Journal of Veterinary Science & Medicine
Download PDF
Review Article
*Address for Correspondence: Peter F. Surai, Department of Microbiology and Biochemistry, Faculty of Veterinary Medicine, Trakia University, Stara Zagora 6000, Bulgaria, E-mail: ppaneri@vet.auth.gr
Citation: Surai PF. Carnitine Enigma: from Antioxidant Actionto Vitagene Regulation Part 1. Absorption, Metabolism, and Antioxidant Activities . J Veter Sci Med. 2015;3(2): 14.
Copyright © 2015 Surai PF. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 03 August, 2015 | Accepted: 03 November, 2015 | Published: 21 November, 2015
Carnitine Enigma: From Antioxidant Action to Vitagene Regulation. Part 1. Absorption, Metabolism, and Antioxidant Activities
Peter F. Surai1-5*
- 1Department of Microbiology and Biochemistry, Faculty of Veterinary Medicine, Trakia University, Stara Zagora 6000, Bulgaria
- 2Department of Animal Nutrition, Faculty of Agricultural and Environmental Sciences, Szent Istvan University, Gödöllo H-2103, Hungary
- 3Department of Veterinary Expertise and Microbiology, Faculty of Veterinary Medicine, Sumy National Agrarian University, Sumy 40021, Ukraine
- 4Odessa National Academy of Food Technology, Odessa 65039, Ukraine
- 5Russian Academy of Science, Moscow, Russia
*Address for Correspondence: Peter F. Surai, Department of Microbiology and Biochemistry, Faculty of Veterinary Medicine, Trakia University, Stara Zagora 6000, Bulgaria, E-mail: ppaneri@vet.auth.gr
Citation: Surai PF. Carnitine Enigma: from Antioxidant Actionto Vitagene Regulation Part 1. Absorption, Metabolism, and Antioxidant Activities . J Veter Sci Med. 2015;3(2): 14.
Copyright © 2015 Surai PF. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 03 August, 2015 | Accepted: 03 November, 2015 | Published: 21 November, 2015
Abstract
L-carnitine (LC) is a small water-soluble molecule playing an important role in fat metabolism and there is a growing interest in the potential uses of LC as a medicinal agent and as a nutritional/dietary supplement. In addition to a great interest from medical sciences, carnitine received a substantial attention from pig and poultry industry. In particular, poultry and pig diets are formulated mainly with plant feed ingredients which are poor sources of carnitine. Furthermore, internal carnitine synthesis depends on many factors and in some cases could be inadequate. Therefore, it seems likely that carnitine dietary supplementation of highly productive and/or stressed animals/birds is of great importance. The molecular mechanisms accounting for the positive effects of LC on farm animals and poultry are not yet determined but many protective effects of LC reported in literature have been related to its antioxidant action.Based on literature review it is concluded that there are several important mechanisms of antioxidant action of carnitine. Firstly, carnitine is shown to directly scavenge free radicals. However, this activity is most likely related to the gut antioxidant defences and has limited relevance to target tissues with relatively low carnitine concentrations. Secondly, carnitine can chelate transition metals (Fe2+and Cu+), preventing their participation in ROS formation via Fenton reaction. However, detailed mechanisms of this process should be further elucidated using modern techniques applied to various biological systems. Again, this carnitine action is very much related to the gut. Thirdly, and more importantly, LC is found to decrease free radical formation by inhibiting specific enzymes (e.g. xanthine oxidase and NADPH oxidase) responsible for free radical production. This carnitine action has a high biological relevance in various stress conditions. Fourthly, and most importantly, carnitine is shown to participate in maintaining the integrity of mitochondria, including electron-transport chain of mitochondria, in stress conditions. Indeed, carnitine can be considered as a mitochondria-specific antioxidant, responsible for mitochondria integrity maintenance and regulation of ROS production and ROS signalling. Fifthly, carnitine can affect vitamin E absorption and metabolism improving the total antioxidant systems. There are important additional mechanisms of carnitine AO activity, including activation/inhibition of various transcription factors and vitagene networks.
Antioxidant activities of carnitine in physiologically relevant concentrations have been well demonstrated in various in vitro systems including cell cultures or isolated cells or organelles. Protective effect of LC and its derivatives on the antioxidant systems of the body are also shown in various models of oxidative stress/toxicity caused by a variety of toxicants and neurotoxic agents. Several lines of evidence from animal experiments and clinical studies indicate that LC supplementation is effective in preventing oxidative stress under various pathological conditions (hypoxia, ischemia-reperfusion, ionizing radiation, hypertension, renal failure and drug-induced nephrotoxicity, over-exercising and ageing) and in patients with various diseases. Antioxidant protective effects were shown by using a range of oxidative stress-related indexes, including lipid peroxidation (MDA, TBARS, and DNA damage), protein oxidation (carbonyls and sulphydryls), activities of antioxidant enzymes and transcription factors (Nrf2 and NF-κB), etc. It is concluded, that depending on conditions carnitine exerts its antioxidant potential in biological systems via a combination of the aforementioned activities.
Keywords
Carnitine; Antioxidant; Antioxidant enzymes; Mitochondria; Vitagenes; Poultry; PigsAbbreviations
ALC: Acetyl-L-Carnitine; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline Phosphatase; BBD: γ-Butyrobetaine dioxygenase; b.w: Body weight; γ-GT: Gammaglutamyl transpeptidase; GR: Glutathione reductase; GSH: Glutathione; GSH-Px: Glutathione peroxidase; GST: Glutathione transferase; HNE-4: Hydroxynonenal; HO: Heme oxygenase; HSP: Heat Shock Protein; i.m: Intramuscular; i.p: Intraperitoneal; i.v: Intravenous; LC- L: Carnitine; LCLT: Carnitine L-tartrate; LDH: Lactate dehydrogenase; L-NAME: N-nitro-L-arginine methyl ester; MDA: Malondialdehyde; MSUD: Maple Syrup Urine Disease; NF-κB: Nuclear Factor-kappa B; Nrf2: Nuclear factor-erythroid-2-related factor 2; OCTN: Organic Cation Transporters; PLC: Propionyl-Lcarnitine; PPARα : Peroxisome Proliferator Activated Receptor Alpha; ROS: Reactive Oxygen Species; RNS: Reactive Nitrogen Species; SHR: Spontaneously Hypertensive Rat; SOD: Superoxide dismutase; STZ: Streptozotocin, TBARS: Thiobarbituric Acid Reactive Substances/species; wt: WeightIntroduction
L-carnitine (LC), a naturally occurring and widely distributed in nature compound was discovered in 1905 by Gulewitsch and Krimberg [1,2]. For the last 50 years this nutrient has received a substantial attention from medical sciences and poultry and pig nutritionists. Main dietary sources of carnitine in poultry/animal nutrition are animal-derived feed ingredients while grains and their by-products are quite poor in carnitine [2]. Because of the cereal grains and their by-products represent the major component of poultry and pig diets and endogenous carnitine synthesis depends on many factors, it could well be that chickens and pigs may face carnitine inadequacy in some situations such as stress and high performance. For example, chicken embryos have a limited capacity to synthesize LC during incubation [3] and freshly laid eggs from hens fed diets of plant origin possess low concentrations of LC [4]. There is also a metabolic need for supplemental carnitine in young pigs [5]. Furthermore, LC in the maternal diet of breeder’s affects carcass traits of their progeny, decreasing carcass fat and increasing breast meat in progeny fed on high nutrient density diets [6].Therefore, several early studies on pigs, fish, foal, quail and broiler chickens demonstrate a growth improvement and other beneficial effects by feeding extra dietary LC [7]. In a recent review it was concluded that in poultry production, LC has a multi-functional purpose, which includes: growth promotion, strengthening the immune system and antioxidant effects [8]. It seems likely that the endogenous synthesis of LC by sows does not cover the amount required for maximum performance during pregnancy and lactation. Indeed, L-carnitine supplementation during pregnancy increased the number of piglets born to sows (+0.1-2.8 piglets), showed heavier litters (+0.3-2.6 kg) and litters of LC supplemented sows gained more weight during the suckling period (+2.6-7.8 kg) than litters of control sows [9]. It is worth mentioning that LC supplementation (400 mg/day) during suckling intensifies the early postnatal skeletal myofibril formation in piglets of low birth weight [10].
The molecular mechanisms accounting for the positive effects of LC on livestock animals and poultry are not yet determined but many protective effects of LC reported in literature have been related to its antioxidant action. Therefore, the aim of this paper is a critical review of recent data related to antioxidant action of carnitine in vitro and in vivo as a possible mechanism of its protective action against various stresses in farm animal and poultry production.
Absorption and Metabolism of Carnitine
Carnitine absorption and metabolism have been reviewed elsewhere [11,12] and can be summarised as follows. Dietary LC is absorbed by active and passive transfer across enterocyte membranes. Bioavailability of dietary LC is about 54-87%, depending on the amount of L-carnitine in the diet. However, bioavailability of LC dietary supplements is substantially lower, comprising about 14-18% of dose [13]. It seems likely that there are species-specific differences in carnitine assimilation from the diet. For example, in supplemented pigs (500 mg/kg diet), LC absorption and degradation in the intestinal tract was estimated at 30-40% and 60-70% of LC respectively [14]. In more recent publication it has been shown that young pigs have a high capacity to absorb carnitine from the diet and plasma and tissue carnitine concentrations in young pigs can be markedly increased by supplementation of carnitine [15]. Indeed, absorption rate of the supplemented carnitine in the small intestine was greater than 95% for the lower doses (25, 50, 100 mg/kg) and greater than 90% for the higher doses (200, 500, 1000 mg/kg). Furthermore, dietary supplementation of carnitine caused a dose-dependent increase of free carnitine, acetyl carnitine (ALC) and total carnitine concentrations in plasma, liver, kidney, heart and skeletal muscle. In fact, at the highest dose of 1000 mg/kg, plasma and tissue total carnitine concentrations were 3-6 fold higher than in the unsupplemented control group [15]. It is interesting to note that a moderate excess of dietary lysine lowers plasma and tissue carnitine concentrations in pigs [16].It has been shown that active carrier-mediated transport maintains high tissue/plasma concentration ratios [13] and carnitine metabolism and homeostasis are controlled by organic cation transporters (OCTN) [17]. For example, active sodium-dependent high affinity OCTN2 is responsible for LC transport in the kidney and other tissues [18] and OCTN2 is regulated by PPARα indicating that lipid also may affect LC transport into tissues [19]. Recent comparative analysis indicates that the role of PPARα as a regulator of carnitine homeostasis is well conserved across different species, including rat, mouse, pig, cattle, chicken, and human [20]. There are species-specific differences in OCTN2 expression. In particular, in humans, OCTN2 is expressed strongly in kidney but only weakly in other tissues [18], whereas in rats OCTN2 is highly expressed in kidney and testis and to a lesser extent in liver [21].
