through abortion, still birth, low herd fertility and comparatively low milk production [40].
Pathogenesis and Pathological Lesions
Brucellosis primarily affects organs rich in the sugar erythritol (breast, uterus, epididymis, etc.). Erythritol plays an important role in the tropism of Brucella for the pregnant uterus of ruminants (Figures 3 and 4) [41]. The establishment of infection is influenced by the size of the infective dose, virulence of the bacteria, age, sex and reproductive status of the animal. B. abortus and B. melitensis penetrate mucous membranes of the pharynx and alimentary tract and multiply particularly in cells of the mononuclear phagocytic system [20]. After penetration, the organisms are phagocytosed by neutrophils and macrophages which carry them to the regional lymph nodes where they multiply and induce a lymphadenitis which may persist for months. Multiplication of organisms in lymph nodes may be followed by bacteremia which may persist for several months, resolve itself, or be recurrent for at least two years in 5 to 10 per cent of animals. Recurrence occurs particularly during pregnancy. During the bacteremic phase, organisms are carried intracellularly in neutrophils and macrophages, or free in the plasma and localize in various organs, especially the pregnant uterus, udder and supramammary lymph nodes and the spleen, and in males in the testes, and male accessory sex glands leading to a severe granulomatous reaction [42,43].
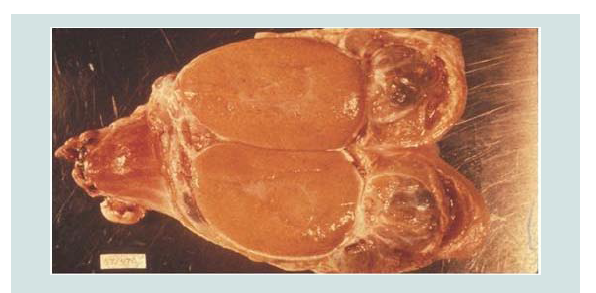
Figure 3: Enlarged epididymis and contains bands of fibrous tissue in Sheep testis (bisected)[41].
Figure 4: A) Enlarged testicle (left) in comparison with the normal (right) in Ram caused by B. ovis and B) Chronic epididymitis with epididymal duct contents (mostly sperm) draining from a spermatic granuloma [41].
Localization of the infection in the endometrium of the pregnant uterus and in fetal membranes appears to be the result of the special affinity of the organism for erythritol present in the placenta and male genital tract of sheep and goats. When invasion of the gravid uterus occurs the initial lesion is in the wall of the uterus but the organism quickly spreads to the lumen, leading to a severe ulcerative endometritis. The allantochorion, fetal fluids and placental cotyledons are invaded next and destruction of the villi, leading to death and expulsion of the fetus. The presence of erythritol in the pregnant uterus results in massive multiplication of Brucella organisms in this organ [41,44].
Invasion of the placenta by Brucella occurs primarily through erythro-phagocytic trophoblasts, with subsequent spread to the cells of the chorioallantoic membrane. Later, Brucella organisms multiply within the rough endoplasmic reticulum (RER) of trophoblasts, causing hypertrophy of the (RER) and subsequent release into the uterine lumen. Placentitis prevents the delivery of nutrients to the fetus and results in fetal stress and death. Vasculitis and other lesions lead to separation of placental trophoblasts and maternal epithelium, resulting in death of the fetus and consequent abortion [45].
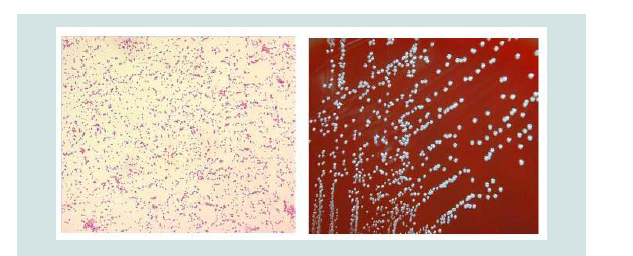
In the ram, an initial bacteremia with a mild systemic reactionis followed by localization of the organism in the epididymis. Theearliest evidence of infection occurring after about two weeks ofinfection is the presence of inflammatory cells in semen. The initialinflammatory reaction destroys the epithelial cells of the epididymis,resulting in the leakage of semen into the interstitial tissues whereit provokes a further inflammatory reaction [34]. Infertility mayresult from total cessation of spermatogenesis or obstruction of theepididymis by granulomas (Figure 5) [46].
Figure 5: Gram-stained Brucella under light microscopy (left) and Brucella spp. Colony Characteristics. The morphology of colonies is pinpoint, smooth, entire translucent and non-haemolytic at 48 h (right)[46].
Gross pathology
Gross lesions in the uterus of infected animals are characterized by brownish fluid with exudates, fibrino necrotic exudates, Erosion, ulceration of the endometrial and multifocal hemorrhages. Aborted fetuses may show increased amounts of bloody fluids in their body cavities ,varying degrees of subcutaneous edema and enlarged spleen and liver. The abomasal content is sometimes turbid, bright yellow and flaky [47].
Infected fetal membranes may be hemorrhagic, edematous and diffuse fibrino necrotic. The lesions in and at the periphery of the cotyledons, as well as those in the intercotyledonary area vary in extent, appearing to be most severe adjacent to cotyledons [47]. The affected cotyledons, or parts of them, lose their blood-red appearance becoming thickened, covered by sticky, odorless, brownish exudates, and are yellowish-grey as a result of necrosis. Parts of the intercotyledonary placenta are thickened, edematous, and yellowgrey and may contain exudates on the surface [34,48].
In male lesions are mainly found in the epididymis, tunica vaginalis and testis. The lesions vary from a slight enlargement of the epididymis to large indurations. Epididymal enlargement can be unilateral or bilateral, and the tail is affected more often than the head or body. The scrotal circumference in these animals may be normal or severely increased. Spermatoceles containing partially inspissated spermatic fluid may be found in the epididymis. The tunica vaginalis is often thickened and fibrous, and can have extensive adhesions. In chronic epididymitis, there is enlargement of the affected parts with reduced mobility of the testes due to fibrous tissue formation [34,41].
Histopathology
Brucella infected animals generally develop granulomatous inflammatory lesions which frequently found in lymphoid tissues and organs such as reproductive organs, udder, supramammary lymph nodes and sometimes joints and synovial membranes. The lesions when present are not pathognomonic. It is characterized by necrotizing placentitis, necrotizing orchitis characterized by multifocal or diffuse necrosis of the testicular parenchyma and epididymitis with subsequent granuloma, necrotizing seminal vesiculitis and prostatitis [41].
