Journal of Veterinary Science & Medicine
Download PDF
Research Article
In vitro and In Situ Evaluationof the Efficacy and Efficiency of Products to Clean, Disinfection and Sterilization Process in Veterinary Environmental
Adiela María Cortés Cortés 1, Jhon Henry Galvis García1*and Marco Tulio Jaramillo Salzar2
- 1Research and Development Department, Alkamedica S.A.S. Villamaria, The Republic of Colombia
- 2University of Caldas, GEAAS Group, The Republic of Colombia
*Address for Correspondence: Jhon Henry Galvis García, Research and Development Department, Technical Director, Alkamedica S.A.S. Villamaria, The Republic of Colombia, E-mail: liderproyectos@alkamedica.com
Citation: Cortés AMC, García JHG, Salzar MTJ. In vitro and In Situ Evaluation of the Efficacy and Efficiency of Products to Clean, Disinfection and Sterilization Process in Veterinary Environmental. J Veter Sci Med. 2017;5(2): 5.
Copyright © 2017 Cortés AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 5, Issue: 2
Submission: September 05, 2017 | Accepted: September 28, 2017 | Published: October 04, 2017
Abstract
Pathogen microorganisms resistance presence in surgical instrument and places where process are performed are the most common right now, this kind of topics are very important and robust on humans, for this reason, the study of bacterial resistance is popular, when is compared with livestock areas, the issue is the same, but less study the trouble, this represent a big annual economic loss, as has been reported different livestock environments that the contamination levels can achieve 82% to periods of until 5 years, this indicates a need for research, for that reason the objective of this study was to evaluate the effectiveness and efficiency of products for the cleaning, disinfection and sterilization process in veterinary environments, the target in this research was to develop different methodologies, in order to carry out previous microbiological identifications, bioassays with four products: 1) monoenzymatic detergent (protease) and quaternary ammonium, 2) high-level disinfectant detergent based on potentiated glutaraldehyde (0.17%) and quaternary ammonium, 3) broad-area disinfectant detergent with quaternary ammonium grapefruit fragrance and 4) disinfectant detergent of furnishings based on quaternary ammonium. In addition, cytotoxic, extracellular and In situ activities were determined, from which it was possible to conclude that all evaluated products have bactericidal, fungicidal and virucidal activity, without affecting the cells or tissues where the microorganisms were found.
Keywords
Antiviral; Cytotoxicity; Glutaraldehyde; Microbicide; Protease; Quaternary ammonium
Introduction
The World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO), the World Organization for Animal Health (OIE) and international experts in public health, animal health and the environment bacterial resistance to antibiotics, along with rabies virus and animal influenza, as the three-major emerging global threats [1]. Since the 1970s, bacterial resistance has been followed by the appearance of new or occult diseases characterized by their zoonotic and pandemic character.
This phenomenon takes on greater dimensions in the hospital environment, due to the pressure exerted using antimicrobials, promoting the selection and accumulation of resistance genes among resident bacterial populations [2]. This situation has caused antibiotic resistance to be considered by some countries and international organisms mentioned above, a global challenge for two reasons: a) the ability of bacteria to transmit genetic information and b) the globalization that facilitates the possibility of spreading such resistance in short periods [3].
In hospitals, this resistance has a higher impact on morbidity and mortality rates, because the therapeutic options are limited and increases costs for alternative therapy and hospital stay. Intra hospital infections are estimated to cost US $ 28.4 to 33.8 billion in the United States and 70% of them are caused by resistant microorganisms at a time when infectious diseases are the leading cause of mortality in low-income countries, both in humans and animals [4].
At the end of the 20th century and until the present, the concern for microbial resistance at the veterinary level and the relationship between antibiotics for animal use and the impact on public health began in the developed countries. As a result, the OIE published in 2003 international standards on antimicrobial resistance, where it provides guidance for resistance monitoring and control program setting [5]. In 2008 a study of 31 accredited veterinary teaching hospitals in the United States by the American Veterinary Medical Association (AVMA), reports that 82% of these hospitals had out breaks of nosocomial infection during the previous 5 years [6].