Chicken enterocytes maintain a steady-state LC gradient of 5 to 1 and 90% of the transported LC remains in a readily diffusive form. In chicken brush-border membrane concentrative LC transport is Na+-, membrane voltage-and pH-dependent and has a high affinity for LC (Km 26-31 μM) [22]. Therefore, the aforementioned transporter has properties similar to those of OCTN2. It is interesting to note that non-proliferating species are also able to cover their increased demand for carnitine during fasting by an increased LC synthesis and uptake into cells. Indeed, fasting increases the activity of gammabutyrobetaine dioxygenase (BBD) in liver and kidney and upregulates the expression of OCTN2 in various tissues of pigs, probably mediated by PPARα activation [23]. It was shown that treatment with a PPARα agonist causes an upregulation of OCTN2 in liver, muscle and enterocytes from small intestine of pigs with following increases carnitine concentrations in liver and muscle probably by enhancing carnitine uptake into cells [24,25].
LC and its short-chain esters do not bind to plasma proteins and excess carnitine is excreted via the kidneys [11]. LС is eliminated from the body mainly via urinary excretion. Meat and meat products are main dietary sources of LC for human, while plant foods are poor sources of carnitine [26]. Carnitine contents in grains (wheat, barley, corn, etc.), comprising major part of poultry diets, is about 5-7 mg/kg, while in soybean meal and sunflower meal carnitine concentrations are 15 and 5 mg/kg respectively [27]. Therefore, in commercial poultry and pig production there is a risk of carnitine deficiency associated with various stress conditions. In general, chicken diet is shown to contain 17.8-22.9 mg carnitine/kg [28].
It has been suggested that in animals and man the liver and kidney are the main sites of LC synthesis. For example, in pigs liver and kidney are shown to be the only tissues with a considerable activity of γ-butyrobetaine dioxygenase (BBD), the last enzyme of carnitine synthesis [29]. It is important to mention, that synthesis of LC requires the essential amino acids lysine and methionine as well as such micronutrients as iron, ascorbic acid, vitamin B6 and niacin. Therefore, various stress conditions as well as an imbalanced diet can create a need for external LC supplementation [30]. Furthermore, carnitine synthesis is a reasonably slow process and does not readily keep up with fast changes of the metabolic requirements in stress conditions [31]. The impact of internal LC synthesis on the carnitine balance is comparatively limited since only about 25% of carnitine comes from its de novo synthesis while about 75% of carnitine is coming from the diet [32]. There are also species-specific differences in rate of carnitine synthesis with pigs having a lower rate of carnitine synthesis in tissues than humans [15]. In fact, carnitine biosynthesis in pigs fed diets without LC supplementation were estimated at 6.71 and 10.63 umol/kg/day in low protein and high protein groups respectively [5,14]. Kinetics of carnitine palmitoyltransferase-I, a major carnitine-dependent regulatory enzyme of lipid metabolism, required for the transport of long-chain fatty acids across the inner mitochondria membrane, were shown to be altered by dietary variables and suggest a metabolic need for supplemental carnitine in young pigs [5].
In animal cells and body fluids, carnitine can be found either as free carnitine, short-chain acyl carnitine, or long-chain acyl carnitine. About 20% of total carnitine in serum and 10-15% in muscle and liver are found as acyl carnitines [33]. In fact, acyl and free carnitine serum concentrations are 12.8 ± 7.4 and 67 ± 21.8 μmol/L, respectively [34] and circulating carnitine comprises only about 0.5% of body carnitine [35]. Animal tissues are characterised by comparatively high amounts of LC (from low μM to low mM), with the highest concentrations found in heart and skeletal muscles. In general, the carnitine concentrations are ≈1 mM in rat skeletal muscle, ≈3 mM in human muscle and up to 15 mM in ruminant muscle [36]. For example, the concentration of LC in fresh semitendinosus muscle from broiler chicken, pig, beef cattle, deer, horse and goat were 0.69, 1.09, 1.86-3.57, 4.57, 4.95 and 11.36 μmol/g wet weight, respectively [37]. In general, carnitine concentrations in tissues of pigs are markedly lower than those reported for humans [15]. For example, free carnitine levels in pig plasma at day 28 are shown to be 18.4 μmol/L and increased up to 37.7 μmol/L after 20 days LC dietary supplementation at 400 mg/ day [38]. Plasma carnitine concentration is comparable with those of other antioxidants, including vitamin E (20-30 μM) and ascorbic acid (26.1-84.6 μM) [39]. In piglets at birth carnitine concentrations in liver, kidney, heart and muscles were (nmol/g) 200 ± 22, 123 ± 27, 316 ± 22 and 284-333, respectively, while in plasma carnitine concentration (μmol/L) was 21.8 ± 3.6 [15]. The authors showed that concentrations of total carnitine in plasma, liver and kidney were highest at birth and thereafter declined until an age of 4 weeks. In contrast, carnitine concentrations in heart and skeletal muscle rose from birth until an age of 3-4 weeks and thereafter declined. In 10 week old pigs total carnitine concentrations (nmol/g) in liver, kidney, skeletal muscle and heart were 33.7 ± 1.6; 110 ± 3; 628 ± 25; 329 ± 12, respectively, while in plasma carnitine concentration was 5.91 ± 0.4 μmol/L [17,23]. A progressive increase in carnitine levels in the liver is seen from birth to 24 h in fed piglets (from 0.14 up to 0.33 mM), the level at 24 h being equal to that of a 3 week-old animal and double that of a 24 h old fasted animal [40]. Heart, kidney and skeletal muscle also contained appreciable levels (0.05-0.2 mM) of carnitine at birth. It is interesting to note that LC concentration in red muscle were significantly higher than those in white muscle suggesting that LC concentration in muscle is related to oxygen metabolism and to myofiber types [37]. Indeed, carnitine concentrations in skeletal muscle of pigs, being around 600 nmol/g (0.6 mM), are comparable with those of rat muscle but they are markedly lower than those of human muscle. Furthermore, concentrations of carnitine in other tissues such as liver, kidney or brain of pigs are also three-five folds lower than those of the respective human tissues suggesting that pigs have generally a lower carnitine status than humans [15,29]. A pig weighing 100 kg, for example, has a LC pool of about 24 g with about 85% of this is present in muscle (20.4 g), about 8% in the gastrointestinal tract (1.92 g), 3.5% in the liver (0.84 g) and only 0.3% in the blood (0.07 g) [41]. Piglets of LC treated sows (125 mg LC/day during pregnancy and 250 mg LC/day during lactation) had higher concentrations of LC in plasma and carcass at birth and on days 10 and 20 of age than control piglets [42]. It is interesting to note that LC supplementation improves the growth performance in light piglets of primiparous sows [43].
There is an intense exchange between the plasma and tissue carnitine pools. In fact, in tissues, carnitine can be acylated, transported back into plasma as acylcarnitine and eventually be excreted via the urine [44]. In fact, acetyl-L-carnitine (ALC), the short-chain ester of carnitine, is endogenously produced within mitochondria and peroxisomes and is involved in the transport of acetyl-moieties across the membranes of these organelles. It was shown that an increase in the plasma carnitine pool, without changing LC concentration in muscles, may be sufficient to influence certain metabolic pathways in skeletal muscle [44]. This is a very important finding explaining protective effects of LC in various stress conditions, including recovery after intense exercise [45]. Indeed, carnitine-supplemented exercise-stressed animals could achieve high physical performance with comparatively low metabolic perturbance [44].
Concentrations of LC and ALC change under altered dietary conditions. ALC is the most extensively investigated derivative of the carnitine formulations, largely due to its pharmacokinetic advantages such as reliable absorption and efficient transport [46]. It seems likely that there are species-specific differences in carnitine assimilation from the diet. For example, in contrast to reports in humans, ALC and propionyl-carnitine (PLC) showed no significant bioavailability as parent substance in mice [44]. Indeed, both acyl carnitines were hydrolysed before reaching the systemic circulation. Similarly, in pigs most orally administered ALC is hydrolysed before reaching the systemic circulation [9]. Comparison of data from various studies with mice, rats, pigs, cows, laying hens and human suggests that carnitine homeostasis is well conserved across different species [20].
First attempts to determine carnitine in chicken egg showed its level comprising < 3 μg/g dry tissue (approximately 0.678 μg/g wet tissue or 4.2 nmol/g) [47], and it was suggested that carnitine is synthesized in sizeable quantities in the growing chick embryo. Similar carnitine concentrations were reported by Chiodi et al. [4]. Indeed, in the egg yolk LC concentration was shown to be 4.64 nmol/g, while ALC was 3 times lower comprising 1.32 nmol/g. Furthermore, LC concentration in the egg albumin was extremely low - 0.029 nmol/g, while ALC was about three fold higher (0.102 nmol/g) [4]. It is interesting to note that at day 2 of embryonic development the carnitine values found in the chick embryos were represented by 12% free carnitine, 29% ALC and 59% long chain acylcarnitines. There is an opportunity to manipulate LC level in eggs. Indeed, LC dietary supplementation (125 mg/kg diet) was associated with a significant (approximately by 30%; from about 13 nmol/g wet yolk to about 17 nmol/g wet yolk. e.g.from 0.013 up to 0.017 mM) increase carnitine concentration in egg yolk [48]. Supplementation of the diets of young broiler breeder hens with 25 mg/kg of LC has been shown to increase LC concentrations in the yolk sacs and livers of 18-d chick embryos and to subsequently influence yolk sac fatty acid β-oxidation [49]. Carnitine in eggs and embryonic tissues could affect chicken embryonic development and have some epigenetic effects such as a decrease in abdominal fat in the progeny [49] and decreased carcass fat and increased breast meat in those progeny fed high nutrient density diets [6]. Indeed, such a manipulation of LC level in egg represents an important model for further studies of health-promoting properties of carnitine in poultry production. In fact, free carnitine concentration in embryonic heart, brain and liver was found to be in a range of 2.98-5.84 μmol/g dry wt. and did not change between days 10 and 21 of the development [3]. The authors also showed that ALC was not detected in any organ until the I7th day of incubation and represented about 20% of the total carnitine in each organ on the day of hatch, while concentrations of long-chain acylcarnitine generally represented from 5 to 10% of the total carnitine in each case. In the embryonic liver total carnitine concentration was about 0.35 μmol/g dry wt. at days 11 and 18 and decreased down to about 0.2 μmol/g dry wt. at time of hatching and increased posthatch up to 0.75 μmol/g dry wt. at day 180 [50]. In terms of carnitine concentration on wet tissue basis it would represent about 50 nmol/g wet tissues at day 11, increasing up to 230 at day 18, decreasing down to 104 at hatch and increasing again up to 240 nmol/g wet tissues. It is interesting to underline that in muscles otal carnitine concentration increased from about 0.6 up to 4 μmol/g dry wt. (from about 120 up to 900 nmol/g wet tissue). In the heart total carnitine concentration at time of hatching and at day 180 posthatch was about 90 and 235 nmol/g wet tissue, respectively, while in the brain it was about 66 and 57 nmol/g wet tissue [50]. In another study, carnitine concentration (nmol/mg non-collagenous protein) in chicken embryonic heart increased from about 1 up to 1.5 between days 7 and 17 with the following sharp decrease down to about 0.5 at time of hatching [51].
