Journal of Veterinary Science & Medicine
Download PDF
Research Article
The Prevalence of Bovine Hydatidosis among Slaughtered Cattle at Debre Berhan Municipal Abattoir, North Shewa Zone, Ethiopia
Dawit Akeberegn1,4, Tewodros Alemneh2,4* andTefera Kassa3
- 1Debre Berhan Town Municipality Office, Meat Inspection and Hygiene, Debre Berhan, Ethiopia
- 2Woreta Town Office of Agriculture and Environmental Protection, Woreta, Ethiopia
- 3Borena Woreda Livestock Association and Development Office, Borena, South Wollo, Ethiopia
- 4University of Gondar, Faculty of Veterinary Medicine, Gondar, Ethiopia
*Address for Correspondence: Tewodros Alemneh, Woreta Town Office of Agriculture and Environmental Protection, University of Gondar, Faculty of Veterinary Medicine, P.O. Box: 196, Gondar, Ethiopia, E-mail: tedyshow@gmail.com
Citation: Akeberegn D, Alemneh T, Kassa T. The Prevalence of Bovine Hydatidosis among Slaughtered Cattle at Debre Berhan Municipal Abattoir, North Shewa Zone, Ethiopia. J Veter Sci Med. 2017;5(1): 5.
Copyright © 2017 Akeberegn D. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 5, Issue: 1
Submission: 31 March 2017 | Accepted: 30 April 2017 | Published: 05 May 2017
Abstract
Background: A cross sectional study was conducted at Debre Berhan Municipalabattoir to determine the occurrence, cyst viability, organ distribution and prevalence of bovine hydatidosis. Routine pre-slaughter examinations and meat inspections were conducted in 384 animals. Visceral organs of 25 slaughtered cattle were detected positive for Hydatidosis with an overall prevalence of 6.51%. Significantly (x2=0.028, p<0.05) higher hydatid cysts prevalence was observed in local breed (9.27%) compared to cross breed cattle (3.35). A prevalence of 14.55% and 4.97% were recorded in female and male cattle, respectively with a significant association (x2=0.011, p<0.05). Regarding to age, ignificantly (x2=0.032, p<0.05) higher prevalence (8.71%) was observed in cattle ≥5 years compared to those < 5 years old (2.80%). Similarly, statistically significant difference (x2=0.013, p<0.05) was found between good (2.86%) and poor (9.57%) body condition score of cattle. The relative prevalence of hydatid cysts in lungs, liver, spleen, heart and kidneys was 4.17%, 1.82%, 0.52%, 0.0% and 0.0%, respectively. The difference was significant (x2=0.001, p<0.05) among organs. Hence, lungs and liver showed the highest rate of organ condemnation in the study area. The present study showed that bovine hydatidosis was revalent in the study area. Due to its impact on animal production and its public health importance, emphasis should be given for the control and prevention of the disease in the study area.
Keywords
Bovine; Debre Berhan; Hydatid cyst; Municipal abattoir; Postmortem inspection; Prevalence
Introduction
Developing countries have nearly two third of the world’s livestock population, but produces less than a third of the world’s meat and a fifth of its milk production. Similarly, Ethiopia has the largest livestock population in Africa, with an estimated 49.3 million heads of cattle, 46.9 million sheep and goats, 7.55 million equines and 2.3 million camels [1]. However, the contribution from these huge livestock resources to the national economy is disproportionately small, owing to several factors such as drought or malnutrition, management problems, poor genetic performance and livestock diseases [2]. Among the many prevalent livestock diseases, parasitism represents a major constraint to livestock development in the tropics in general and hydatidosis is among the major parasitic diseases contributing to low productivity of meat production due to carcass or organ condemnation, in particular [3].