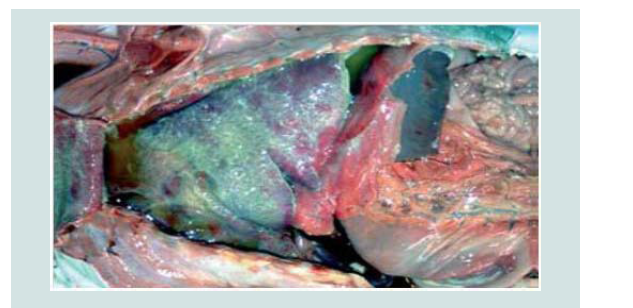
The organism provokes a regional lymphadenitis which is characterized by reticuloendothelial cell and lymphoid hyperplasia, as well as infiltration of large numbers of mononuclear cells and some neutrophils, and few eosinophils and plasma cells. Other lymph nodes in the body and the spleen may be affected later in the course of the infection but to a lesser degree (Figure 6) [49].
Figure 6: B. abortus-infected fetus with an acute diffuse severe fibrinous pleuritis [49].
Microscopically, the uterus shows ulcerative endometritis which is characterized by multifocal desquamation of surface epithelium and its basement membrane. As the disease progresses lesions advance from acute to chronic endometritis. Endometrial stroma is diffusely edematous separating the uterine elements from each other’s. Atrophy of uterine glands. The epithelial lining of some uterine glands are completely destroyed, necrotized and sloughed in the lumen. The blood vessels appeared markedly dilated and congested. Granulomatous endometritis which is characterized by presence of multiple granulomatous structures leading to loss of the architecture of uterus. The granuloma consists of a central area of caseous necrosis associated with massive calcification surrounded with zone of mononuclear inflammatory cells and finally encircled with thick fibrous tissue capsule. The endometrium is infiltrated by lymphocytes and plasma cells and some neutrophils [50,51].
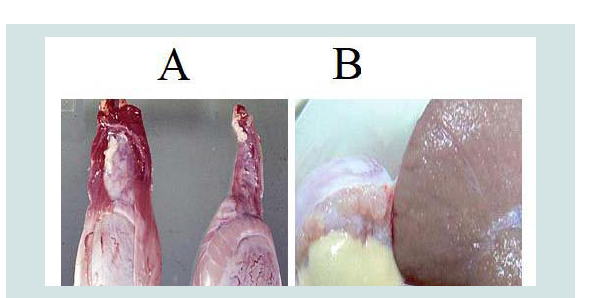
The pathologic changes in the caruncles and cotyledons prevent normal separation and expulsion of the placenta. The placenta of aborted sheep shows necrosis and sloughing of trophoblastic cells of the chorionic villi with neutrophil infiltration and massive calcification in the villous stroma and intervillus space as well as in necrotic area (Figure 7A). In other animals, the placenta revealed necrotic the chorionic plate, in addition large multiple area of dystrophic calcification. The decidual area expressed congestion of the blood vessels with neutrophils in their lumen and necrosis of extratrophoblastic cells and neutrophils infiltration in the necrotic area [20,52].
The liver of aborted fetus shows necrosis of the hepatocytes or microgranulomas, mononuclear cells aggregation in the portal area and vacuolar degeneration of hepatocytes (Figure 7B) [49]. In other section severe fibrin networks deposition in the liver parenchyma as well as aggregation of mononuclear cells scattered through liver parenchyma. The lung of aborted fetus expressed congestion of the blood vessels with neutrophils in their lumen and severe hemorrhage in the alveolar space (Figure 8). In other case, fibrin network in the alveolar space with mononuclear cells aggregation in the interstitial tissues, in the interalveolar septa and in the wall of the bronchi [52].
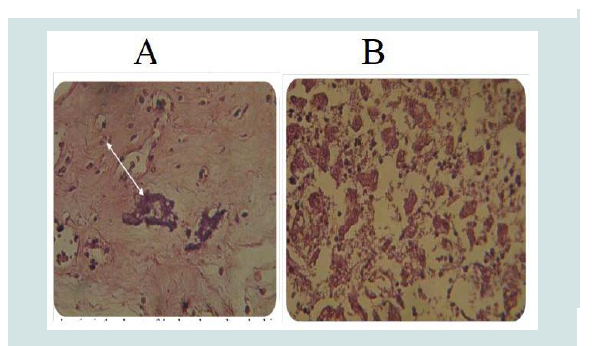
Figure 7: A) Calcium deposition in the Placenta of the aborted ewe with congestion of blood vessels (H&E stain, 400X). B) Degeneration of hepatocytes in the Liver of aborted fetus with inflammatory cells in dilatedsinusoids (H&E stain 400X) [52].
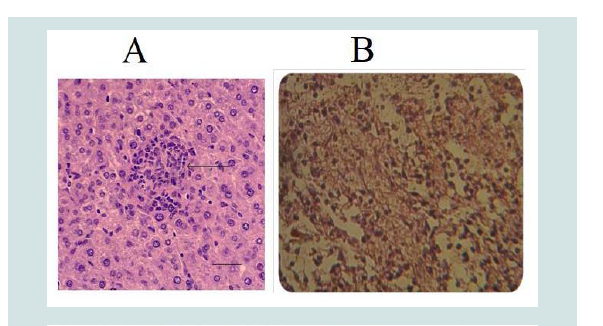
Figure 8: A) CGranuloma in liver containing predominantly macrophages and neutrophil infected by Brucella (arrow) H&E (1) and B) Degeneration of hepatocytes in the Liver of aborted fetus with inflammatory cells in dilated sinusoids (H&E stain 400X) [52].
Diagnosis
Diagnosis of brucellosis is the corner stone for control and eradication program. Unequivocal diagnosis of Brucella infections can be made only by the isolation and identification of etiological agent from the aborted fetus, vaginal discharge or milk [15]. There is no single test by which a bacterium can be identified as Brucella. A combination of growth characteristics, serological, bacteriological and/or molecular methods is usually needed (Figures 9 and 10) [53-55].
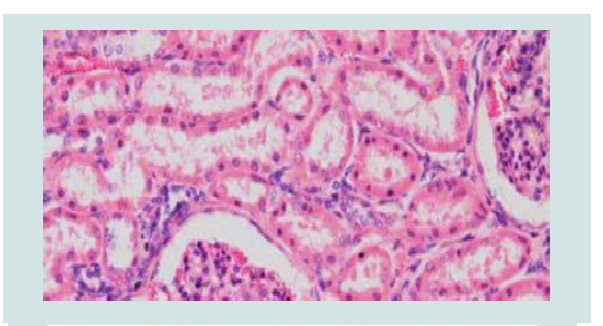
Figure 9: A) Moderate congestion of the glomerulus and mild glomerulonephritis with infiltration of mainly neutrophilic cells. H&E stain, 200X. B) Diffuse and moderately strong immunoperoxidase stain reactions in the epithelial cells of the renal tubules and in the cytoplasm of inflammatorycells infiltrating the glomeruli. H&E stain, 200X [53].