As LC is not regarded as an essential nutrient, no values for dietary reference intake or recommended daily allowance have been set. Main carnitine function in the body include [52]: a) transport of activated long-chain fatty acids from the cytosol to the mitochondrial matrix, where β-oxidation takes place; b) transfer of the products of peroxisomal β-oxidation, including acetyl-CoA, to the mitochondria for oxidation to CO2 and H2O in the Krebs cycle; c) modulation of the acyl-CoA/CoA ratio; d) storage of energy as acetyl-carnitine; ) modulation of toxic effects of poorly metabolized acyl groups by excreting them as carnitine esters. In addition, the preservation of membrane integrity and mitochondria functions as well as apoptosis inhibition is also important carnitine action [53]. Indeed, recent evidence suggests that LC and ALC supplementation can modulate the antioxidant systems responsible for attenuation of oxidative stress, and mitochondrial dysfunction associated to various pathological conditions [54].
Antioxidant Systems of the Cell and Whole Body
During evolution, living organisms have developed specific antioxidant protective mechanisms to deal with reactive oxygen species (ROS) and reactive nitrogen species (RNS) [55]. Therefore, it is only the presence of natural antioxidants in living organisms which enable them to survive in an oxygen-rich environment [56]. The general term “antioxidant systems” describes these mechanisms, which are diverse and responsible for the protection of cells from the actions of free radicals. Therefore, there are three major levels of antioxidant defence building antioxidant systems of the living cell [57-64].The first level of defence includes three antioxidant enzymes, namely superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and Catalase plus metal-binding proteins responsible for prevention of free radical formation by removing precursors of free radicals or by inactivating catalysts. However, because of huge number of ROS produced, the first level of antioxidant defence in the cell is not sufficient to completely prevent free radical formation. Therefore, the second level of defence consists of mainly chainbreaking antioxidants, such as vitamin E, ubiquinol, carotenoids, vitamin A, ascorbic acid, uric acid, and some other antioxidants. Glutathione (GSH) and thioredoxin systems play an important role in the second level of antioxidant defence. It is difficult to avoid any damages at the cellular level due to ROS and the third level of defence is based on systems that eliminate damaged molecules or repair them. The cooperative interactions between antioxidants and various signaling molecules in the cell are crucial for effective protection from the deleterious effects of free radicals and toxic products of their metabolism. Therefore, antioxidant systems of the living cells are responsible for maintenance of cell membrane integrity, effective cell signaling and adaptation to various stresses.
Antioxidant Action of Carnitine
The cytoprotective effects of carnitine in various stress conditions are believed to be due to a decrease in oxidative stress-related cell damages. Main antioxidant mechanisms of carnitine have been described recently [65] and here additional evidence will be provided to substantiate molecular mechanisms of antioxidant action of carnitine in vitro and in vivo.Direct free radical scavenging
As can be seen from the data on carnitine absorption and metabolism presented above, it seems likely that it is difficult to achieve an effective carnitine concentration in target tissues to have direct AO effect. Therefore, the early studies in this area [66-69] were not successful. However, later publications [70-72] presented data indicating a possibility of the direct AO action of carnitine in physiologically relevant concentrations, but it seems likely that the main such action could be related to the gut [64], since LC concentration there could be quite high. Indeed, the antioxidant properties of carnitine in the gut need further elucidation and clearly there is a need for more research in this area.
Chelating properties of carnitine
It is necessary to take into account that iron and copper are powerful promoters of free radical reactions and therefore their availability in “catalytic” forms is carefully regulated in vivo [56]. Indeed, reactions of Fe2+ and Cu+ with H2O2 are a source of the most powerful hydroxyl radical (*OH) in the Fenton reaction:
H2O2 + Fe2+/Cu+ → *OH + OH- + Fe3+/Cu2+
Transition metal ions also accelerate the decomposition of lipid hydroperoxides into cytotoxic products such as aldehydes, alkoxyl radicals and peroxyl radicals:
LOOH + Fe2+ → LO* + Fe3+ + OH-
LOOH + Fe3+ → LOO* + Fe2+ + H+
Therefore, organisms have evolved to keep transition metal ions safely sequestered in storage or transport proteins. In this way, the metal-binding proteins prevent formation of hydroxyl radical by preventing metals from participation in radical reactions. In addition, metal chelating is another process involved in prevention of Fe and Cu from participation in ROS formation. Therefore, metal chelating properties of carnitine [66,68,70] could contribute to its antioxidant action. Indeed, Reznick et al. showed that L-Propionyl L-Carnitine (PLC) at quite high concentration (75 mM) suppressed hydroxyl radical production in the Fenton system, probably by chelating the iron required for the generation of hydroxyl radicals [66]. The authors suggested that decrease in hydroxyl radical generation was due to chelating ability of PLC. However, in the same system, deferoxamine, a well-known chelating agent, was effective at 0.1 mM concentration ndicating that PLA is mild iron chelator. Furthermore, in the systems based on iron-induced ascorbate oxidation only very modest chelating ability of PLC was seen at 10 mM concentration and no chelating by LC even at 100 mM was observed. In the same system deferoxamine was much more effective at 0.1 mM [66]. It is interesting to note that 14 years later quite strong chelating ability of LC was shown by using spectrophotometric assay with ferrozine, which can quantitatively form complexes with Fe2+ [70]. Therefore, in the presence of chelating agents, the complex formation is disrupted, resulting in a decrease in the red colour of the complex. In particular, it was shown that the metal chelating effects of LC was concentration-dependent between 5 to 30 μg/mL (approximately 0.03-0.19 mM). It is very important to note that the chelating ability of LC was comparable with that of EDTA, since LC exhibited 98.9% chelation of ferrous ion at 30 mg/mL (0.19 mM) concentration, while the percentages of metal chelating capacity of 30 μg/mL (approx. 0.1 mM) EDTA was found to be 80.7%. The chelating effect of LC is suggested to connect with the complex formation between the hydroxyl and carboxylate groups of LC molecule and metal ion [70]. Indeed, the discrepancy of chelating ability of LC obtained by Gulcin and Reznick et al. can be explained by different methodological approaches but, clearly, this issue needs further investigation using modern analytical techniques applied to various biological systems [66,70].
Protective effects of carnitine on mitochondria
It is well known that mitochondria are the primary cellular consumers of oxygen and contain numerous redox enzymes capable of transferring single electrons to oxygen, generating the ROS superoxide [73]. Furthermore, ROS induce protein modifications, lipid peroxidation and mitochondrial DNA damage, which ultimately results in mitochondrial dysfunction [74]. In recent years more and more attention has been paid to signaling role of ROS and their participation in cell adaptation to stress [75]. Our recent review on protective effects of carnitine on mitochondria [65] indicates that in the case of oxidative stress carnitine protects/repairs mitochondria by triggering pro-survival cell signaling. Indeed, LC and its derivatives can affect expression of a range of genes responsible for a synthesis of various proteins in the cell. In fact ALC prevented age-related changes in rat liver mitochondria, including maintenance of the antioxidant systems [76]. LC prevented free fatty acid induced oxidative stress [77], while ALC provided acetyl groups for protein acetylation and affected the amount of mitochondrial proteins [78]. Therefore, LC can protect against mitochondrial dysfunctions associated with oxidative stress caused by various damaging agents. Carnitine is shown to be a mitochondria-specific antioxidant, responsible not only for mitochondria integrity maintenance but also for regulation of ROS production and ROS signaling. It could well be that LC can participate in crosstalk between mitochondria and various transcription factors, including Nrf2, a crucial element of adaptation to stress [79,80].
Inhibition of free-radical generating enzymes by carnitine
Our recent review [65] clearly showed that LC can inhibit main ROS-producing enzymes, namely xanthine oxidase (XO) and NADPH oxidase and in this way contributing to improved antioxidant defences.
In vitro antioxidant effects of carnitine
Clear evidence of protective effect of carnitine against oxidative stress caused by various chemicals came from in vitro studies with cell culture, isolated cells or organelles. This includes human dermal fibroblasts [81], cerebellar granular cell culture [82], LDL [83], human hepatocytes [84], cultured porcine oocytes [85], neuroblastoma cells [86] and neurones of newly born rats [87]. LC was also able to decrease DNA damage caused by toxicants in various experimental systems [88-90]. Effective LC concentrations showing antioxidant protective effects were within the physiological range of LC concentrations varying from 9-25 μM [85], 30-100 μM [87], 0.1-1 mM [83,84,86,88] up to 1-10 mM [82]. Indeed, the aforementioned data confirmed antioxidant action of carnitine in physiologicallyrelevant concentrations in various in vitro systems.
Antioxidant effects of carnitine against oxidative stress in vivo: Protection against toxicants
Protective effect of LC and its derivatives on the antioxidant systems of the body are shown in various models of oxidative stress/ toxicity caused by CCl4 [91-93], cisplantin [94-96], ethanol [97-101], 3-Nitropropionic acid [102], valproate [103], diethylnitrosamine [104], doxorubicin [105], adriamycin [106], tamoxifen [107], indomethacin [108], acetaminophen [109], Cd [100], aflatoxin [111], thioacetamide [112], methotrexate [113]. LC and its derivatives were also effective in decreasing oxidative stress caused by neurotoxic agents such as glutamate [114], quinolinic acid or 3-nitropropionic acid [115], rotenone [116], dexamethasone [117], aminoglycosides [118], scopolamine [119], 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [120] and silver nano-particles [121].
Carnitine was also effective in prevention of oxidative stress in the L-buthionine-sulfoximine-induced cataract model [122], selenite-induced cataractogenesis [123,124], LPS-treated rats [125], high dietary copper in laying hens [126], high fat diet in rats [127], fructose-fed rats [128-131], experimental glaucoma [132], acetic acidinduced colitis [133,134] and atherosclerotic rats [135]. Furthermore, carnitine showed clear antioxidant protective activities in caeruleininduced acute pancreatitis [136], adjuvant arthritis [137] and in hyperthyroid rats [138] as well as in a mouse model of non-alcoholic steatohepatitis [139], in mice with familial amyotrophic lateral sclerosis [140], streptozotocin-induced diabetes in rats [141,142], ifosfamide-induced Fanconi syndrome [143], chemically induced model of maple syrup urine disease [144] and unilateral urethral obstruction-induced oxidative stress [145,146].
Antioxidant effects of carnitine against oxidative stress in vivo: Established clinical models
The possibility of modulating with natural antioxidants the progression of various diseases with ROS species-related pathogenesis has been widely discussed in the literature. It has been established that nutritional antioxidants play an important role in decreasing detrimental consequences of the oxidative stress-related pathologies. Therefore, several lines of evidence from animal experiments and clinical studies exist indicating that LC supplementation is effective in preventing oxidative stress under various pathological conditions.