The disease cause decreased livestock production and condemnation of offal containing hydatid cysts in slaughter houses. Despite the large efforts that have been put in to the research and control of hydatidosis, it remains endemic in livestock rearing areas of the world and is inflicting public health problems in the Middle East, Mediterranean, Central and South America, Asia and Africa including Ethiopia [4]. Several reports from different parts of Ethiopia indicated that hydatidosis is highly prevalent and leads to huge economic losses [2].
Hydatidosis and Echinococosis are terms often used interchangeably, to describe the zoonotic infection caused by a cestode of genus Echinococcus with species Echinococcus granulosus (E. granulosus) [5]. Echinococosis has a worldwide distribution; the reason is mainly due to ability of this tape worm to adapt to a wide variety of domestic and wild intermediate hosts [6]. A wide variety of animal species, both domestic and wild, that act as intermediate hosts have made E. granulosus to be widely distributed across theglobe and at least 10 genetically distinct populations exist within the complex E. granulosus [5].
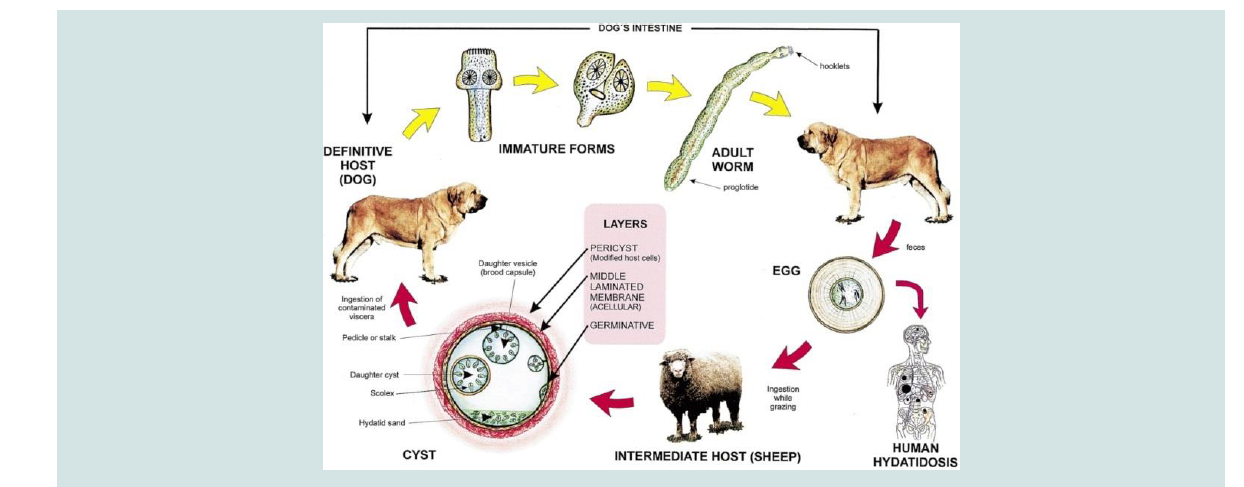
The life cycle consists of the definitive and intermediate hosts. The definitive hosts are carnivores which harbor mature tapeworm in the intestine and excrete the parasite eggs along with their faces and play a major role in the epidemiology of the disease, while livestock and humans are intermediate hosts (Figure 1) [7,8]. The transmission of Echinococcus species from intermediate to definitive host is the result of predator pre- relationship existing between hosts; however, it can be modified by human behavioral factors for synathropic cycles [5] and man is usually a dead end intermediate host (Figure 1) [5,9].
The outcome of infection in humans and animals is the development of hydatid cysts in lung, liver or other organs [9]. In domestic animals disease due to hydatid cyst is rare, but in human being it is more dangerous. The significance of domestic animals as host of this parasite is therefore mainly that they act as the reservoir of the infection for man [10]. As the cysts gradually increase in size, they may impair the health status of the host and causes dyspnea when they occur in the lung or digestive disturbance and possible ascites when the liver is affected [11].
In most Ethiopian abattoirs and slaughter houses, bovine hydatidosis is one of the major causes of organ condemnation and leads to huge economic losses. Human cases of hydatidosis were frequently reported from different parts of the country [12].