In Germany, an increase in the prevalence of multidrug-resistant Acinetobacter baumannii has been identified in hospitalized animals in the veterinary clinic of the Justus-Liebig University of Giessen and other clinics in the city for a period of 9 years, where records from 2000 to 2008 of the Department of Microbiology of the Faculty of Veterinary Medicine of this University shows that of 137 hospitalized animals of different species, 56 acquired Acinetobacter spp. Infections, identified and differentiated by a combination of genotypic methods and susceptibility to antimicrobials [7].
Sources of infection associated with hospitals include medical staff,the patient’s own flora and inanimate objects. Research conducted by the Veterinary College of Ontario, Canada (CVO) determine the prevalence of contamination of cell phones carried by CVO health science staff with methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP),where 1.6% corresponded to MRSP and 0.8% to MRSA [7].
Agents that cause infectious diseases, or pathogens, are very varied. The most common are viruses, bacteria and protozoa, in addition to parasites that are not microorganisms, such as mites, helminths or worms. In veterinary area, the diagnosis of many of these infectious disea to be identified in time and ensure a correct treatment to guarantee theses is of great importance, since they allow the causal pathogen good health of the animal, which is the reason for the consultation [8,9].
Among the diseases most frequently diagnosed are: Feline Viral Leukemia, Canine Distemper, Canine Parvovirus, Ehrlichia, Anaplasma, Feline Immunodeficiency (FIV or VIF), Brucellosis, Equine Encephalitis, Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae, Aphtovirus and Aujeszky’s Disease, intestinal parasites, ear infections, skin and urine infection. In the present, there is a variety of quick tests to identify the causative agent of the infection, which has made it possible to know the prevalence of many of these diseases, mainly due to the sterilization practices of the veterinary environment [10,11].
Infections from hospital origin are a major problem, which has been documented in the case of human medicine, however, has not been well documented in the case of veterinary medicine. Microorganisms associated with the hospital environment are generally resistant to a significant number of antibiotics and products used for cleaning and disinfection, causing significant costs associated with their treatment, as well as increasing hospitalization periods [12].
Therefore, it is essential to ensure a clean environment, providing the best attention possible to all patients, as well as protection to the staff that performs their tasks. The professionalization of production sectors and the scientific studies that prove their usefulness have led these sectors and administrations themselves to include programs of national scope, often mandatory, for the control and eradication of many infectious diseases. Biosecurity includes a set of measures focused at preventing the entry of pathogens into a farm and to curb or prevent their spread therein and to other neighbors, as well as to minimize the risk (if occur) for employees. Cleaning and disinfection practices are a major part of the programs and are becoming more important every day [12].
According to the foregoing, the present investigation aimed at evaluating the efficacy and efficiency of products for the cleaning, disinfection and high-level disinfection process in veterinary environments, whose main composition is quaternary ammoniums of the last generation, enzymes (protease) and Glutaraldehyde.
Materials and Methods
Study area
This study was carried out in 6 clinics and veterinary reference centers in Manizales city, which for confidentiality reasons, the information corresponding to the stuff or social name of the institutions that supported the research will not be divulged. In each place, the samples (swabs) were taken, for triplicate from areas such as external consultation, hospitalization, surgery, laboratory, nursery, hairdressing clinic and veterinary centers, following the notation and methodology of the Standard Methods for the Examination of Water and Wastewater [13].
Partial microorganism’s identification
The prevalence of microorganisms was determined in the study places, which were responsible for the main clinical manifestations, specifically, in dogs, cats, among others. The identifications were made according to the notation and method of Madigan et al. [14].
In vitro bioassays
Cytotoxic activity: In vitro cytotoxic activity was evaluated with quaternary ammonium-based products, enzymes (Protease) and glutaraldehyde by In vitro bioassays at a concentration of 100,000 cells/ml in RPMI-1640 culture medium, each product P1(Bactericidal monoenzyme detergent, water soluble dose of 20 g), P2(High Level Disinfectant, Potentialized Glutaraldehyde at 0.17%, pH=6), P3(Disinfectant detergent, fifth generation quaternary ammonium 30,50 g/Kg, pH=5,0 in 750 ml) and P4(Surface disinfectant detergent, fifth generation quaternary ammonium, water soluble dose of 5 ml with grapefruit scent), which were prepared according to the manufacturer’s data sheet. The effect was determined by spectrophotometry at 570 nm emission. As viability control, cultured cells were used in the absence of the products [12,15].