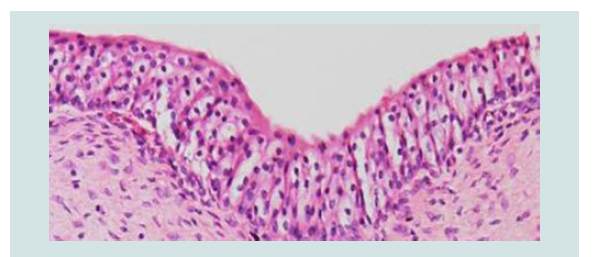
Figure 10: A) Cystitis with macrophages and few lymphocytes in the connective tissue layer of the urinary bladder. H&E stain, 200X. B) Strong golden brown immunoperoxidase staining observed intracellularly in macrophages and in the transitional epithelial cells of the urinary bladder. IP, × 200 [53].
Direct microscopic examination and cultural methods
The presumptive bacteriological diagnosis of B. melitensis can be made by means of the microscopic examination of smears from vaginal swabs, placentas or aborted fetuses are stained with Gram stain and Stamp modified Ziehl-Neelsen method. The morphology of Brucella is fairly constant, except in old cultures where pleomorphic forms may be evident. They are not truly acid-fast, but are resistant to decolorization by weak acids and stains red against a blue background in tissue sections and smears by the Stamp’s modification of the Ziehl–Neelsen’s method. However, morphologically related microorganisms such as Chlamydia psittaci or caxilla burneti can mislead the diagnosis. Accordingly, the isolation of Brucella on appropriate culture media is recommended for an accurate diagnosis [56].
Brucella melitensis does not require serum or CO2 for growth and can be isolated on ordinary solid media under aerobic conditions at 37 °C. Use of nonselective media cannot be recommended because of the overgrowing contaminants usually present in field samples, and selective media are needed for isolation purposes. The use of selective media such as Farrell’s media and modified Thayer-Martin’s may substantially enhance the chances of isolation by inhibiting the growth of contaminants, although the growth of Brucella may be markedly slower. For this reason, the cultures should be incubated for five days or longer before being discarded as negative [46]. After 3-5 days incubation on selective serum agar, pinpoint, smooth, glistening, bluish, translucent colonies appear. Smooth colonies in a clear growth medium such as serum-dextrose agar, are convex, entire-edged, have a smooth shiny surface and are pale yellowishbrown when viewed under transmitted light. Smooth forms are often markedly pathogenic whereas the rough variants are usually less pathogenic [46].
Animal inoculation:Guinea pigs are the most sensitive laboratory animals, two guinea pigs are inoculated intramuscular 0.5- 1.0 ml of suspected tissue homogenate and are sacrificed at three and six weeks post inoculation and serum are taken along with spleen and other abnormal tissues for serology and bacteriological examination, respectively [12,54].
Serological diagnosis:Several serological tests have been evaluated for the diagnosis of B. melitensis infection in sheep and goats [57]. Consideration should be given to all factors that impact on the relevance of the test method and test results for a specific diagnostic interpretation or application. Accordingly, RBT as a screening test and the CFT as the confirmatory test are the most widely used tests for the serological diagnosis of brucellosis in sheep and goats [58]. Serological tests may show cross-reactions with other Gram-negative organisms such as Salmonella group, Eschericia coli O: 157, E. coli O: 116, and Pseudomonas maltophilia. However, the most notable cross-reaction is between smooth lipopolysacharide (SLPS) found in Brucella and Yersinia enterocolitica O: 9 making diagnosis difficult due to the sharing of antigenic determinants in the O-polysaccharide (OPS) molecule, which is the basis for most serological tests [47,59].
Rose Bengal Plate Test (RBPT)
The RB test is internationally recommended for the screening of brucellosis in small ruminants [60]. The test detects specific antibodies of the Ig M and IgG types and is more effective in detecting antibodies of the IgG 1type than IgM and IgG 2 types. The antigen stained with Rose Bengal stain, is buffered at a pH of 3.65. At this level of activity non-specific agglutinins are destroyed and IgG, the most abundant antibody in the serum of infected animals, agglutinates strongly. The test does not need special laboratory facilities and it is simple and easy to perform it is used to screen sera for Brucella antibodies. It is highly sensitive for individual diagnosis especially in cattle vaccinated with strain 19 and can be performed in the field [61].
Complement Fixation Test (CFT)
The CFT is the most widely used test for the serological confirmation of brucellosis in cattle, sheep and goats. It is very specific and sensitive and is regarded throughout the world as being the confirmatory test of choice for serological detection of infected animals. CFT detects specific antibodies of the IgM and IgG 1 types and it is more sensitive to the IgG1 type than IgM type. Since antibodies of the IgG 1type usually appear after antibodies of the IgM type control and surveillance for brucellosis is best done with SAT and CFT [62]. The CFT has many drawbacks such as complexity, variability of reagents, anti-complementary activity of sera, difficulty to perform with hemolysed sera, false negative results with the IgG 2 type antibodies and subjectivity of the interpretation of low titers. The CFT is recommended by the OIE as the test prescribed for international trade and is often used as a secondary test for confirmation of RBT positive samples [63].
Enzyme Linked Immunosorbent Assay (ELISA) methods
ELISAs are methods that involve the immobilization of one of the active components on a solid phase. The large majority ELISA use in brucellosis diagnosis are indirect ELISAs (iELISA). IELISAs are those in which the antigen is bound to a solid phase, usually a polystyrene micro titer plate so that antibody, if present in a sample, binds to the immobilized antigen and may be detected by an appropriate anti-globulin-enzyme conjugate which in combination with a chromogenic substrate gives a colored reaction indicative of the presence of antibody in the sample. An antigen coated on a solid phase combine with the patient’s serum containing antibody, the antigen antibody complex will interact with conjugate (enzyme labeled with anti-animal immunoglobulin) then a color change isobserved up on addition of a substrate [47].
All the wells of the microplate are coated with the lipopolysaccharide (LPS) of Brucella. The samples to be tested are diluted and incubated in the wells. Any antibodies specific to Brucella present in the sample will form a LPS-antibody immune-complex and remain bound in the wells. After washing, a Peroxidase conjugated anti-ruminant IgG is added to the wells. This conjugate will bind to the immune-complex and the enzyme substrate is added to the conjugate, forming a blue compound becoming yellow after blocking. The intensity of the color is a function of the rate of antibodies present in the sample to test [64]. However, it is not as specific as the other tests and it cannot distinguish vaccine stimulated antibodies from antibodies produced from a field infection. Therefore, it should be considered more as a screening test than a confirmatory test in the testing of vaccinated herds affected by false-positive results [16,59].