Many potential risk factors might influence the prevalence of bovine hydatidosis and play a role in transmission of the disease. The presence of large stray dog population is thought to contribute significantly to the prevalence of the disease. In spite of this, no indepth studies were adopted to investigate the association of these risk factors with the prevalence of this important zoonotic disease. Hence, it is essential to obtain baseline data concerning prevalence of the disease before contemplating any rational control programs.
Therefore, the aim of this study was to determine the prevalence, occurrence and cyst viability and organ distribution of haydatid cysts in cattle, and to assess some of the determinant factors involved in bovine hydatidosis at Debre Berhan Municipal abattoir, Ethiopia.
Materials and Methods
Study area
The study was undertaken in Debre Berhan Town, North Shoa Zone of Amhara Regional State, located at 130 km North of Addis Ababa. The area is found at an altitude of 2780 meters above sea level. The climatic condition of the area is 50% highland, 46% mid altitude, 2% lowland and 2% wurchi. It has an annual rainfall and temperature ranging from 814-1080 mm and 10-200 c, respectively. The rainfall is bi-modal with the short rainy season from February to May and long rainy season from June to September. Agriculture is the main occupation of the population in the area. The agricultural activities are mainly mixed type with cattle rearing and crop production. Extensive livestock management system predominate the area and dogs are commonly used for control and guarding of herds of cattle and flocks of sheep and goats.
Study animals
A total of 384 (62 female and 322 male) cross breed and indigenous zebu cattle were brought from various localities to Debre Berhan Municipal abattoir for slaughter. It was difficult to precisely indicate the geographical origin of all animals slaughtered at the abattoir and relate the findings on hydatidosis to a particular area. Nevertheless, attempts made in this regard revealed that majority of them were brought from nearby markets. The majority (85%) of cattle that were slaughtered in the abattoir were adult male having poor and good body conditions and were below and above than 5 years old.
Study design
A cross sectional study design was conducted to collect data related with the prevalence of hydatidosis in Debre Berhan Municipal abattoir.
Sample collection methods
During postmortem examinations, each visceral organ particularly the liver, lungs, heart, kidneys and spleen were systematically inspected by visual inspection, palpation and incisions for the presence of hydatid cyst and total numbers of hydatid cysts were collected and counted per infected organ [13].
Sample size determination
The sample size was determined based on the formula by Thrusfield for random sampling as follows [14]:

where, Pexp = Expected prevalence; N = Total number of sample size; d = Desired absolute precision.
The reported prevalence of hydatidosis in the study area was 7.2% [15]. N = (1.96)2(0.072 x 0.928)/ (0.05)2 =103. However, to increase the level of accuracy of determining the prevalence, the study sample size had been increased to 384.
Sampling techniques
384 cattle were selected by using random sampling technique and collect organs (liver, lung, heart, kidney and spleen) from the study animals. Regular visits (three days per week) were made to conduct ante and postmortem examinations of slaughtered cattle. During ante mortem examinations, sex, age, breed and body condition of each animal was recorded. The age of cattle was estimated on the basis of the dentation as described by Kelly which comprises two age groups; below and above five years [16]. Body condition scoring was conducted according to Nicholson and Butter Worth and classified in to two groups; poor and good [17].
Data analysis
Data collected during inspection was entered in to MS Excel work sheet. The analysis was conducted using SPSS 16.0. Prevalence of bovine hydatidosis was expressed as percentage with 95% confidence interval (CI) by dividing the total number of animals positive to hydatid cyst to the total number of animals examined.
Significance differences between the prevalence of hydatid cysts were determined using Chi-square test. Age, sex, breed, body conditions and visceral organs were considered as risk factors to see their association with the prevalence.