Antiviral activity:Evaluation of the antiviral activity was performed in chicken embryo culture tissue supplemented with SFB (10%), by determination of cellular viability by the Neutral Red method using plates of 96 wells [16,17]. In this assay 100 ml of culture medium containing formulations of the products to be evaluated were added. After 90 min of incubation at 37 °C, 10 ml/well of the viral suspensions (ATCC CCL-34 and ATCC VR-2209) were added. The cultures were incubated at 37 °C in 5% CO2 atmosphere for 5 days [12,18].
Extracellular antiviral activity:To determine the direct inactivation of the ATCC CCL-34 and ATCC VR-2209 particles, equal volumes of each of the (independent) strains and double concentrations of the products to be evaluated were mixed and incubated for 30 min at 37 °C At the end of this time, each mixture was added to chicken embryo cell cultures (50 ml / well) in 96-well plates, using 4 replicates per concentration. For 5 days of incubation at 37 °C in 5% CO2 atmosphere, the antiviral activity was determined by the Neutral Red method (EC50). The experiment was performed in triplicate [12,18].
In situ bioassay
Products were applied in each of the areas of veterinary centers and institutions environments. The formulations were made according to the technical data of the products; additionally the protocol of use was made according to the specifications and recommendations of the manufacturer. To evaluate the prevalence (presence/absence) of microorganisms, samples were collected (swab) before and after the application of the products, evaluating cleaning and disinfection by luminometry (System Sure Plus) [19,20].
The swabs were stored according to the notation and methodology of the Standard Methods for the Examination of Water and Wastewater, which were transported to the Applied Microbiology Laboratory of the Caldas University [13]. The samples were incubated on agar and nutrient broth, both formulated with the treatments of interest, for 24 hours at 37 °C. As a control of effectiveness, both agar and nutrient broth were formulated with Ampicillin-Sulbactam and Amphotericin B® and as viability control agar was formulated in the absence of the evaluated products [14,15]. After the incubation period, in the solid medium, the growth of microorganisms was evaluated by means of colonies [14]. In the liquid medium, microbial growth was assessed by turbidity measured by spectrophotometry according to the 0.5 scale of Mc Farland [21].
Results
Preliminary bacterial identification
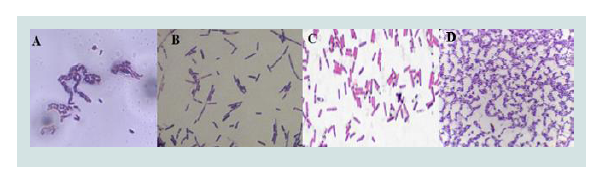
Samples identification was obtained from critical areas (e.g. hospitalization, surgery, and outpatient clinic). Incubation was carried out at 35 °C for 72 hours on nutritive agar. At the end of this time, qualitative identification of these microorganisms was achieved by gram staining. Brown tones, characteristic of microorganisms of the genus Bacillus and Staphylococcus, were observed. Likewise, specific red/violet staining of gram positive bacteria was observed. Additionally, the position of the spore was recognized to identify the probable Bacillus species present in the analyzed samples (Figure 1). All the bacteria presented the spore in central position, of oval or cylindrical form and non-distended sporangium (Table 1).
Figure 1:Gram staining identification of Bacillus. A. Hospitalization, B. Surgery, C. External consult and D. Sacrificing room.