Another method of diagnosis is the competitive ELISA (cELISA). In this test, Brucella antigen is immobilized on the plate as with the indirect ELISA. Following that, the serum under test and a monoclonal antibody directed against an epitope on the antigen are incubated at 37 °C. This anti-brucella monoclonal antibody is conjugated to an enzyme, the presence is detected if it binds to the antigen. This will only occur if there is no antibody in the serum sample which is bound preferentially [56].
Since the reliability of serological tests to detect Brucellosis depends on antibodies that may or may not be present at the time of examination, inevitably some infected animals may elude detection. Because the skin-delayed-type-hypersensitivity (SDTH) test is independent of circulating antibodies it should be added to the serological tests to improve detection of brucellosis. The SDTH test confirms serologic test results, confirms brucellosis in animals with ambivalent serologic test results and detects latent carriers of Brucella. Furthermore, the SDTH test does not sensitize animal for several consecutive SDTH tests. Therefore, the SDTH test should be the test of choice in developing countries, as small ruminant in those countries are usually not tagged so that serological test results could be related to the individual animal [65].
Gel precipitation test
The specificity of the i-ELISA is quite low when testing sera from sheep and goats subcutaneously vaccinated with the live B. melitensis Rev.1 vaccine. Under these conditions, only the GD or the RID tests with native hapten (NH) as antigen are specific enough to discriminate the immune responses of sheep and goats infected with B. melitensis. As demonstrated by the contrasting results of the i-ELISA and the c-ELISA, the superior diagnostic specificity of the latter in vaccinated sheep can be due to the elimination by the competing anti-C monoclonal antibody of the low avidity antibodies, supposed to be dominant in the sera from vaccinated animals. This antibody avidity, rather than epitopic differences in the antigens used, is also likely to account for the high specificity of the NH precipitation tests. The higher specificity of the precipitation tests with NH may result from the higher threshold avidity required in precipitation tests as compared to that of i-ELISAs. This could explain why NH fails to react with sera from vaccinated animals in the former but not in the latter assay, if low avidity antibodies are predominant at a given time after vaccination [56].
Serum Agglutination Test (SAT)
An agglutinin when combined with homologous antigen (agglutinogen) under the properly controlled conditions is capable of causing agglutination. A suspension of Brucella possessing active antigen will agglutinate when exposed to homologous Brucella antibody. This agglutination forms clumps of bacteria which become macroscopically visible. Stained, standardized, smooth suspensions of killed bacteria (Brucella) are agglutinated when mixed with samples containing specific antibodies to Brucella. The SAT is serological test used to detect brucellosis, measures agglutinating antibodies of the Ig M, IgG 1, IgG 2, and IgA types. The SAT can be used to detect acute infections, as antibodies of the IgM type usually appear first after infection and are more reactive in the SAT than antibodies of the IgG 1and IgG 2 types. However, because the SAT may yield both false negative or false positive results it effectively detects brucellosis only on a herd basis [66].
Anti-globulin (coombs) test
The anti-globulin test or coombs’ test was developed to detect antibodies which combine with antigens of Brucella do not give rise to agglutination. The presence of these so-called “incomplete agglutinins” can be detected by using an antibody directed against the IgG fraction of the animal species being tested [62]. The anti-globulin test detects antibodies of the IgG 2 type and is used to confirm SAT results. The Coombs test is particularly important when the SAT is positive and CFT results are negative or inconclusive [31,67].
The direct anti-globulin test (DAT) is used to determine whether red blood cells (RBCs) have been coated with immunoglobulin (IgG antibodies) and/or complement (C3b, C3d, and C4). Red cells coated with complement or IgG antibodies do not agglutinate directly when centrifuged. Anti-globulin is used to detect non-agglutinating red cell antibodies (indirect anti-globulin test, IAT) or sensitized red cells (direct anti-globulin test, DAT). Most non-agglutinating (incomplete) antibodies are IgG, although some antibodies are IgM. These antibodies do not spontaneously cause agglutination due to a strong electronegative charge on the red cell surface that prevents the cells from coming into close proximity. The anti-globulin reagent is able to bridge these negative forces. Current anti-globulin reagent (coombs reagent) preparations contain a “cocktail” of monoclonal antibodies directed against IgG and C3. The direct anti-globulin test is used most commonly to investigate hemolytic transfusion reactions,hemolytic disease of the fetus and newborn (HDFN) [62].
Fluorescence polarization assay
Fluorescence polarization immunoassay (FPA) makes use of molecular rotational properties, considered a homogenous test due to measuring antibody binding to antigen directly. The principle of the method relies on a fluorescent dye attached to a small antigen (or antibody fragment) that is excited by plane-polarized light at the appropriate wavelength. The rate of rotation of the antigen molecule is reduced when its molecular size is increased by its binding to antibody (or antigen) and resulting in a higher polarization value [68]. A small molecule will rotate rapidly while larger molecules rotate more slowly. By attaching a fluorescing molecule to a small molecular weight antigen molecule, the time of rotation through a given angle can be measured using polarized light. For brucellosis serology, a small molecular weight subunit of OPS, labeled with fluorescent isothiocyanate is used as the antigen. If antibody to the OPS is present in diluted serum, milk or whole blood to which the antigen has been added, the rate of rotation of the labeled antigen will be reduced. The FPA is a homogeneous assay, requiring no steps to remove unreacted reagents, and can therefore be performed in minutes, even outside the laboratory, and is very cost effective [64].
Immunocapture test
The immunocapture test is a one-step technique, very easy to perform, able to detect antibodies of medium to high affinity against Brucella, and suitable for simple standardization and automation. The test is based on a blue colored cellular antigen of B. melitensis and on anti-total species immunoglulin coated polystyrene micro titer plates of 96 U wells. Recently, the use of the immunocapture test for the recognition of Brucella melitensis infection in sheep has been evaluated in experimental models of vaccination and infection [69]. The test revealed an optimal sensitivity in the detection of animals with active brucellosis (abortion and excretion) and adequate specificity in Rev.1 immunized animals that were protected against challenge with the standard virulent strain 53H38 of B. melitensis. Moreover, the specificity of the immunocapture test is higher than RBT and CFT either in lambs or in adult animals vaccinated with Rev.1 by conjunctival route [51,70].
Immunohistochemistry: Immunohistochemistry (IHC) technique is a sensitive and specific test that detects Brucella antigen. The organism could be detected in the cytoplasm of inflammatory cells, especially the neutrophils and macrophages of fixed tissues. IHC techniques have been to detect the location of Brucella organisms in formalin-fixed, paraffin-embedded tissues of goat and sheep. Location of Brucella antigens in tissue sections of naturally aborted foetuses will be evaluated by utilising anti-Brucella polyclonal antibody using the avidin-biotin-peroxidase (ABC) methods [71,72].