Hypoxia: Hypoxia can occur under both physiological (e.g., exercise, embryonic development, high altitude. etc.) and pathological conditions (e.g., inflammation, solid tumour formation, lung disease, myocardial infarction, etc.). Substantial evidence indicates increased oxidative stress under moderately hypoxic conditions [147-149] with a specific role for the functional respiratory chain in the generation of ROS. Indeed, under conditions of oxygen deficiency, oxygen molecules in the mitochondrial respiratory chain can accept only one electron, instead of four under normal conditions, producing the superoxide anion radical [150]. In such conditions various antioxidants, including carnitine, are shown to be beneficial. For example, carnitine (400 mg/kg b.w., orally) showed a protective effect against intermittent hypobaric hypoxia-induced oxidative stress in rat erythrocytes indicative by reduction of MDA and increase in protein sulphydryls [151]. LC (100 mg/kg b.w.) significantly decreased the levels of the MDA and carbonylated proteins and significantly increased the serum antioxidant capacity (GSH) in rats exposed to hypobaric hypoxia [152]. Rats pre-treated with LC had lower TBARS levels after the exposure to hypobaric hypoxia and the protective effect of LC was comparable with the effect of α-tocopherol [153]. Supplementation with ALC (75 mg/kg b.w., orally) improves spatial working memory deficits, reduces oxidative stress and lipid peroxidation and inhibits apoptotic cascade induced by hypoxia [154] with a concomitant increase in thioredoxin and GSH levels in the hippocampal neurons [155]. While hypoxia itself can be recognised as conditionally a cause of injury, it is the transition from hypoxia to normoxia (hypoxia-reoxygenation or ischaemia-reperfusion) that causes the most cell damage and tissue failure, known as reperfusion injury [150].
Ischemia-reperfusion: Ischemia-reperfusion (I/R) injury is a complex biological process involving compromised antioxidant defences, oxidative stress and cell death. Indeed, I/R injury is directly related to the formation of ROS, endothelial cell injury, increased vascular permeability, and the activation of neutrophils and platelets, cytokines, and the complement system [156,157]. Because of the strong evidence for the importance of ROS in I/R injury, a number of different intervention strategies have been successfully used in multiple models of ischemia with reperfusion. Furthermore, various nutritional antioxidants have been tested as a means of decreasing oxidative stress and detrimental consequences of I/R injury [158] and protective role of carnitine also received substantial attention. For example, LC pre-treatment (100 mg/kg, i.v.) of rats exposed to gastricI/R increased the tissue catalase activity to the levels of sham-operated rats [159]. Similarly, LC (100-500 mg/kg, i.p.) is shown to protect rat kidney tissue against I/R injury, decrease lipid peroxidation [160] and prevented SOD activity decrease [161]. LC (500 mg/kg, i.p.) showed a protective effect on testicular I/R injury in rats [162] via activation of antioxidant enzymes (SOD, GSH-Px, Catalase) and increased expression of HSP70 [163]. The protective effects of exogenous LC (200 mg/kg, i.v.) on lipid peroxidation in plasma and liver tissue in an experimental warm hepatic I/R injury model in rats were also shown [164,165]. LC was also proven to have a protective effect against oxidative stress resulted from I/R of heart [166] and brain [167]. Therefore, from the data presented above it is clear that LC and its derivatives have antioxidant protective effects in hypoxia and I/R injury indicative by preservation of AO enzyme activities as well as maintaining levels of small molecular weight antioxidants (GSH).
Ionizing radiation: Ionizing radiation (IR) was reported to compromise antioxidant defences and generate excessive amount of ROS. Indeed, IR is responsible for cellular damage and impaired intracellular homeostasis and intracellular signaling system through the damage of cellular macromolecules including DNA, protein, and lipid [168,169]. Natural antioxidants, including carnitine, have been extensively studied for radioprotective activity in various model systems. For example, LC supplementation (200 mg/kg b.w./day, i.p.) was associated with decreased MDA levels and increased SOD and Catalse activities in the plasma of gamma-irradiated rats [170]. LC administration (100 mg/kg b.w./day) significantly decreased the MDA level and increased the activity of SOD and GSH-Px enzymes in lenses of gamma-irradiated rats [171]. Administration of LC (200 mg/kg b.w.) to rats receiving 15 Gy external radiotherapy significantly reduced the severity of brain and retinal damages and decreased the MDA levels and increased the activity of SOD and Catalase in the brain [172]. LC (1.5 mg/kg/day, i.p.) has protective effects against 2.45-GHz-induced blood lipid peroxidation in rats indicative by increased GSH and GSH-Px in erythrocytes [173]. LC showed a protective effect on the 2.45 GHz-induced lipid peroxidation in cortex brain of rats [174]. LC (200 mg/kg b.w.) administered prior to the irradiation reduced the severity of mucosal damage and prevented fall in tissue total sulfhydryl levels in rat ileum [175]. It seems likely that ALC is as effective as LC. Indeed, administration of ALC (250 mg/kg, i.p.) to rats for 5 consecutive days resulted in a significant increase in the activities of both SOD and GSH-Px and the level of GSH in lung and liver tissues which were reduced by gammairradiation treatment [176]. Furthermore, pre-treatment with ALC (250 mg/kg, i.p.) of rats prevented the radiation-induced increase in plasma MDA [177]. From the data presented above it is clear that LC and ALC have antioxidant protective effect against oxidative stress caused by irradiation.
Spontaneously hypertensive rats: The spontaneously hypertensive rat (SHR) has been widely used for the last 50 years as an animal model of hypertension and accompanying metabolic disturbances. A great body of evidence accumulated for the last 20 years clearly indicates that the increased oxidative stress and inadequate activity of endogenous antioxidant defences are the main pathogenic mechanisms for the development of hypertensioninduced damage of target organs [178]. Therefore, dietary antioxidants, including carnitine, are among important elements to decrease negative consequences of the oxidative stress in SHR. For example, LC (200 mg/kg b.w.in drinking water) was shown to prevent lipid peroxidation and GSH-Px activity decrease in liver and cardiac tissues of SHR [179]. Similarly, treatment with LC in the same dose leads to an increase in hepatic and cardiac antioxidant defence (GSH/ GSSG ratio, GSH-Px activity and total antioxidant potential) and ameliorated oxidative stress in liver, heart and plasma (TBARS) of SHR [180]. LC or PLC (200 mg/kg b.w. in drinking water) decreased basal and NADPH-stimulated O2 - production in SHR toward values observed in normotensive rats [181] and improved endothelial function through SOD-dependent mechanisms [182]. LC (300 mg/kg b.w. in drinking water) augmented the antioxidant defence capacity in SHRs. This effect was mediated by an upregulation of antioxidant enzymes, an increase in plasma total antioxidant capacity and a reduction of lipid peroxidation and superoxide anion production in the heart [183]. The authors suggested that the molecular regulation of antioxidant enzymes through an inhibition of the renin-angiotensin system and a modulation of the NF-κB/IκB system to be responsible for this antioxidant effect. After the chronic administration of LC (300 mg/kg b.w. in drinking water for 12 weeks), the activities of GSH-Px, GR, and SOD increased significantly in the erythrocytes of both SHR and L-NAME-treated rats, reaching in some cases values similar to those obtained in the corresponding control, normotensive animals [184,185]. In hypertensive rats LC (400 mg/kg b.w.) improves the oxidative stress response through a specific modulation of NF-κB, Nrf2, and PPARα transcription factors [186]. Therefore, in SHR or L-NAME-treated rats LC was shown to be effective in improving antioxidant defences by upregulating antioxidant enzymes and affecting redox signaling.
Exercise: It has been shown that regular moderate training is beneficial for animal and human health. Conversely, acute exercise leads to compromised antioxidant defences and increased oxidative stress and supporting endogenous defences with additional oral antioxidant supplementation may represent a suitable non-invasive tool for preventing or reducing oxidative stress during training [187]. In skeletal muscle, ROS and NRS are physiologically synthesized at low levels and are required for normal force production. However, when ROS production overtakes tissue antioxidant protection, oxidative stress takes place activating pathophysiologic signaling leading to proteolysis and apoptosis within the myofibers [188]. In this regard, carnitine received substantial attention. For example, LC supplementation (0.5% of dry diet) decreases oxidative stress in exercised rats by reducing lipid peroxidation, redistributing GSH from liver to blood and muscle and increasing GSH-Px activity [189] and increasing SOD, GSH-Px and Catalase activities in the liver and skeletal muscles [190]. LC administration (300 mg/kg) to rats prevented reduction in total antioxidant activity and protein concentration induced by forced swimming [191]. In young rats submitted to exhaustive exercise stress LC treatment (5 mg/kg for 7 days) increased the renal levels of GSH and decreased lipid peroxidation [192]. LC applied at a 3 g dose (via glass of fruit juice) provides strong antioxidant action by increasing the GSH and NOx level and decreasing the TBARs level after exhaustive exercise in young soccer players [193]. Two-week daily oral supplementation of LC has alleviating effects on lipid peroxidation and muscle damage markers following an acute bout of exercise in active healthy young men [194]. Indeed, antioxidant action of LC in exercise models was clearly shown.
Renal failure and drug-induced nephrotoxicity: In recent years, oxidative stress has been identified to contribute to druginduced liver, heart, renal and brain toxicity [195] and improvement of antioxidant defences is a key for prevention of such detrimental effects. Firstly, LC (500 mg/kg, i.p) decreased oxidative stress by prevention of reductions in renal GSH levels as well as plasma SOD, Catalase, and GSH-Px activities, and prevented increases in kidney lipid peroxidation (MDA) in rats with chronic renal failure [196]. Secondly, LC significantly ameliorates drug-induced nephrotoxicity and inhibited ROS generation, lipid peroxidation and apoptosis [118]. In fact, LC treatment (100-200 mg/kg, i.p.) improves antioxidant defences (increased SOD, GSH-Px, Catalase and GSH in the kidney tissue) and protects against functional, biochemical and morphological damage and iron accumulation in glycerol-induced myoglobinuric acute renal failure in rats [197,198]. Similarly, LC (200 mg/kg, i.p.) is shown to prevent the development of ifosfamide induced nephrotoxicity via downregulation of oxidative stress (GSH-Px and Catalase activities) and nitrosative apoptotic signaling (caspase-9 and caspase-3) in kidney tissues [143]. In rats with contrast media-induced nephropathy LC (500 mg/kg, i.p.) increased GSH in renal, spleen and lung tissues, while MDA concentration decreased in renal, liver and lung tissues and Catalase activity increased in the spleen [199]. LC (500 mg/kg, i.m.) also had a partial protective effect against renal tissue damage after experimental pyelonephritis by increasing Catalase activity and alleviating oxidative stress [200]. Fructose-fed rats exhibited increased levels of peroxidation end products, diminished antioxidant status, increased 4-hydroxy-2-nonenal, 2,4-dinitrophenol and 3-nitrotyrosine protein adducts and lipid accumulation in kidney. LC administration (300 mg/kg) attenuated these pathological renal alterations in rats caused by fructose-induced metabolic syndrome including increased level of peroxidation end products and diminished AO status [129]. PLC (250 mg/kg, i.p.) was also effective by decreasing ifosfamide-induced oxidative stress and restored GSH level in cardiac tissues to the control values [201]. Therefore, LC has a substantial antioxidant protective effect against oxidative stress caused by renal failure and drug-induced nephrotoxicity.