Results
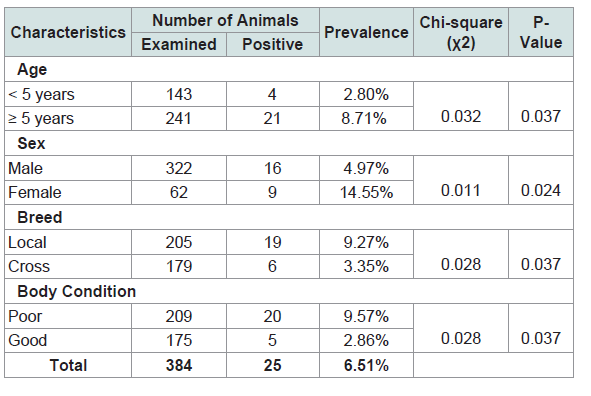
Altogether, 384 cattle were slaughtered and examined during the study period with an overall prevalence of 6.51%. A majority, 242 (21%) were within the age group of > 5 years with a prevalence rate of 8.71%. Three hundred and twenty two (83.9%) were males. Of the 322 male cattle, 16 (4.97%) were observed to be infected with hydatidosis while 9 (14.55%) out of 62 female cattle were observed to be infected with hydatidosis.
Of the 384 cattle examined, 205 (53.4%) were local breed while 6 (1.56%) cross breed of the 205 local breed, 19 (9.27%) were observed to be infected by hydatidosis while 3.35% out of the 6 cross breed were observed to be infected with hydatidosis.
The Chi-square test showed that there was significantly (p=0.037) higher prevalence of hydatidosis detected in cattle within age group ≥ 5 years (8.71%) as compared to those in ˂5 years of age (2.80%)(Table 1 and Figure 2).
Table 1:The prevalence of hydatid cysts on the basis of body condition score, age, sex and breed of cattle.
The difference between sexes in the prevalence of hydatidosis was significant (p=0.024) (Table 1 and Figure 2).
There was a significant association between hydatid cyst infection and breed of cattle (p=0.037) (Table 1 and Figure 2).
An assessment made on the body condition score of cattle revealed that the prevalence of hydatidosis was significantly ( 2=0.013, p˂0.05) higher in poor body condition cattle (9.57%) than those in good condition (2.86%) (Table 1 and Figure 1).
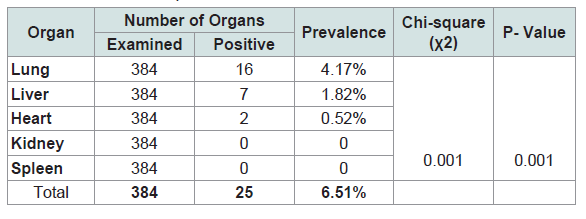
Among the infected organs, the highest prevalence was detected in lungs followed by liver, which counted for 4.17%, and 1.82%, respectively (Table 2 and Figure 3).
Table 2:The prevalence of hydatidosis in various organs of slaughtered Cattle at Debre Berhan Municipal Abattoir.
Discussion
The overall infection rate recorded in this study (6.51%) was comparable with previous studies conducted at the same abattoirs (7.2%) by Tsegaye [15]; however, it was very low as compared to the results reported by Tibebu et al. (37%) [12]. Similarly, the current result was very low compared with the results that had been reported from certain parts of Ethiopia by various authors, such as 16% prevalence of bovine hydatidosis reported at Wolayta Sodo municipality abattoir [18], 15.2% at Birre-Sheleko and Dangila abattoir [2,19], 32.1% in Tigray Region of Ethiopia [20], 46.8% at Hawassa Municipal abattoir [21]. This is due to changes in cultures and believes of the society that denied the presence of large number of dog populations, which are major contributory for the dissemination of the cyst. Similarly, slaughtering of animals at backyard practice might be minimizedin the study area. In Debre Berhan Municipal abattoir, condemned organs and carcasses were also properly disposed and buried. Good fence of the abattoir and immediate response against stray dogs were the major reasons for the decreased in the prevalence of hydatidosis in the current study. Hydatidosis infection rate increases due to the presence of some stray dogs and wild carnivores which are the major contributory for the maintenance of the life cycle of E. granulosus. The wide spread tradition of offering uncooked offal’s to pet animals around home stead, absence of proper treatment for adult E. granulose in dogs, poor public awareness about the disease, absence of proper fencing (when dogs and other carnivores get an easy access), absence of proper meat inspection procedures in the abattoir, the bad habit of disposing dead wild or domestic animals unburied and left open for scavenging carnivores create favorable conditions for the dissemination of the disease.