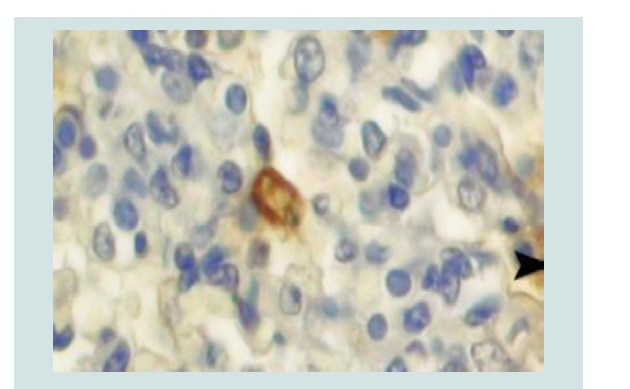
For IHC analysis, sections 4 µm thick will deparaffinised in xylene and hydrated through graded alcohols. Endogenous peroxidase activity will be blocked by incubation with 3% H2O2 in methanol for 15 min. After washing the slides with phosphate-buffered saline (PBS), all sections will be incubated with 5% normal goat serum for 30 min at room temperature in order to block non-specific binding. The slides will then be incubated overnight at 4 °C with rabbit anti-Brucella polyclonal antibody diluted 1:50 in PBS [70,71]. The sections will be incubated for 30 min at room temperature with biotinylated goat anti-rabbit immunoglobulin G diluted 1:200 in PBS. Hematoxylin is used as the counter stain. Control sections were incubated with normal rabbit serum instead of primary antibody. Brucella antigen in macrophages ranged from single to aggregates that filled the cytoplasm (Figure 11) [72-74].
Figure 11: Immunoreactivity to the anti-Brucella polyclonal antibody in several macrophages (arrow head) and cellular debris (arrow) in a foetal lung. ABC method [74].
Molecular diagnosis:Molecular techniques are important tools for diagnosis and identification of species and biotypes of Brucella spp., allowing differentiation between virulent and vaccine strains. Molecular detection of Brucella sp. can be done directly on clinical samples without previous isolation of the organism [50].
Polymerase Chain Reaction (PCR)
The development of PCR has offered a new dimension in the diagnosis of different microorganisms, enabling to perform tests in just few hours. In principle, identification of Brucella at the genus level is sufficient to initiate therapy, however, further differential diagnosis at the species/biovar level is useful for elucidation of epidemiological aspects in order to take appropriate actions [75]. Molecular detection of Brucella DNA can be a sign of acute or chronic brucellosis and can also be detected in asymptomatic subjects with a history of brucellosis [76,77].
The basis for PCR diagnostic applications in microbiology is the detection of infectious agents and the discrimination of non pathogenic from pathogenic strains by virtue of specific genes. The technique allows a small amount of the DNA molecule to be amplified many times, in an exponential manner [75]. The PCR is commonly carried out in a reaction volume of 10-200 ml in small reaction tubes (0.2-0.5 ml volumes) in a thermal cycler. The thermal cycler heats and cools the reaction tubes to achieve the temperatures required at each step of the reaction. Thin-walled reaction tubes permit favourable thermal conductivity to allow for rapid thermal equilibration. To prevent evaporation of the reaction mixture (typically volumes between 15-100 l per tube), a heated lid is placed on top of the reaction tubes or layer of oil is put on the surface of the reaction mixture [76].
PCR is the amplification of a DNA sequence to high copy number. This amplification involves two oligonucleotide primers that hybridize to opposite strands of the target sequence and repeated heating cycles to denature the DNA and subsequent annealing of the primers to their complementary sequences. This is followed by extension of annealing primers with DNA polymerase a heat stable polymerase derived from a bacterium adapted to hot springs. The successive cycling doubles the amount of DNA synthesized to amplification greater than 105, which permits the detection of a small number of bacteria including Brucella. A prerequisite for PCR is that the nucleotide sequence for the DNA of interest is known. The advantage of PCR are simple design, are very robust, highly sensitive, very specific, rapid, and easily detect slow growing bacteria including Brucella [78].
Interferon-Gamma-Test: Tests for the in vitro detection of cell mediated immunity (lymphocyte transformation and proliferation assays) showed a lack of acceptable efficacy in order to be applied for the large scale routine diagnosis of Brucella infection. Phagocytes play a key role in initiating T-cell responses by processing and presenting antigens. IFN-gamma is one of the most important T-cell stimulated cytokines in the course of an infection. It is a potent activator of macrophages and monocytes and up-regulates their metabolic activities to produce oxidative metabolites and other microbicidal molecules. The use of IFN-gamma assay in the diagnosis ovine brucellosis and bovine brucellosis has been explored [79].
The performance of the IFN-gamma test is culturing of whole blood samples from donors are stimulated with specific antigen, and then IFN-gamma released is measured by immunodetection with a sandwich ELISA. In principle, such versatile design could lead to the use of the test for different purposes by using different stimulating antigens (S-LPS, cytosolic proteins) [79].
Treatment
The essential element in the treatment of all forms of brucellosis is the administration of effective antibiotics, and treatment should be implemented at an early stage. Control by treatment is mostly not successful because of the interacellular sequestration of the organisms in the lymph nodes, the mammary glands and reproductive organs. If deemed necessary the treatments often given are sulphadiazine, streptomycin, chlortetracycline and chloramphenicol [15,37].
Control and Prevention of Brucellosis
Prevention and control of brucellosis can be adopted realistically through understanding of local and regional variations in animal husbandry practices, social customs, infrastructures and epidemiological patterns of the disease. The common approaches used to control brucellosis includes, quarantine of imported stock, handling hygienic disposal of aborted fetuses, fetal membrane and discharges with subsequent disinfection of contaminated area and decide for or against immunization of negative animals [80]. Another important control strategy is improving the quality of veterinary services movement control within and outside herds and implementing appropriate diagnostic services. This includes standardization of quality control of diagnostic kits/ reagents andvaccines [20].
Immunization
Control of brucellosis can be achieved by using vaccination to increase the population’s resistance to the disease [73]. Vaccination increases immunity to infection, thus minimizing the risk of abortion and spread of the infection. Currently there are two vaccines available that are effective enough and inexpensive. These are B. melitensis strain Rev.1 for small ruminants given by the conjunctival route, and B. abortus strain 19 for cattle given individually subcutaneously or conjunctively [78]. The live B. melitensis Rev. 1 vaccine is considered the best vaccine available for the prophylaxis of B. melitensis infection in sheep and goats. Both vaccines can be hazardous to humans and also may induce abortion when vaccinating pregnant animals [15,81,82].
Elimination of infected animals by test-and slaughter
Eradication by test and slaughter principle based on the magnitude of disease prevalence and economic status of countries. When the sero-prevalence of brucellosis is reduced to less than 2% animals which are positive to both RBPT and CFT, slaughter of positive reactors are possible. For the implementation of such a program it is essential that the flocks are under strict surveillance and movement control. Animals must be individually identified and well-organized veterinary service for surveillance and laboratory testing must be in place [80]. The flock sizes as well as the prevalence of brucellosis are the most important factors of this strategy which has been shown to be ineffective and unreliable when attempted in large flocks with a high prevalence of brucellosis. The limited reliability of the diagnostic tests used are unable to reveal all infected animals and which may give false negative results due to incubation period, latency or due to criteria used to interpret the results must also be considered [80].