Ageing model: Oxidative stress from increased production of ROS has been identified as a major contributing factor to ageing [202]. Reduction of molecular oxygen through electron leakage within the electron transport chain or other cellular sources leads to the generation of highly reactive ROS including the superoxide anion, hydrogen peroxide, hydroxyl radical, peroxyl radicals and other minor species [203]. This relates to decreased antioxidant defences and oxidative stress in aged animals. Therefore, a comparison between young and aged animals became an important model for understanding antioxidant system regulation in the body. Indeed, it was shown, that aged rats underwent significant perturbation of the antioxidant defence system, including depletion of GSH content, decreased AO enzyme activities, increased lipid peroxidation in various tissues. It is interesting to note that more than 10 years ago it was found that such AO system modifications were enhanced by long-term alcohol exposure and they were significantly reduced with ALC supplements [204]. Similarly, long-term supplementation with ALC significantly reduced GSH depletion in the brain regions of hippocampus and ALC treatment increased GR and arginase activities [205]. Supplementation of LC (300 mg/kg b.w., i.p.) to aged (24-month-old) rats improved the antioxidant status (GSH, ascorbate and vitamin E; [206,207]; SOD and GSH-Px activities [208], Catalase and GR [209] of various rat brain regions in a duration dependent manner. LC (300 mg/kg b.w, i.p.) has also inhibiting effect on the accumulation of age-related oxidative DNA damage in rat brain cerebral cortex, striatum and hippocampus [210] or heart [211]. LC supplementation (150 mg/kg b.w in drinking water) to aged rats was associated with a decrease protein carbonyl, 3-nitrotyrosine and protein-bound HNE levels in various brain regions [212] and suppressed the oxidation of methionine residues [213]. It is quite obvious that carnitine has rejuvenating effect on aged rats by restoring activity of antioxidant enzymes, improving vitamin E and C status and restoring mitochondria integrity and functions.
Antioxidant effects of carnitine against oxidative stress in vivo: Patients with various diseases
Promising antioxidant activities of carnitine have been found following supplementation in patients with cardiovascular diseases such as peripheral arterial disease, chronic heart failure, or stable angina [214], coronary artery disease patients [215,216], ischemic cardiomyopathy [217], renal disease with hemodialysis [218,219], multiple sclerosis [220], mild cognitive impairment and mild Alzheimer’s disease [221], age related macular degeneration [222], disorders of propionate metabolism [223], major surgery 224], alcoholic steatohepatitis [225], phenylketonuria [226,227] and MSUD patients [228-230]. In a recent meta-analysis 74 reports testing carnitine and its derivatives were considered, including 18 trials related to kidney disease with success ratio 0.58. Furthermore, trails with other diseases were more successful with success ratio to be 1, including 13 reports on diabetes trials, 9 - heart and vessel disease, 6 - liver disease, 9 - neurological diseases and 6 - genetic diseases [231].
It is also interesting to mention that carnitine supplementation can be beneficial in healthy subjects as well. For example, single dose administration of LC (2.0 g, orally) was shown to improve antioxidant activities in healthy subjects. Indeed, there was a gradual increase in plasma concentrations of SOD, GSH-Px, Catalase and total antioxidative capacity (T-AOC) in the first 3.5 h following LC administration. Furthermore, a positive correlation was found between LC concentration and the antioxidant index of SOD (r = 0.992, P < 0.01), GSH-Px (r = 0.932, P < 0.01)
Conclusions
Antioxidant properties of carnitine have been demonstrated in vitro and in vivo using various model systems as well as clinical observations in patients with various diseases. In the aforementioned in vitro and in vivo studies, the antioxidant properties of LC and its derivatives are demonstrated by:• restoration of the endogenous AO enzymes (SOD, Catalase,GSH-Px, GR and GST) and non-enzymatic antioxidants (vitamins E and C) in the liver and other tissues of stressed animals;
• increased intracellular concentration of GSH in liver and other tissues;
• decreased lipid and protein oxidation, detected as reduced MDA/TBARS and carbonyl content;
• decreased DNA fragmentation/damage and apoptosis;
• reduced secretion of ALT, AST, ALP, γ-GT from the liver into the plasma due to hepatic injuries caused by ROS;
• restored Nrf2 and HO-1 activities;• reduced NF-κB expression and concentration of proinflammatory cytokines, including tumour necrosis factor.
• vitagene activation and increased synthesis of HSPs, thioredoxins and sirtuins.
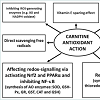
Indeed, antioxidant protection provided by carnitine was significant and the aforementioned studies consistently demonstrated that LC and its derivatives supplementation exert strong antioxidant and tissue-protecting effects independent of the model used (Figure 1). It seems likely that in biological system in vivo all the aforementioned mechanisms are involved and their interactions provide an important place for carnitine to be a crucial part of the integrated antioxidant systems of the animal and human body. Taking into account low carnitine content in grains and poultry and pig diet formulations with limited amounts of animal proteins, carnitine requirement and possible inadequacy in commercial poultry and pig nutrition should receive more attention. Furthermore, protective roles of carnitine in stress conditions of commercial poultry and pig production are of great physiological and economical importance. Therefore, a development of carnitine-containing antioxidant compositions seems an important way forward in decreasing detrimental consequence of various stresses in poultry and pig production.
References
- Gulewitsch WI, Krimberg R (1905) Zur kenntnis der extraktivstoffe der muskeln. Z Physiol Chem 45: 326-330.
- Borum PR (1983) Carnitine. Annu Rev Nutr 3: 233-259.
- Casillas ER, Newburgh RW (1969) Carnitine and derivatives in embryonic chick tissue. Biochim Biophys Acta 184: 566-577.
- Chiodi P, Ciani B, Kentroti S, Maccari F, Vernadakis A, et al. (1994) Carnitine and derivatives in the central nervous system of chick embryo. Int J Biochem 26: 711-720.
- Heo K, Lin X, Odle J, Han IK (2000) Kinetics of carnitine palmitoyltransferase-I are altered by dietary variables and suggest a metabolic need for supplemental carnitine in young pigs. J Nutr 130: 2467-2470.
- Kidd MT, McDaniel CD, Peebles ED, Barber SJ, Corzo A, et al. (2005) Breeder hen dietary L-carnitine affects progeny carcase traits. Br Poult Sci 46: 97-103.
- Szilágyi M (1998) L-carnitine as essential methylated compound in animal metabolism. An overview. Acta Biol Hung 49: 209-218.
- Golzar Adabi SH, Cooper RG, Ceylan N, Corduk M (2011) L-carnitine and its functional effects in poultry nutrition. Worlds Poult Sci J 67: 277-296.
- Eder K (2005) Effects of L-carnitine supplementation in sows. Monatsh Chem 136: 1535-1544.
- Lösel D, Kalbe C, Rehfeldt C (2009) L-Carnitine supplementation during suckling intensifies the early postnatal skeletal myofiber formation in piglets of low birth weight. J Anim Sci 87: 2216-2226.
- Rebouche CJ (2004) Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci 1033: 30-41.
- Evans AM, Fornasini G (2003) Pharmacokinetics of L-carnitine. Clin Pharmacokinet 42: 941-967.
- Pochini L, Scalise M, Galluccio M, Indiveri C (2013) OCTN cation transporters in health and disease: role as drug targets and assay development. J Biomol Screen 18: 851-867.
- Heo KN, Odle J, Han IK (2000) Effects of dietary L-carnitine and protein level on plasma carnitine, energy and carnitine balance, and carnitine biosynthesis of 20 kg pigs. Asian-Australas J Anim Sci 13: 1568-1575.
- Fischer M, Varady J, Hirche F, Kluge H, Eder K (2009) Supplementation of L-carnitine in pigs: absorption of carnitine and effect on plasma and tissue carnitine concentrations. Arch Anim Nutr 63: 1-15.
- Fischer M, Hirche F, Kluge H, Eder K (2009) A moderate excess of dietary lysine lowers plasma and tissue carnitine concentrations in pigs. Br J Nutr 101: 190-196.
- Ringseis R, Eder K (2009) Influence of pharmacological PPARalpha activators on carnitine homeostasis in proliferating and non-proliferating species. Pharmacol Res 60: 179-184.
- Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, et al. (1998) Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273: 20378-20382.
- Wen G, Ringseis R, Eder K (2010) Mouse OCTN2 is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) via a PPRE located in the first intron. Biochem Pharmacol 79: 768-776.
- Ringseis R, Wen G, Eder K (2012) Regulation of genes involved in carnitine homeostasis by PPARα across different species (rat, mouse, pig, cattle, chicken, and human). PPAR Res 2012: 868317.
- Spaniol M, Brooks H, Auer L, Zimmermann A, Solioz M, et al. (2001) Development and characterization of an animal model of carnitine deficiency. Eur J Biochem 268: 1876-1887.
- Durán JM, Peral MJ, Calonge ML, Ilundáin AA (2002) Functional characterization of intestinal L-carnitine transport. J Membr Biol 185: 65-74.
- Ringseis R, Wege N, Wen G, Rauer C, Hirche F, et al. (2009) Carnitine synthesis and uptake into cells are stimulated by fasting in pigs as a model of nonproliferating species. J Nutr Biochem 20: 840-847.
- Ringseis R, Luci S, Spielmann J, Kluge H, Fischer M, et al. (2008) Clofibrate treatment up-regulates novel organic cation transporter (OCTN)-2 in tissues of pigs as a model of non-proliferating species. Eur J Pharmacol 583: 11-17.
- Ringseis R, Lüdi S, Hirche F, Eder K (2008) Treatment with pharmacological peroxisome proliferator-activated receptor alpha agonist clofibrate increases intestinal carnitine absorption in rats. Pharmacol Res 58: 58-64.
- Krahenbuhl S (2000) L-carnitine and vegetarianism. Ann Nutr Metabol 44: 81-82.
- Arslan C (2006) L-Carnitine and its use as a feed additive in poultry feeding a review. Rev Méd Vét (Toulouse) 157: 134-142.
- Mast J, Buyse J, Goddeeris BM (2000) Dietary L-carnitine supplementation increases antigen-specific immunoglobulin G production in broiler chickens. Br J Nutr 83: 161-166.
- Fischer M, Keller J, Hirche F, Kluge H, Ringseis R, et al. (2009) Activities of gamma-butyrobetaine dioxygenase and concentrations of carnitine in tissues of pigs. Comp Biochem Physiol A Mol Integr Physiol 153: 324-331.
- Walter P (2000) Introduction. Ann Nutr Metabol 44: 77.
- Bohles H (2000) Basic concept of L-carnitine supplementation. Ann Nutr Metabol 44: 77-78.
- Lohninger A, Pittner G, Pittner F (2005) L-Carnitine: New aspects of a known compound- A brief survey. Monatsh Chem 136: 1255-1268.
- Ferrari R, Di Mauro S, Shervood WG (1992) L-Carnitine and its role in medicine: from function to therapy. London, England, Academic Press.
- Rubio JC, de Bustos F, Molina JA, Jiménez-Jiménez FJ, Benito-León J, et al. (1998) Cerebrospinal fluid carnitine levels in patients with Alzheimer’s disease. J Neurol Sci 155: 192-195.
- Stanley CA (2004) Carnitine deficiency disorders in children. Ann N Y Acad Sci 1033: 42-51.