This study showed significant association between hydatid cyst infection and sex of cattle (p=0.024). The rate of infection within females was 14.55%, and that of males was 4.97%. Both males and females can be infected by hydatidosis, but the prevalence was significantly higher in females. This finding agreed with previous report of Okolugbo et al. [22]. This observation might be due to the fewer number of females slaughtered as compared to males. Again this significant association of hydatidosis to female cattle might be associated with loss of body weight during physiological activities like pregnancy, lactation, parturition and malnutrition, which leads to immunosuppression. These factors were probably pre-condition (exposure) for increasing infestation rate of hydatid cysts in females than in males [23].
In this study, the prevalence of hydatidosis revealed significance association with age and body condition scores of cattle. Animals above five years of age were highly infected. This could be mainly due to their longer exposure to E. granulosus and to lower immunity against the infection. In addition, the reason for lower prevalence in cattle below five years might be early culling of the infected young cattle through slaughtering before they reach to adult and old age. Animals with poor body condition were also found highly infected with hydatidosis. This could be due to the retarded growth, weight loss and moderate to severe infection by other concurrent diseases in such animals. The highest infection rate in cattle with lean bodycondition might probably indicate the effect of high cyst burden.
The breed of cattle and the prevalence of hydatid cyst had been investigated. The infection rate was statistically significant (p <0.05) between breeds with higher prevalence detected in local than cross breeds. This is because local breeds were usually kept in pasture, whereas cross breeds were kept in in-doors.
The lungs and liver were found the most commonly infected organs. The kidneys, heart and spleen were the least affected organs in the study animals. Similar findings were also obtained by various authors and it had indicated that the liver and lungs were the most commonly affected organs with hydatid cysts. This could justified by the fact that lungs and liver possess grater capillary field, which allow these organs to efficiently filter the ingested oncospher from the blood. Liver and lungs undergo sequential from portal veins which is followed by pulmonary filtering action before other organs are invaded only those oncospheres which travels then will reach the systemic circulation and other tissues [24].
In conclusion, hydatidosis is a parasitic disease widely recognized and very important in ruminants; however, surveys of hydatid disease in bovine in the study area have revealed that the disease is endemic.
Conclusions
In general; although the prevalence was low, this study disclosed that bovine hydatidosis was among the major significant diseases needing special considerations to prevent and control in the study area. This high attributed disease in animals with a high economic lose indicated a plan of hydatid cycts control in the study area that involves due attention on Veterinary interventions such as improvement of slaughtering hygiene’s and proper meat inspections. Further studies need to be carried out in dogs and other intermediate hosts including humans to make clear the cycle of transmission thatcould aid to design appropriate controlling measures.
References
- CSA (2008) Federal Democratic Republic of Ethiopia. Report on livestock and livestock characteristics (Private peasant holdings). Statistical Bulletin, pp. 1-183.
- Kebede N, Gebre-Egziabher Z, Tilahun G, Wossene A (2011) Prevalence and financial effects of hydatidosis in cattle slaughtered in Birre-Sheleko and Dangila Abattoirs, Northwestern Ethiopia. Zoonoses Public Health 58: 41-46.
- Eckert J, Gemmell MA, Meslin FX, Pawłowski ZS (2001) World health organization/Office international des Epizootics manual on echinococcus in humans and animals: a public health problem of global concern. Paris, France, pp. 1-126.