The most rational approach for preventing human brucellosis is the control and elimination of the infection in animal reservoirs [18]. In addition to this, there is a need to educate the Public not to drink raw milk or products made from unpasteurized or untreated milk, Farmer to take care in handling and disposing of aborted fetus, fetal membrane and discharges [80].
Zoonotic importance
Human brucellosis is widely distributed all over the world. It is considered by the FAO, the WHO and the OIE as one of the widest spread zonooses in the world [18]. Almost all human cases of brucellosis are acquired from animals, in particular goats and sheep. In humans, ovine/caprine brucellosis caused by B. melitensis is the most important clinically apparent disease and remains one of the most common zoonotic diseases worldwide, with more than 500,000 human cases reported annually [83,84].
The disease is primarily an occupational risk in exposed professions, i.e. veterinarians, farmers, laboratory technicians, abattoir workers, and others who work with animals and their products. The primary source is the animal and infection is transmitted either by direct or indirect contact through the skin or mucous membranes, inhalation of infectious aerosols with invasion occurring through the mucosa of the upper respiratory tract and ingestion of contaminated products, especially consumption of fresh unpasteurized milk from sheep and goats. Brucella spp. persists for several days in milk (even when it turns sour). It may also persist for weeks in ice cream and months in butter. Meat of animals with brucellosis may also be a source of infection if eaten when insufficiently cooked. The maximum danger is during the lambing or kidding period when infected animals, whether they have aborted or not, may discharge billions of bacteria from the uterus in birth products and the discharges that follow. Abattoir workers handling infected sheep or goats are also at risk, especially from the contents of the uteri and udders [60]. Inhalation is often responsible for a significant percentage of cases in abattoir employees. Contamination of skin wounds may be a problem for persons working in slaughterhouses or meat packing plants or for veterinarians [20,82,85].
Brucellosis is most common in rural areas and it also occurs in urban settings where animals are kept in compounds around houses and among meat packers and veterinarians. Human brucellosis is a disease with non-pathognomonic signs and characterized by acute illness with undulant fever, which may progress to a more chronic form and can also produce a serious complication affecting the musculoskeletal, cardiovascular and central nervous system [86].
Conclusion and Recommendations
Brucellosis in sheep and goats is an important animal diseasewhich affects various countries of the world where small ruminants are the predominant species of domestic animals. B. melitensis infection is responsible for brucellosis in sheep and goats. It causes disease only in adult (sexually mature) females and males. Young animals may be infected but do not show any clinical sign. Brucellosis is primarily affecting organs rich in the sugar erythritol (breast, uterus, epididymis, etc). It is important cause of abortion and infertility in the sheep and goats. Diagnosis of brucellosis is the corner stone for control and eradication program. No specific serological test for B. melitensis infections of small ruminants has been developed, and it is assumed that serological tests used for B. abortus infections in cattle are adequate for the diagnosis of brucellosis in small ruminants. Control of the disease in animals is a prerequisite to reduce its zoonotic spread. The live B. melitensis Rev.1 strain is the best classical vaccine available for prophylaxis of brucellosis in small ruminants t, it induces abortion when vaccinating pregnant animals. For successful eradication, organization of veterinary services, the strict control of animal movements and the provision of adequate conomic compensation to affected farmers are compulsory. Very limited researches have been done on small ruminant brucellosis.
Based on the above conclusion the followings recommendationsare forwarded:
1. Specific serological tests for the diagnosis of B. melitensis should be developed which enable the differentiation between vaccinated and infected sheep and goats. 2. Efforts should be made to develop a new vaccine against brucellosis in sheep and goats based on rough strains which is devoid of the disadvantages of the Rev.1 vaccine. 3. Regular testing of the goats and sheep for brucellosis is important to take measure for prevention, control and in order to protect human health. 4. Cooperation with farmers is essential to succee
References
- Bernard F, Vincent C, Matthieu L, David R, James D (2005) Tuberculosis and brucellosis prevalence survey on dairy cattle in Mbarara milk basin (Uganda). Prev Vet Med 67: 267-281.
- Pastoralist Forum Ethiopia (2003) Background to the Ethiopian livestock industry. In: 3rd National conference on pastoral development in pastoralism and sustainable pastoral development. 23-24 December, Addis Ababa University, Ethiopia, pp. 78-79.
- Alemu Y, Markel RC (2008) Ethiopia sheep and goat productivity improvement program, Addis Ababa University, Ethiopia, pp. 2-6.
- Ademosun AA (1994) Constraints and prospects for small ruminant research and development in Africa. ILCA, Addis Ababa University, Ethiopia, pp. 1-5.
- Livestock marketing authority (2001) Information on Ethiopian livestock resource base and its trade. Addis Ababa University, Ethiopia.
- Al-Talafhah AH, Lafi SQ, Al-Tarazi Y (2003) Epidemiology of Ovine brucellosis in Awassi sheep in northern Jordan. Prev Vet Med 60: 297-306.
- Bekele T, Kasali OB (2014) Brucellosis in sheep and goats in central Ethiopia. Bull Anim Hlth Prod Afr 38: 23-25.
- Golo D, Mulugeta T, Mekonnen A (2013) Small ruminant brucellosis: Serological survey in Yabello District, Ethiopia. Asia J Anim Sci 7: 14-21.
- Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, et al (2011) Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med 102: 118-131.
- Banai M (2002) Control of small ruminant brucellosis by use of Brucella melitensis Rev.1 vaccine: laboratory aspects and field observations. Vet Microbiol 90: 497-519.
- Seleem MN, Boyle SM, Sriranganathan N (2010) Brucellosis: a re-emerging zoonosis. Vet Microbiol 140: 392-398.
- Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, et al. (2002) Brucella species. In: Veterinary microbiology and microbial disease (2ndedn). Willey-Black Well Science. pp. 928.
- Bekele M, Mohammed H, Tefera M, Tolosa T (2011) Small ruminant brucellosis and community perception in Jijiga district, somali regional state, Eastern Ethiopia. Trop Anim Health Prod 43: 893-898.
- Assessment of risk factors and seroprevalence of small ruminant brucellosis in Adamitulu-Jido-Kombolcha district, Oromia regional state, Ethiopia. Res Cen J Intl 9: 1338-1344.
- Radostitis OM, Gay CC, Blood DC, Hinchcliff KW (2000) Bovine mastitis: In: Veterinary medicine a text book of the diseases of cattle, sheep, pigs, goats and horses, (9thedn). W.B. Sounders Company Ltd, London, Uk.