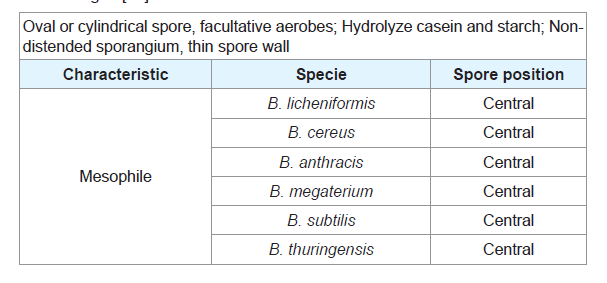
Table 1: Characteristics of representative species of the genus Bacillus taken from Madigan[14].
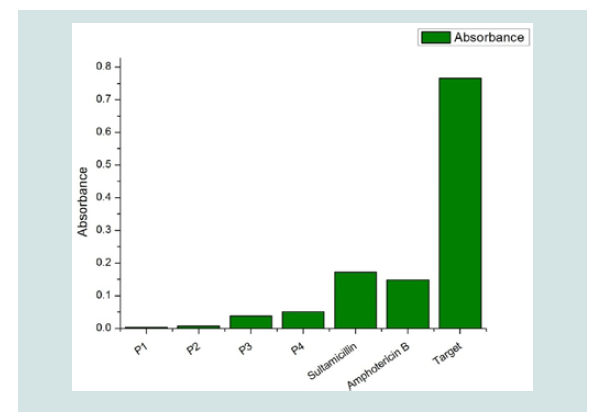
The evaluated products presented antimicrobial potential, as wellas the effectiveness controls (Ampicillin/Sulbactam and Amphotericin B®). Higher inhibition was reported in contact with P1(Bactericidal monoenzyme detergent, water soluble dose of 20 g), P2(High- Level Disinfectant, Potentialized Glutaraldehyde at 0.17%, pH=6), P3(Disinfectant detergent, fifth generation quaternary ammonium 30,50 g/Kg, pH=5,0 in 750 ml) and P4(Surface disinfectant detergent, fifth generation quaternary ammonium, water soluble dose of 5 ml with grape fruit scent). The greater activity of t(Figure 2). Regarding the target, microbial growth is evidenced in the solution lacking the evaluated products and the effectiveness controls.
Figure 2: Antimicrobial activity of the treatments evaluated. P1(Bactericidal monoenzyme detergent, water soluble dose of 20 g), P2(High-Level Disinfectant, Potentialized Glutaraldehyde at 0.17%, pH=6), P3(Disinfectant detergent, fifth generation quaternary ammonium 30,50 g/Kg, pH=5,0 in 750 ml) and P4(Surface disinfectant detergent, fifth generation quaternary ammonium, water soluble dose of 5 ml with grape fruit scent).
Bioassays
In vitro and In situ biological activity:The cultures of the product were performed in non-selective culture media (RPMI 1640, agar and nutrient broth) for in vivo and In situ analyzes. After the incubation time (24 hours and 5 days at 37 °C), bactericidal, fungicidal and antiviral activity of all the evaluated products was evidenced, being the mono-enzymatic product (Bactericidal monoenzyme detergent, water soluble dose of 20 g.), the formulation that presented greater activity, in relation to the time and the evaluated activity, followed by the potentiated glutaraldehyde based product (0.17%), furniture quaternary ammonium and surface disinfectant detergent.
The effectiveness controls used for antibacterial activity (Ampicillin/Sulbactam) and for the antifungal activity (Amphotericin B®), formulated according to the notation and method proposed by the European Committee on Antimicrobial Susceptibility Testing, evidenced the absence of growth of the microorganisms collected by means of the swab, after 10 minutes of the bioassay [22].
In vitro antiviral activity:This experiment showed no growth of the evaluated strains (ATCC CCL-34 and ATCC VR-2209), reporting EC50 (Mean Effective Concentration) from 0.0186 μg/ml to 0.0901 μg/ml of the evaluated products. According to the data of the LC50 (Mean Lethal Concentration), the low toxicity generated on the chicken embryo cells, used for the establishment of the test organisms, was evidenced with respect to the untreated cultures (p<0.05) (Table 2).