- Shambesh MA, Craig PS, Macpherson CN, Rogan MT, Gusbi AM, et al. (1999) An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in northern Libya. Am J Trop Med Hyg 60: 462-468.
- Thompson RC, McManus DP (2002) Aetiology: parasites and life cycles. In: WHO/OIE Manual in echinococcosis in humans and animals. WHO/OIE, Paris, pp. 1-226.
- Torgerson P (2002) Transmission dynamics of taeniid parasites in animal hosts: In: Craig P, Pawlowski Z (Eds), Cestode zoonoses: echinoccosis and cysticercosis: An emergent and global problem. IOS Press Amsterdam, Netherlands, pp: 1-395.
- Khuroo MS (2002) Hydatid disease: current status and recent advances. Ann Saudi Med 22: 56-64.
- Seimenis A (2003) Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta Trop 85: 191-195.
- Zhang W, Li J, McManus DP (2003) Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev 16: 18-36.
- Taylor MA, Coop RL, Wall RL (2007) Veterinary parasitology, (3rdedn). Wiley Blackwell Publishing Ltd, Oxford, UK.
- Eckert J, Delplazes P (2004) Biological, epidemiological and clinical aspects of echinococosis, a zoonosis of increasing concern. Clin Microbiol Rev 17: 107-135.
- Tibebu S, Kinfe M, Tegegne B (2016) The prevalence, cyst viability, organ distribution and economic importance of bovine hydatidosis in cattle slaughtered at Debre Berhan Municipal Abattoir, North Shewa Zone, Amhara Regional State, Ethiopia. Adv Biol Res 10: 315-322.
- Eckert J, Gemmell MA, Matyas Z, Soulsby EJ, World Health Organization FAO/UNEP/WHO (1994) Guidelines for surveillance, prevention and control of Echinococcosis/Hydatidosis, (2ndedn). World Health Organization FOA, Geneva, pp. 147.
- Thrusfield MV (2005) Veterinary Epidemiology, (3rdedn). Wiley. pp. 584.
- Tsegaye T (1995) Epidemiology of bovine fasciolosis and hydatidosis in debre brehan region. DVM Thesis, FVM, Debre Zeit, Ethiopia, pp. 56.
- Kelly W (1975) Age determination by teeth, in veterinary clinical diagnosis, (2ndedn). Bailliere Tindall, London, pp. 12-15.
- Nicholson MJ, Buttrworth MH (1986) A guide condition scoring of Zebu cattle. International livestock Centre for Africa, Ethiopia, pp. 18-39.
- Kebede N, Mekonnen H, Wossene A, Tilahun G (2009) Hydatidosis of slaughtered cattle in Wolaita Sodo Abattoir, southern Ethiopia. Trop Anim Heal Prod 41: 629-633.
- Bekele J, Butako B (2011) Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, Southern Ethiopia. Trop Anim Health Prod 43: 221-228.
- Berhe G, Tadesse G, Kiros H, Abebe N (2010) Concurrent infection of hydatidosis and fasciolosis in cattle slaughtered at mekelle municipal abattoir, Tigray Region. Ethiop Vet J 14: 39-49.
- Getaw A, Beyene D, Ayana D, Megersa B, Abuna F (2010) Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopian. Acta Trop 113: 221-225.
- BeOkolugbo B, Luka S, Ndams I (2014) Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatidosis in camels (Camelus dromedarius) and cattle in sokoto, Northern Nigeria. Int J Infect Dis 13: 1-6.
- Zhang LH, Joshi DD, Mcmanus DP (2000) Three genotype of Echinococcus granulosus identification in Nepal using mithochdrial DNA markers. Trans R Sac Trop Med Hyg 94: 258-268.
- Mathis A, Deplazes P, Eckert J (1996) An improved test system for PCR-based specific detection of Echinococcus multicularis eggs. J Heleminthol 70: 219-222.