- Oie (2004) Manual of the diagnostic tests and vaccines for terrestial animals, (5thedn). Office International Des Epizooties, Paris, France 1: 409-438.
- Garin-Bastuji B, Blasco JM, Greyon M, Verger JM (1998) Brucella melitensis infection in sheep: present and future. Vet Res 29: 255-274.
- Acha PU, Szyfers B (2001) Zoonosis and communicable disease common to man and animals, (3rdedn). Pan America Health Organization. Washington, DC, USA, pp. 1-395.
- Bamaiyi PH, Hassan L, Khairani-Bejo S, Zainal Abidin M, Ramlan M, et al. (2015) The prevalence and distribution of Brucella melitensis, in goats in Malaysia from 2000 to 2009. Prev Vet Med 119: 232-236.
- Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, et al. (2005) From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res 36: 313-326.
- McDermott JJ, Arimi SM (2002) Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol 90: 111-134.
- Hadush A, Pal M (2013) Brucellosis- an infectious re-emerging bacterialzoonosis of global importance. Int J Livest Res 3: 28-34.
- Teklue T, Tolosa T, Tuli G, Beyene B, Hailu B (2013) Sero-prevalence and risk factors study of brucellosis in small ruminants in Southern Zone of Tigray Region, Northern Ethiopia. Trop Anim Health Prod 45: 1809-1815.
- Teshale S, Muhie Y, Dagne A, Kidanemariam A (2006) Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of Eastern Ethiopia: the impact of husbandry practice. Revue Med Vet 11: 557-563.
- Yibeltal M (2005) A seroprevalence study of small ruminant brucellosis in selected sites of Afar and somali regions, DVM thesis, Faculty of veterinary medicine, Addis Ababa University, Ethiopia..
- Ashenafi F, Teshale S, Ejeta G, Fikru R, Laikemariam Y (2007) Distribution of brucellosis among small ruminants in the pastoral region of Afar, Eastern Ethiopia. Rev Sci Tech 26: 731-739.
- Mohammed H (2009) Seroprevalence of small ruminant brucellosis in and around Jijiga. DVM thesis, School of veterinary medicine, Jimma University, Ethiopia.
- Mengistu M (2007) Seroepidemiology of brucellosis in small ruminants in southern Ethiopia. Addis Ababa University, Faculty of veterinary medicine, Ethiopia.
- Tsegay A, Tuli G, Kassa T, Kebede N (2017) Seroprevalence and risk factorsof brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoir, Central Ethiopia. BMC Infect Dis 17: 101.
- Lemu D, Mamo H, Deressa A, Pal M (2014) A study on seroprevalence of brucellosis in goats and sheep in East Shewa, Ethiopia. Ethio Int J Multidis Res 1: 14-18.
- Nielsen K, Duncan JR (2017) Animal brucellosis. In: Brucella melitensis. Taylor & Francis group. CRC press Boston, USA, pp. 463.
- Adugna KE, Agga GE, Zewde G (2013) Seroepidemiological survey of Bovine brucellosis in cattle under a traditional production system in Western Ethiopia. Rev Sci Tech 32: 765-773.
- Tadesse G (2016) Brucellosis seropositivity in animals and humans in Ethiopia: a meta-analysis. Plos Negl Trop Dis 10: e0005006.
- Coetzer JA, Thomson GR, Tustin RC (1994) Brucella ovis infection. In: Infectious diseases of livestock with special reference to Southern Africa. Oxford University Press, New York, USA, pp. 2: 1065.
- Tuon FF, Gondolfo RB, Cerchiari N (2017) Human-to-human transmission of Brucella- a systematic review. Trop Med Int Health 22: 539-546.
- Grillo MJ, Barberan M, Blasco JM (1997) Transmission of Brucella melitensis from sheep to lambs. Vet Rec 140: 602-605.
- Kose S, Serin Senger S, Akkoclu G, Kuzucu L, Ulu Y, et al (2014) Clinical manifestations, complications, and treatment of brucellosis: evaluation of 72 cases. Turk J Med Sci 44: 220-223.
- Sutra L, Caffin JP, Dubray G (1986) Role of immunoglobulins in the Brucella milk ring test. Vet Microbial 12: 359-366.
- Yang HX, Feng JJ, Zhang QX, Hao RE, Yao SX, et al. (2018) A case report of spontaneous abortion caused by Brucella melitensis biovar 3. Infect Dis Poverty 7: 31.
- Gessese AT, Mulate B, Nazir S, Asmare A (2014) Seroprevalence of brucellosis in camels (Camelus dromedaries) in South East Ethiopia. J Vet Sci Med Diagn 3.
- Xavier MN, Paixao TA, Poester FP, Lage AP, Santos RL (2009) Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and Fetuses experimentally infected with Brucella abortus. J comp Pathol 140: 149-157.
- Sutherland SS, Searson J (1990) The immune response of Brucella abortus: the humoral response, In animal brucellosis (K Nielsen & R Duncan, eds). CRC Press, boca raton, Florida pp.65-81.
- Poester FP, Samartino LE, Santos RL (2013) Pathogenesis and pathobiology of brucellosis in livestock. Rev Sci Tech 32: 105-115.
- Sangari FJ, Aguero J, Garcia-Lobo JM (2000) The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology 146: 487-495..
- Thoen CO, Enright FM, Cheville FN (1993) Brucella. In: pathogenesis of bacterial infections in animals (C.L. Gyles & CO Thoen Eds). Iowa State University pp. 236
- Quinn P, Markey B, Carter M, Carter GR (2013) Clinical Veterinary Microbiology. Mosby International Ltd, Edinburgh pp. 720.
- Nielsen K, Gall D, Smith P, Bermudez R, Moreno F, et al. (2005) Evaluationof serological tests for detection of caprine antibody to Brucella melitensis.Small Rumin Res 56: 253-258.
- Narnaware SD, Dahiya SS, Kumar S, Tuteja FC, Nath K, et al. (2017) Pathological and diagnostic investigations of abortions and neonatal mortality associated with natural infection of Brucella abortus in dromedary camels. Comp Clin Pathol 26: 79-85.
- Bricker BJ, Halling SM (1994) Differentiation of Brucella abortus bv.1,2 and 4, Brucella melitensis,, Brucella ovis, and Brucella Suis biovar bv. 1 by PCR. J Clini Microbial 32: 2660-2666.
- Carvalho CA, Moustacas VS, Xavier MN, Costa EA, Costa LF, et al (2012) Andrological, pathologic, morphometric, and ultrasonographic findings in rams experimentally infected with Brucella ovis. Small Rum Res 102: 213-222.
- Ferrero MC, Hielpos MS, Carvalho NB, Barrionuevo P, Corsetti PP, et al. (2014) Key role of toll-like receptor 2 in the inflammatory response and major histocompatibility complex class ii downregulation in Brucella abortusinfected alveolar macrophages. Infect Immun 82: 626-639.