The variability in virucidal activity may be due to the concentration variant of the components present in the products. Virulentextracellular action, given by the fact that at low concentrations of the product (0.0186 ± 0.1 mg/ml), indicates inhibition in viral replication. Due to the non-toxicity on the culture cells for the case of the monoenzymatic detergent (water soluble dose of 20 g), it was not possible to calculate the Selectivity Index.
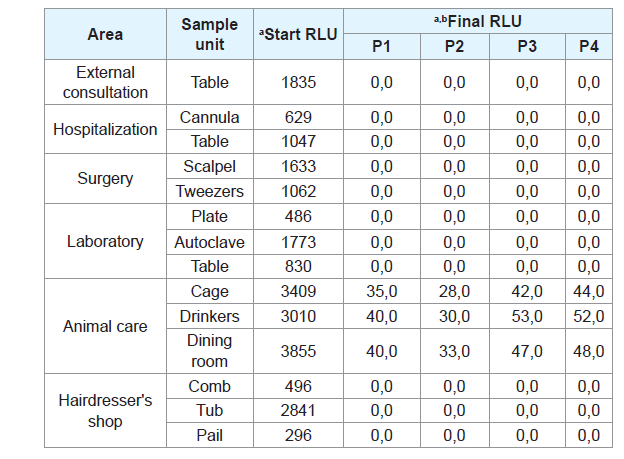
Respect to the cleaning and disinfection process evaluated In situ by luminometry, the elimination of microorganisms in different veterinary environments is evidenced (external consultation, hospitalization, surgery, laboratory, nursery and hairdressing), where the collected swabs of mesons, feeding areas (drinkers and feeders) and surgical instruments, reporting initial values of 3855 RLU (Prior to the use of the products) and 28.0 RLU in the end (after the application of the products, without the minimum contact time) (Table 3).
Table 3: Evaluation of cleaning and disinfection processes of evaluated products in different areas of clinics and veterinary centers environments. a. RLU: Relative Light Units; b. Products evaluated outside the time indicated by the data sheet for the processes of cleaning and disinfection (<1 min of application of the products).
Discussion
The present research demonstrates the In vitro biological activity of products for cleaning, disinfection and sterilization against bacteria, fungi and viruses that cause pathologies in veterinary, where one of the criteria most used to consider the effectiveness of an antiviral product, is to obtain a selectivity index value above 10,18.
The fact that the Selectivity Index (SI) is higher in the virucidal experiment, when the cells are incubated for 1 hour prior to infection, demonstrates the strong virucidal action of each product. These results indicate the ability to reduce the infective title in 2 logarithms (Log2). Other possible mechanisms of inhibition are not eliminated, along with the fact of the quantity of active compounds in the evaluated products in the tests.
According to the data reported by luminometry, the high efficiency and efficacy in the elimination of organic matter and microorganisms in different locations in the study places is evidenced, emphasizing that the products were not evaluated in the required time and indicated by the technical data, however, determined the decrease in the activity evaluated, where, for veterinary environments, the maximum luminometric measurement (3855 RLUs) verified a decrease in organic matter and microorganisms up to 98,76% (48, 0 RLUs), denoting a reduction in organic matter and microorganisms present on the surfaces.
The fact that at 2 minutes at the beginning of the bioassay and 3 days of treatment, the test to which the mono-enzymatic detergent was supplied in 20 g (0.5%) water-soluble dosage (manufacturer’s recommendations), showed both an inhibition of the viruses, as a significant reduction of the replication with respect to the control group, this suggests the presence of a protective effect, before the aroused infection, which supports the antiviral activity obtained.
In future studies, in vivo evaluation is recommended, verifying the route of administration.
Acknowledgment
The authors thank the centers and veterinary clinics for providing spaces, time and confidence for the dissemination of the results, without compromising the terms of confidentiality agreed. Specially acknowledgement to Caldas University.
References
- FAO, OIE, OMS (2011) Communiqué No 11/317. UN Bulletin: Headquarters of the high level technical meeting on zoonotic diseases. Mexico, Cuba,Dominican Republic: United Nations Information Center.