- Bremer J (1983) Carnitine--metabolism and functions. Physiol Rev 63: 1420-1480.
- Shimada K, Sakuma Y, Wakamatsu J, Fukushima M, Sekikawa M, et al. (2004) Species and muscle differences in L-carnitine levels in skeletal muscles based on a new simple assay. Meat Sci 68: 357-362.
- Lösel D, Rehfeldt C (2013) Effects of L-carnitine supplementation to suckling piglets on carcass and meat quality at market age. Animal 7: 1191-1198.
- Hu ML (2011) Dietary polyphenols as antioxidants and anticancer agents: more questions than answers. Chang Gung Med J 34: 449-460.
- Bieber LL, Markwell MA, Blair M, Helmrath TA (1973) Studies on the development of carnitine palmitoyltransferase and fatty acid oxidation in liver mitochondria of neonatal pigs. Biochim Biophys Acta 326: 145-154.
- Hermeyer J (2002) The physiological role of L-Carnitine. Lohman Inf 27: 1-8.
- Birkenfeld C, Doberenz J, Kluge H, Eder K (2006) Effect of L-carnitine supplementation of sows on L-carnitine status, body composition and concentrations of lipids in liver and plasma of their piglets at birth and during the suckling period. Anim Feed Sci Technol 129: 23-38.
- Birkenfeld C, Ramanau A, Kluge H, Spilke J, Eder K (2005) Effect of dietary L-carnitine supplementation on growth performance of piglets from control sows or sows treated with L-carnitine during pregnancy and lactation. J Anim Physiol Anim Nutr (Berl) 89: 277-283.
- Morand R, Bouitbir J, Felser A, Hench J, Handschin C, et al. (2014). Effect of carnitine, acetyl-, and propionylcarnitine supplementation on the body carnitine pool, skeletal muscle composition, and physical performance in mice. Eur J Nutr 53: 1313-1325.
- Volek JS, Kraemer WJ, Rubin MR, Gómez AL, Ratamess NA, et al. (2002) L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am J Physiol Endocrinol Metab 282: E474-E482.
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ (2009) Vitagenes, cellular stress response, and acetylcarnitine: relevance to hormesis. Biofactors 35: 146-160.
- 47. Fraenkel G (1953) Studies on the distribution of vitamin BT (Carnitine). Biol Bull 104: 359-371.
- Zhai W, Neuman SL, Latour MA, Hester PY (2008) The effect of male and female supplementation of L-carnitine on reproductive traits of white leghorns. Poult Sci 87: 1171-1181.
- Peebles ED, Kidd MT, McDaniel CD, Tanksley JP, Parker HM, et al. (2007) Effects of breeder hen age and dietary L-carnitine on progeny embryogenesis. Br Poult Sci 48: 299-307.
- Rinaudo MT, Curto M, Bruno R, Piccinini M, Marino C (1991) Acid soluble, short chain esterified and free carnitine in the liver, heart, muscle and brain of pre and post hatched chicks. Int J Biochem 23: 59-65.
- Kargas SA, Bruyere HJ, Gilbert EF, Shug AL (1985) Changes in carnitine levels in the embryonic chick heart during development. Comp Biochem Physiol B 82: 525-527.
- Vaz FM, Wanders RJ (2002) Carnitine biosynthesis in mammals. Biochem J 361(Pt 3): 417-429.
- Karlic H, Lohninger A (2004) Supplementation of L-carnitine in athletes: does it make sense? Nutrition 20: 709-715.
- Ribas GS, Vargas CR, Wajner M (2014) L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533: 469-476.
- Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Third Edition. Oxford University Press, Oxford.
- Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70: 257-265.
- Niki E (1996) -Tocopherol. In: Cadenas E, Packer L, Dekker M (eds). Handbook of antioxidants, New York, London. pp. 3-25.
- Niki E (2014) Antioxidants: basic principles, emerging concepts, and problems. Biomed J 37: 106-111.
- Surai PF (1999) Vitamin E in avian reproduction. Poult Avian Biol Rev 10: 1-60.
- Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham, UK.
- 61. Surai PF (2006) Selenium in nutrition and health. Nottingham University Press, Nottingham, UK.
- Surai PF (2015) Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants 4: 204-247.
- Surai PF (2014) Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol Anim Nutr (Berl) 98: 19-31.
- 64. Surai PF, Fisinin VI (2015) Antioxidant-prooxidant balance in the intestine: Applications in chick placement and pig weaning. J Veter Sci Med 3: 1-16.
- Surai PF (2015) Antioxidant action of carnitine: molecular mechanisms and practical applications. EC Veter Sci 2: 66-84.
- Reznick AZ, Kagan VE, Ramsey R, Tsuchiya M, Khwaja S, et al. (1992) Antiradical effects in L-propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch Biochem Biophys 296: 394-401.
- Di Giacomo C, Latteri F, Fichera C, Sorrenti V, Campisi A, et al. (1993) Effect of acetyl-L-carnitine on lipid peroxidation and xanthine oxidase activity in rat skeletal muscle. Neurochem Res 18: 1157-1162.
- Vanella A, Russo A, Acquaviva R, Campisi A, Di Giacomo C, et al. (2000) L -propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol 16: 99-104.
- Bertelli A, Conte A, Ronca G (1994) L-propionyl carnitine protects erythrocytes and low density lipoproteins against peroxidation. Drugs Exp Clin Res 20: 191-197.
- Gülçin I (2006) Antioxidant and antiradical activities of L-carnitine. Life Sci 78: 803-811.
- Solarska K, Lewińska A, Karowicz-Bilińska A, Bartosz G (2010) The antioxidant properties of carnitine in vitro. Cell Mol Biol Lett 15: 90-97.
- Kolodziejczyk J, Saluk-Juszczak J, Wachowicz B (2011) L-Carnitine protects plasma components against oxidative alterations. Nutrition 27: 693-699.
- Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787-795.
- Sekine S, Ichijo H (2015) Mitochondrial proteolysis: its emerging roles in stress responses. Biochim Biophys Acta 1850: 274-280.
- Calabrese V, Cornelius C, Stella AM, Calabrese EJ (2010) Cellular stress responses, mitostress and carnitine insufficiencies as critical determinants in aging and neurodegenerative disorders: role of hormesis and vitagenes. Neurochem Res 35: 1880-1915.
- Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, et al. (2011) Rat liver mitochondrial proteome: changes associated with aging and acetyl-L-carnitine treatment. J Proteomics 74: 2536-2547.
- Jun DW, Cho WK, Jun JH, Kwon HJ, Jang KS, et al. (2011) Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int 31: 1315-1324.
- Kerner J, Yohannes E, Lee K, Virmani A, Koverech A, et al. (2015) Acetyl-L-carnitine increases mitochondrial protein acetylation in the aged rat heart. Mech Ageing Dev 145: 39-50.
- Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88(Pt B): 179-88.
- Itoh K, Ye P, Matsumiya T, Tanji K, Ozaki T (2015) Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr 56: 91-97.
- Dhaunsi GS, Al-Essa M, Ozand PT, Moosa A (2004) Carnitine prevents cyclic GMP-induced inhibition of peroxisomal enzyme activities. Cell Biochem Funct 22: 365-371.
- Tastekin A, Gepdiremen A, Ors R, Emin Buyukokuroglu M, Halici Z (2005) L-carnitine protects against glutamate- and kainic acid-induced neurotoxicity in cerebellar granular cell culture of rats. Brain Dev 27: 570-573.
- Augustyniak A, Stankiewicz A, Skrzydlewska E (2008) The influence of L-carnitine on oxidative modification of LDL in vitro. Toxicol Mech Methods 18: 455-462.
- Li JL, Wang QY, Luan HY, Kang ZC, Wang CB (2012) Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J Biomed Sci 19: 32.
- Yazaki T, Hiradate Y, Hoshino Y, Tanemura K, Sato E (2013) L-carnitine improves hydrogen peroxide-induced impairment of nuclear maturation in porcine oocytes. Anim Sci J 84: 395-402.
- Ye J, Han Y, Chen X, Xie J, Liu X, et al. (2014) L-carnitine attenuates H2O2-induced neuron apoptosis via inhibition of endoplasmic reticulum stress. Neurochem Int 78: 86-95.
- Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, et al. (2013) Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci 131: 548-557.
- Mescka CP, Wayhs CA, Guerreiro G, Manfredini V, Dutra-Filho CS, et al. (2014) Prevention of DNA damage by L-carnitine induced by metabolites accumulated in maple syrup urine disease in human peripheral leukocytes in vitro. Gene 548: 294-298.
- Deon M, Landgraf SS, Lamberty JF, Moura DJ, Saffi J, et al. (2015) Protective effect of L-carnitine on phenylalanine-induced DNA damage. Metab Brain Dis 30: 925-933.
- Liu F, Rainosek SW, Sadovova N, Fogle CM, Patterson TA, et al. (2014) Protective effect of acetyl-L-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 42: 49-57.
- Annadurai T, Vigneshwari S, Thirukumaran R, Thomas PA, Geraldine P (2011) Acetyl-L-carnitine prevents carbon tetrachloride-induced oxidative stress in various tissues of Wistar rats. J Physiol Biochem 67: 519-530.
- Cetinkaya A, Kantarceken B, Bulbuloglu E, Kurutas EB, Ciralik H, et al. (2013) The effects of L-carnitine and N-acetylcysteine on carbontetrachloride induced acute liver damage in rats. Bratisl Lek Listy 114: 682-688.
- Shaker ME, Houssen ME, Abo-Hashem EM, Ibrahim TM (2009) Comparison of vitamin E, L-carnitine and melatonin in ameliorating carbon tetrachloride and diabetes induced hepatic oxidative stress. J Physiol Biochem 65: 225-233.
- Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, et al. (2006) Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res 53: 278-286.
- El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A (2011) Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies. Eur J Pharmacol 650: 335-341.
- Morigi M, Perico L, Rota C, Longaretti L, Conti S, et al. (2015) Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715-726.
- Haorah J, Floreani NA, Knipe B, Persidsky Y (2011) Stabilization of superoxide dismutase by acetyl-l-carnitine in human brain endothelium during alcohol exposure: novel protective approach. Free Radic Biol Med 51: 1601-1609.
- Arafa HM, Sayed-Ahmed MM (2003) Protective role of carnitine esters against alcohol-induced gastric lesions in rats. Pharmacol Res 48: 285-290.
- Dokmeci D, Akpolat M, Aydogdu N, Doganay L, Turan FN (2005) L-carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacol Rep 57: 481-488.
- Dobrzyńska I, Szachowicz-Petelska B, Skrzydlewska E, Figaszewski Z (2010) Effect of L-carnitine on liver cell membranes in ethanol-intoxicated rats. Chem Biol Interact 188: 44-51.
- Augustyniak A, Skrzydlewska E (2010) The influence of L-carnitine supplementation on the antioxidative abilities of serum and the central nervous system of ethanol-induced rats. Metab Brain Dis 25: 381-389.
- Binienda ZK, Ali SF (2001) Neuroprotective role of L-carnitine in the 3-nitropropionic acid induced neurotoxicity. Toxicol Lett 125: 67-73.