- Hameed-Abdul, AL-tememy H, AL-jubort KH, Ban A (2013) Pathological and molecular diagnosis of Brucella melitensis in the fetal and placental tissues of aborted ewes in Al-Najaf city. J Vet Medic Sci 4.
- Mazlina M, Khairani-Bejo S, Hazilawati H, Tiagarahan T, Shaqinah NN, et al. (2018) Pathological changes and bacteriological assessments in the urinary tract of pregnant goats experimentally infected with Brucella melitensis. BMC Vet Res 14: 203.
- Walker RL (1999) Brucella. In: Hirsh DC, Zee YC (Eds): Veterinary Microbiology. USA: Blackwell Science Inc. pp. 196-203.
- Smirnova EA, Vasin AV, Sandybaev NT, Klotchenko SA, Klotchenko SA, et al. (2013) Current methods of human and animal brucellosis diagnostics. Adv Infect Dis 3: 177-184.
- Marin CM, Jimenez de Bagues MP, Barberan M, Blasco JM (1996) Comparison of two Selective media for the isolation of Brucella melitensis from naturally infected sheep and goats. Vet Rec 138: 409-411.
- Minas A, Stournara A, Minas M, Papaioannou A, Krikelis V, et al. (2005) Validation of fluorescence polarization assay and comparison with other tests used for diagnosis of B. melitensis infection in sheep. Vet Microbiol 111: 211-221.
- Ramirez-Pfeiffer C, Nielsen K, Marin-Ricalde F, Rodriguez-Padilla C, Gomez- Flores R (2006) Comparison of fluorescence polarization assay with card and complement fixation test for the diagnosis of goat brucellosis in a high prevalence area. Vet Immunol Immunopathol 110: 121-127.
- Nielsen K (2002) Diagnosis of brucellosis by serology. Vet Microbiol 90: 447- 459.
- WHO (2006) Brucellosis in humans and animals. Geneva. pp. 102.
- Reviriego FJ, Moreno MA, Dominguez L (2000) Risk factors for brucellosis Seroprevalence of sheep and goat flocks in Spain. Prev Vet Med 44: 167-173.
- MacMillan AP, Greiser-Wilke I, Moennig V, Mathias LA (1990) A competition enzyme immunoassay for brucellosis diagnosis. Dtsch Tierarztl Wochenschr 97: 83-85.
- Blasco JM, Garin-Bastuji B, Marin CM, Gerbier G, Finlo J (1994) Efficacy of different rose bengal and complement fixation antigens for the diagnosis of Bruella melitensis infection in sheep and goats. J Vet Med 134: 415-420.
- Nielsen K, Gall D, Jolley M, Leishman G, Balsevicius S (2001) A homogeneous fluorescence polarization assay for detection of antibody to Brucella abortus. J Immunol Methods 195: 161-168.
- Bercovich Z (1999) The use of skin delayed-type hypersensitivity as an adjunct test to diagnose brucellosis in cattle: a review. Vet Q 22: 123-130.
- Corbel MJ, MacMillan A (2000) Bovine brucellosis. In: Manual of standards for diagnostic a tests and vaccines, Office International des Epizooties. Paris..
- Hanci H, Igan H, Uyanik MH (2017) Evaluation of a new and rapid serologic test for detecting brucellosis: Brucella Coombs gel test. Pak J Biol Sci 20: 108-112.
- Hajdu S (2003) Efficiency of the fluorescence method in the serological diagnosis of cattle and swine brucellosis. Arch Exp Vet Med 20: 293-306.
- Vanzini VR, Aquirre NP, Valentini BS, Torioni de ES, et al (2001) Comparison of an indirect ELISA with the Brucella milk ring test for the detection of antibodies to Brucella abortus in milk samples. Vet Microbiol 82: 55-60.
- Duran-Ferrer M, Mendoza J, Osuna A, Caporale V, Lucas A, et al. (2000) Evaluation a new immunocapture-test for the diagnosis of ovine brucellosis caused by Brucella melitensis. BMJ J.
- Yazicioglu O, Haziroglu R (1997) Pathological and immunoperoxidase studies of the foetal lesions of ovine brucellosis. Ankara Univ Vet Fak Derg. Terky.
- Emikpe BO, Sabri MY, Ezeasor CK, Tanko PN (2013) Immunohistochemical detection of Brucella mellitensis and Coxiella burnetii antigens in formalinfixed tissues of west African dwarf goats. Arch Clin Microbiol 4: 2.
- Ilhan, F, Yener Z (2008) Immunohistochemical Detection of Brucella melitensis antigens in cases of naturally occurring abortions in sheep. J Vet Diagin Invest 20: 803-806.
- Ramos-Vara JA (2005) Technical aspects of immunohistochemistry. Vet Pathol 42: 405-426.
- Cetinkaya B, Ongor H, Muz A, Ertas HB, Kalender H, et al. (1999) Detection of brucella species DNA in the stomach content of aborted sheep fetuses by PCR. Vet Rec 144: 239-240.
- Leal-Klevezas DS, Lopez-Merino A, Martinez-Soriano JP (1995) Molecular detection of Brucella spp: rapid identification of B. abortus biovar i using PCR. Arch Med Res 26: 263-267.
- Neha, Verma AK, Kumar A, Ahmed I (2017) Comparative efficacy of serological diagnostic methods and evaluation of polymerase chain reaction for diagnosis of bovine brucellosis. Iran J Vet Res 18: 279-281.
- Fekete A, Bantle JA, Halling SM, Sanborn MR (1990) Preliminary development of diagnostic test for Brucella using polymerase chain reaction. J Appl Bacteriol 69: 216-227..
- Weynants V, Godfroid J, Limbourg B, Saegerman C, Letesson JJ (1995) Specific bovine brucellosis diagnosis based on in vitro antigen-specific gamma interferon production. J Clin Microbiol 33: 706-712.
- Advanced veterinary public health lecture note. pp. 1-18.
- Byndloss MX, Tsolis RM (2016) Brucella spp. virulence factors and immunity. Annu Rev Anim Biosci 4: 111-27.
- Li T, Tong Z, Huang M, Tang L, Zhang H, et al. (2017) Brucella melitensis M5-90Δbp26 as a potential live vaccine that allows for the distinction between natural infection and immunization, Can J Microbiol 63: 719-729.
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6: 91-99.
- Garcell HG, Garcia GE, Pueyo PV, Martin IR, Arias AV, et al (2016) Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J Infec Public Health 9: 523-527..
- Johansen MV, Welburn SC, Dorny P, Brattig NW (2017) Control of neglected zoonotic diseases. Acta Trop 165: 1-2.
- Lopes L, Nicolino RR, Haddad JP (2010) Brucellosis-risk factors and prevalence: a review. J Vet Sci 4: 72-84.