- Sahoo KC, Tamhankar AJ, Johansson E, Lundborg CS (2010) Antibiotic use, resistance development and environmental factors: a qualitative study among healthcare professionals in Orissa, India. BMC Public Health 10: 629.
- Andrews B (2013) Antimicrobial use in animal husbandry and its relationship to resistant bacteria in human health. In: OIE International standards on antimicrobial resistance. Paris.
- Scott RD (2009) The direct medical costs of healthcare-associated Infections in US hospitals and the benefits of prevention. Atlanta, GA: division of healthcare quality promotion national center for preparedness, detection, and control of infectious diseases coordinating center for infectious diseases centers for disease control and prevention.
- International Committee of the OIE (2003) Resolutions. Guidelines for the control of antimicrobial resistance. Paris.
- Benedict KM, Morley PS, Van Metre DC (2008) Characteristics of biosecurity and infection control programs at veterinary teaching hospitals. J Am Vet Med Assoc 233: 767-773.
- Zordan S, Prenger-Berninghoff E, Weiss R, van der Reijden T, van den Broek P, et al. (2011) Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg Infect Dis 17: 1751-1755.
- Castro NA (2008) Retrospective epidemiological study of feline viral leukemia- LVF-of the cases referred to the veterinary clinical laboratory Zoolab in the city of Santiago de Cali between February of 2006 and June of 2008.
- González CM, Villegas CL (2013) Prevalence of gastrointestinal parasites of canines and felines of the city of Cali sent to the veterinary clinical laboratory Zoolab.
- Schudel AA (2002) Infectious diseases of animals. Science journal todayonline. 11: 143-156.
- Beyli ME, Brunori J, Campagna D, Cottura G, Crespo D, et al. (2012) Buenas prácticas pecuarias (BPP) para la producción y comercialización porcina familiar. Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO), Ministerio de Agricultura, Ganadería y Pesca. Instituto Nacional de Tecnología Agropecuaria (INTA), 19-33.
- Haenni M, Ponsin C, Metayer V, Medaille C, Madec JY (2012) Veterinary hospital-acquired infections in pets with a ciprofloxacin-resistant CTX-M-15- producing Klebsiella pneumoniae ST15 clone. J Antimicrob Chemother 67:770-771.
- APHA, AWWA, WEF (2005) Standard Methods for the Examination of Water and Wastewater (21stedn), American Public Health Association, Washington, USA.
- Madigan MT, Martinko JM, Dunlap PV, Clark DP (2003)Brock: biología de los microorganismos. Pearson prentice hall editores. Madrid.
- Sundstrom C, Nilsson K (1976) Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer 17: 565-577.
- Wyde PR, Ambrose MW, Meyerson LR, Gilbert BE (1993) The antiviral activity of SP-303, a natural polyphenolic polymer against respiratory syncytial and parainfluenza type 3 viruses in cotton rats. Antiviral Res 20: 145-154.
- Swierkosz E,Biron K (1995) Antiviral susceptibility testing. En: David A. Lennette, Edwin H, editors. Diagnostic procedures for viral, rickettsial and clamydial infections (7thedn). Am Public Heal Ass pp. 139-154
- Takeuchi H, Baba M, Shigeta S (1991) An application of tetrazolium (MTT) The authors thank the centers and veterinary clinics for providing spaces, time and confidence for the dissemination of the results, without compromising the terms of confidentiality agreed. Specially acknowledgement to Caldas University. Acknowledgment colorimetric assay for the screening of anti-herpes simplex virus compounds. J Virol Methods 33: 61-71.
- Boyce JM, Havill NL, Dumigan DG, Golebiewski M, Balogun O, et al. (2009) Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect Control Hosp Epidemiol 30: 678-684.
- Dvvilla-Ramriez FA, Díaz-Villamil NT, Fajardo-Granados D, Jiménez-Cruz C (2014) Quality of cleaning on surgery room by adenosin triphosfate luminometry. Rev Gerenc Polít Salud 13: 266-273.
- Mc Farland J (1907) The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA XLIX: 1176-1178.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2011) Breakpoint tables for interpretation of MICs and zone diameters. pp. 1-68.