- Bykov IL, Mal'tsev AN, Gurinovich VA, Nefedov LI (2004) Biochemical basis of valproic acid toxicity: role of oxidative stress and effects of L-carnitine. Biomed Khim 50: 384-389.
- Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, et al. (2009) Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol 15: 1373-1380.
- Alshabanah OA, Hafez MM, Al-Harbi MM, Hassan ZK, Al Rejaie SS, et al. (2010) Doxorubicin toxicity can be ameliorated during antioxidant L-carnitine supplementation. Oxid Med Cell Longev 3: 428-433.
- Sayed-Ahmed MM, Salman TM, Gaballah HE, Abou El-Naga SA, Nicolai R, et al. (2001) Propionyl-L-carnitine as protector against adriamycin-induced cardiomyopathy. Pharmacol Res 43: 513-520.
- Ibrahim AB, Mansour HH, Shouman SA, Eissa AA, Abu El Nour SM (2014) Modulatory effects of L-carnitine on tamoxifen toxicity and oncolytic activity: in vivo study. Hum Exp Toxicol 33: 968-979.
- Erkin B, Dokmeci D, Altaner S, Turan FN (2006) Gastroprotective effect of L-carnitine on indomethacin-induced gastric mucosal injury in rats: a preliminary study. Folia Med (Plovdiv) 48: 86-89.
- Yapar K, Kart A, Karapehlivan M, Atakisi O, Tunca R, et al. (2007) Hepatoprotective effect of L-carnitine against acute acetaminophen toxicity in mice. Exp Toxicol Pathol 59: 121-128.
- Liu T, He W, Yan C, Qi Y, Zhang Y (2011) Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicol Ind Health 27: 249-256.
- Citil M, Gunes V, Atakisi O, Ozcan A, Tuzcu M, et al. (2005) Protective effect of L-carnitine against oxidative damage caused by experimental chronic aflatoxicosis in quail (Coturnix coturnix). Acta Vet Hung 53: 319-324.
- Túnez I, Muñoz MC, Medina FJ, Salcedo M, Feijóo M, et al. (2007) Comparison of melatonin, vitamin E and L-carnitine in the treatment of neuro- and hepatotoxicity induced by thioacetamide. Cell Biochem Funct 25: 119-127.
- Sener G, Ekşioğlu-Demiralp E, Cetiner M, Ercan F, Sirvanci S, et al. (2006) L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol 22: 47-60.
- Nagesh Babu G, Kumar A, Singh RL (2011) Chronic pretreatment with acetyl-L-carnitine and ±DL-α-lipoic acid protects against acute glutamate-induced neurotoxicity in rat brain by altering mitochondrial function. Neurotox Res 19: 319-329.
- Elinos-Calderón D, Robledo-Arratia Y, Pérez-De La Cruz V, Pedraza-ChaverríJ, Ali SF, et al. (2009) Early nerve ending rescue from oxidative damage and energy failure by L:-carnitine as post-treatment in two neurotoxic models in rat: recovery of antioxidant and reductive capacities. Exp Brain Res 197: 287-296.
- Zaitone SA, Abo-Elmatty DM, Shaalan AA (2012) Acetyl-L-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson's disease therapy. Pharmacol Biochem Behav 100: 347-360.
- Assaf N, Shalby AB, Khalil WK, Ahmed HH (2012) Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem 68: 77-90.
- Jafari A, Dashti-Khavidaki S, Khalili H, Lessan-Pezeshki M (2013) Potential nephroprotective effects of l-carnitine against drug-induced nephropathy: a review of literature. Expert Opin Drug Saf 12: 523-543.
- Wang X, Wang LP, Tang H, Shan WY, Wang X, et al. (2014) Acetyl-L-carnitine rescues scopolamine-induced memory deficits by restoring insulin-like growth factor II via decreasing p53 oxidation. Neuropharmacology 76 Pt A: 80-87.
- Loots DT, Mienie LJ, Bergh JJ, Van der Schyf CJ (2004) Acetyl-L-carnitine prevents total body hydroxyl free radical and uric acid production induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the rat. Life Sci 75: 1243-1253.
- Liu F, Mahmood M, Xu Y, Watanabe F, Biris AS, et al. (2015) Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci 9: 115.
- Elanchezhian R, Sakthivel M, Isai M, Geraldine P, Thomas PA (2009) Evaluation of lenticular antioxidant and redox system components in the lenses of acetyl-L-carnitine treatment in BSO-induced glutathione deprivation. Mol Vis 15: 1485-1491.
- Geraldine P, Sneha BB, Elanchezhian R, Ramesh E, Kalavathy CM, et al. (2006) Prevention of selenite-induced cataractogenesis by acetyl-L-carnitine: an experimental study. Exp Eye Res 83: 1340-1349.
- Elanchezhian R, Ramesh E, Sakthivel M, Isai M, Geraldine P, et al. (2007) Acetyl-L-carnitine prevents selenite-induced cataractogenesis in an experimental animal model. Curr Eye Res 32: 961-971.
- Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, et al. (2009) Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid Med Cell Longev 2: 73-81.
- Güçlü BK, Kara K, Çakır L, Çetin E, Kanbur M (2011) Carnitine supplementation modulates high dietary copper-induced oxidative toxicity and reduced performance in laying hens. Biol Trace Elem Res 144: 725-735.
- Amin KA, Nagy MA (2009) Effect of Carnitine and herbal mixture extract on obesity induced by high fat diet in rats. Diabetol Metab Syndr 1: 17.
- Rajasekar P, Anuradha CV (2007) Effect of L-carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. Exp Diabetes Res 2007: 72741.
- Rajasekar P, Viswanathan P, Anuradha CV (2008) Renoprotective action of L-carnitine in fructose-induced metabolic syndrome. Diabetes Obes Metab 10: 171-180.
- Balasaraswathi K, Rajasekar P, Anuradha CV (2008) Changes in redox ratio and protein glycation in precataractous lens from fructose-fed rats: effects of exogenous L-carnitine. Clin Exp Pharmacol Physiol 35: 168-173.
- Rajasekar P, Palanisamy N, Anuradha CV (2007) Increase in nitric oxide and reductions in blood pressure, protein kinase C beta II and oxidative stress by L-carnitine: a study in the fructose-fed hypertensive rat. Clin Exp Hypertens 29: 517-530.
- Calandrella N, De Seta C, Scarsella G, Risuleo G (2010) Carnitine reduces the lipoperoxidative damage of the membrane and apoptosis after induction of cell stress in experimental glaucoma. Cell Death Dis 1: e62.
- Kurutas EB, Cetinkaya A, Bulbuloglu E, Kantarceken B (2005) Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators Inflamm 2005: 390-394.
- Cetinkaya A, Bulbuloglu E, Kantarceken B, Ciralik H, Kurutas EB, et al. (2006) Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci 51: 488-494.
- Dayanandan A, Kumar P, Panneerselvam C (2001) Protective role of L-carnitine on liver and heart lipid peroxidation in atherosclerotic rats. J Nutr Biochem 12: 254-257.
- Arafa HM, Hemeida RA, Hassan MI, Abdel-Wahab MH, Badary OA, et al. (2009) Acetyl-L-carnitine ameliorates caerulein-induced acute pancreatitis in rats. Basic Clin Pharmacol Toxicol 105: 30-36.
- Tastekin N, Aydogdu N, Dokmeci D, Usta U, Birtane M, et al. (2007) Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res 56: 303-310.
- Yildirim S, Yildirim A, Dane S, Aliyev E, Yigitoglu R (2013) Dose-dependent protective effect of L-carnitine on oxidative stress in the livers of hyperthyroid rats. Eurasian J Med 45: 1-6.
- Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T, Koike K, et al. (2014) L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS One 9: e100627.
- Kira Y, Nishikawa M, Ochi A, Sato E, Inoue M (2006) L-carnitine suppresses the onset of neuromuscular degeneration and increases the life span of mice with familial amyotrophic lateral sclerosis. Brain Res 1070: 206-214.
- Irat AM, Aktan F, Ozansoy G (2003) Effects of L-carnitine treatment on oxidant/antioxidant state and vascular reactivity of streptozotocin-diabetic rat aorta. J Pharm Pharmacol 55: 1389-1395.
- Uysal N, Yalaz G, Acikgoz O, Gonenc S, Kayatekin BM (2005) Effect of L-carnitine on diabetogenic action of streptozotocin in rats. Neuro Endocrinol Lett 26: 419-422.
- Sayed-Ahmed MM, Hafez MM, Aldelemy ML, Aleisa AM, Al-Rejaie SS, et al. (2012). Downregulation of oxidative and nitrosative apoptotic signaling by L-carnitine in Ifosfamide-induced Fanconi syndrome rat model. Oxid Med Cell Longev 2012: 696704.
- Mescka C, Moraes T, Rosa A, Mazzola P, Piccoli B, et al. (2011) In vivo neuroprotective effect of L-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26: 21-28.
- Moosavi SM, Ashtiyani SC, Hosseinkhani S (2011) L-carnitine improves oxidative stress and suppressed energy metabolism but not renal dysfunction following release of acute unilateral ureteral obstruction in rat. Neurourol Urodyn 30: 480-487.
- Moosavi SM, Ashtiyani SC, Hosseinkhani S, Shirazi M (2010) Comparison of the effects of L: -carnitine and alpha-tocopherol on acute ureteral obstruction-induced renal oxidative imbalance and altered energy metabolism in rats. Urol Res 38: 187-194.
- Schumacker PT (2002) Hypoxia, anoxia, and O2 sensing: the search continues. Am J Physiol Lung Cell Mol Physiol 283: L918-L921.
- Movafagh S, Crook S, Vo K (2015) Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem 116: 696-703.
- 149. Kumar H, Choi DK (2015) Hypoxia inducible factor pathway and physiological adaptation: a cell survival pathway? Mediators Inflamm 2015: 584758.
- Silachev DN, Plotnikov EY, Pevzner IB, Zorova LD, Babenko VA, et al. (2014) The mitochondrion as a key regulator of ischaemic tolerance and injury. Heart Lung Circ 23: 897-904.
- Devi SA, Vani R, Subramanyam MV, Reddy SS, Jeevaratnam K (2007) Intermittent hypobaric hypoxia-induced oxidative stress in rat erythrocytes: protective effects of vitamin E, vitamin C, and carnitine. Cell Biochem Funct 25: 221-231.
- Bodea F, Bocea A, Decea N (2010) L-carnitine decreases oxidative stress induced by experimental hypobaric hypoxia. Pediatr Endocrinol Diabetes Metab 16: 78-81.
- Rauchová H, Vokurková M, Koudelová J (2012) Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol Res 61: S89-S101.
- Barhwal K, Singh SB, Hota SK, Jayalakshmi K, Ilavazhagan G (2007) Acetyl-L-carnitine ameliorates hypobaric hypoxic impairment and spatial memory deficits in rats. Eur J Pharmacol 570: 97-107.
- Barhwal K, Hota SK, Jain V, Prasad D, Singh SB, et al. (2009) Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience 161: 501-514.
- Eltzschig HK, Eckle T (2011) Ischemia and reperfusion--from mechanism to translation. Nat Med 17: 1391-1401.
- Ferrari RS, Andrade CF (2015) Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev 2015: 590987.
- Jaeschke H, Woolbright BL (2012) Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 26: 103-14.
- Derin N, Izgut-Uysal VN, Agac A, Aliciguzel Y, Demir N (2004) L-carnitine protects gastric mucosa by decreasing ischemia-reperfusion induced lipid peroxidation. J Physiol Pharmacol 55: 595-606.
- Görür S, Bağdatoğlu OT, Polat G (2005) Protective effect of L-carnitine on renal ischaemia-reperfusion injury in the rat. Cell Biochem Funct 23: 151-155.
- Liu Y, Yan S, Ji C, Dai W, Hu W, et al. (2012) Metabolomic changes and protective effect of (L)-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press Res 35: 373-381.
- Dokmeci D, Inan M, Basaran UN, Yalcin O, Aydogdu N, et al. (2007) Protective effect of L-carnitine on testicular ischaemia-reperfusion injury in rats. Cell Biochem Funct 25: 611-618.
- Guan Y, Zheng XM, Yang ZW, Li SW (2009) Protective effects of L-carnitine upon testicular ischemia-reperfusion damage in rats. Zhonghua Yi Xue Za Zhi 89: 1858-1861.
- Canbaz H, Akca T, Tataroglu C, Caglikulekci M, Dirlik M, et al. (2007) The effects of exogenous l-carnitine on lipid peroxidation and tissue damage in an experimental warm hepatic ischemia-reperfusion injury model. Curr Ther Res Clin Exp 68: 32-46.
- Çekın AH, Gür G, Türkoğlu S, Aldemır D, Yilmaz U, et al. (2013) The protective effect of L-carnitine on hepatic ischemia-reperfusion injury in rats. Turk J Gastroenterol 24:51-56.
- Xie J, Zeng Q, Wang L (2006). The protective effect of L-carnitine on ischemia-reperfusion heart. J Huazhong Univ Sci Technolog Med Sci 26: 188-191.
- Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, et al. (2015) L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab 35: 382-391.
- Santivasi WL, Xia F (2014) Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal 21: 251-259.
- Szumiel I (2015) Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol 91: 1-12.
- Uçüncü H, Ertekin MV, Yörük O, Sezen O, Ozkan A, et al. (2006) Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced oral mucositis and myelosuppression: a controlled study in a rat model. J Radiat Res 47: 91-102.
- Kocer I, Taysi S, Ertekin MV, Karslioglu I, Gepdiremen A, et al. (2007) The effect of L-carnitine in the prevention of ionizing radiation-induced cataracts: a rat model. Graefes Arch Clin Exp Ophthalmol 245: 588-594.
- Sezen O, Ertekin MV, Demircan B, Karslioğlu I, Erdoğan F, et al. (2008) Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg Rev 31: 205-213.
- Gumral N, Naziroglu M, Koyu A, Ongel K, Celik O, et al. (2009) Effects of selenium and L-carnitine on oxidative stress in blood of rat induced by 2.45-GHz radiation from wireless devices. Biol Trace Elem Res 132: 153-163.
- Naziroğlu M, Gümral N (2009) Modulator effects of L-carnitine and selenium on wireless devices (2.45 GHz)-induced oxidative stress and electroencephalography records in brain of rat. Int J Radiat Biol 85: 680-689.
- Akpolat M, Gulle K, Topcu-Tarladacalisir Y, Safi Oz Z, Bakkal BH, et al. (2013) Protection by L-carnitine against radiation-induced ileal mucosal injury in the rat: pattern of oxidative stress, apoptosis and cytokines. Int J Radiat Biol 89: 732-740.
- Mansour HH (2006) Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res 54: 165-171.
- Babicová A, Havlínová Z, Hroch M, Rezáčová M, Pejchal J, et al. (2013) In vivo study of radioprotective effect of NO-synthase inhibitors and acetyl-L-carnitine. Physiol Res 62: 701-710.
- Hirooka Y (2008) Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci 142: 20-24.
- Gómez-Amores L, Mate A, Revilla E, Santa-María C, Vázquez CM (2006) Antioxidant activity of propionyl-L-carnitine in liver and heart of spontaneously hypertensive rats. Life Sci 78: 1945-1952.
- Gómez-Amores L, Mate A, Miguel-Carrasco JL, Jiménez L, Jos A, et al. (2007) L-carnitine attenuates oxidative stress in hypertensive rats. J Nutr Biochem 18: 533-540.
- Alvarez de Sotomayor M, Bueno R, Pérez-Guerrero C, Herrera MD (2007) Effect of L-carnitine and propionyl-L-carnitine on endothelial function of small mesenteric arteries from SHR. J Vasc Res 44: 354-364.
- de Sotomayor MA, Mingorance C, Rodriguez-Rodriguez R, Marhuenda E, Herrera MD (2007) l-carnitine and its propionate: improvement of endothelial function in SHR through superoxide dismutase-dependent mechanisms. Free Radic Res 41: 884-891.
- Miguel-Carrasco JL, Monserrat MT, Mate A, Vázquez CM (2010) Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur J Pharmacol 632: 65-72.
- Mate A, Miguel-Carrasco JL, Monserrat MT, Vázquez CM (2010) Systemic antioxidant properties of L-carnitine in two different models of arterial hypertension. J Physiol Biochem 66: 127-136.
- Mate A, Miguel-Carrasco JL, Vázquez CM (2010) The therapeutic prospects of using L-carnitine to manage hypertension-related organ damage. Drug Discov Today 15: 484-492.
- Zambrano S, Blanca AJ, Ruiz-Armenta MV, Miguel-Carrasco JL, Revilla E, et al. (2013) The renoprotective effect of L-carnitine in hypertensive rats is mediated by modulation of oxidative stress-related gene expression. Eur J Nutr 52: 1649-1659.
- Pingitore A, Lima GP, Mastorci F, Quinones A, Iervasi G, et al. (2015) Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 31: 916-922.
- D'Antona G, Nabavi SM, Micheletti P, Di Lorenzo A, Aquilani R, et al. (2014) Creatine, L-carnitine, and ω3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscle. Biomed Res Int 2014: 613890.
- Karanth J, Jeevaratnam K (2005) Oxidative stress and antioxidant status in rat blood, liver and muscle: effect of dietary lipid, carnitine and exercise. Int J Vitam Nutr Res 75: 333-339.
- Pandareesh MD, Anand T (2013) Ergogenic effect of dietary L-carnitine and fat supplementation against exercise induced physical fatigue in Wistar rats. J Physiol Biochem 69: 799-809.
- Tsakiris T, Angelogianni P, Tesseromatis C, Tsakiris S, Schulpis KH (2008) Effect of L-carnitine administration on the modulated rat brain protein concentration, acetylcholinesterase, Na+K+-ATPase and Mg2+-ATPase activities induced by forced swimming. Br J Sports Med 42: 367-372.
- Bucioli SA, De Abreu LC, Valenti VE, Vannucchi H (2012) Carnitine supplementation effects on nonenzymatic antioxidants in young rats submitted to exhaustive exercise stress. J Strength Cond Res 26: 1695-1700.
- Atalay Guzel N, Erikoglu Orer G, Sezen Bircan F, Coskun Cevher S (2015) Effects of acute L-carnitine supplementation on nitric oxide production and oxidative stress after exhaustive exercise in young soccer players. J Sports Med Phys Fitness 55: 9-15.
- Parandak K, Arazi H, Khoshkhahesh F, Nakhostin-Roohi B (2014) The effect of two-week L-carnitine supplementation on exercise -induced oxidative stress and muscle damage. Asian J Sports Med 5: 123-128.
- Pereira MD, Ksiazek K, Menezes R (2012) Oxidative stress in neurodegenerative diseases and ageing. Oxid Med Cell Longev 2012: 796360.
- Sener G, Paskaloğlu K, Satiroglu H, Alican I, Kaçmaz A, et al. (2004) L-carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol 43: 698-705.
- Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, et al. (2006) Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin Exp Pharmacol Physiol 33: 119-124.
- Ustundag S, Sen S, Yalcin O, Ciftci S, Demirkan B, et al. (2009) L-Carnitine ameliorates glycerol-induced myoglobinuric acute renal failure in rats. Ren Fail 31: 124-133.
- Boyacioglu M, Turgut H, Akgullu C, Eryilmaz U, Kum C, et al. (2014) The effect of L-carnitine on oxidative stress responses of experimental contrast-induced nephropathy in rats. J Vet Med Sci 76: 1-8.
- Gurocak S, Ure I, Cumaoglu A, Gonul II, Sen I, et al. (2010) Renal tissue damage after experimental pyelonephritis: role of antioxidants and selective cyclooxygenase-2 inhibitors. Urology 76: 508.e1-5.
- Sayed-Ahmed MM, Darweesh AQ, Fatani AJ (2010) Carnitine deficiency and oxidative stress provoke cardiotoxicity in an ifosfamide-induced Fanconi syndrome rat model: Oxid Med Cell Longev 3: 266-274.
- Briehl MM (2015) Oxygen in human health from life to death - An approach to teaching redox biology and signaling to graduate and medical students. Redox Biol 5: 124-139.
- Biala AK, Dhingra R, Kirshenbaum LA (2015) Mitochondrial dynamics: Orchestrating the journey to advanced age. J Mol Cell Cardiol 83: 37-43.
- Scapagnini G, Ravagna A, Bella R, Colombrita C, Pennisi G, et al. (2002) Long-term ethanol administration enhances age-dependent modulation of redox state in brain and peripheral organs of rat: protection by acetyl carnitine. Int J Tissue React 24: 89-96.
- Calabrese V, Scapagnini G, Latteri S, Colombrita C, Ravagna A, et al. (2002) Long-term ethanol administration enhances age-dependent modulation of redox state in different brain regions in the rat: protection by acetyl carnitine. Int J Tissue React 24: 97-104.
- Arockia Rani PJ, Panneerselvam C (2001) Carnitine as a free radical scavenger in aging. Exp Gerontol 36: 1713-1726.
- Rani PJ, Panneerselvam C (2002) Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J Gerontol A Biol Sci Med Sci 57: B134-B137.
- Juliet PA, Joyee AG, Jayaraman G, Mohankumar MN, Panneerselvam C (2005) Effect of L-carnitine on nucleic acid status of aged rat brain. Exp Neurol 191: 33-40.
- Thangasamy T, Jeyakumar P, Sittadjody S, Joyee AG, Chinnakannu P (2009) L-carnitine mediates protection against DNA damage in lymphocytes of aged rats. Biogerontology 10: 163-172.
- Haripriya D, Sangeetha P, Kanchana A, Balu M, Panneerselvam C (2005) Modulation of age-associated oxidative DNA damage in rat brain cerebral cortex, striatum and hippocampus by L-carnitine. Exp Gerontol 40: 129-135.
- Savitha S, Panneerselvam C (2007) Mitigation of age-dependent oxidative damage to DNA in rat heart by carnitine and lipoic acid. Mech Ageing Dev 128: 206-212.
- Poon HF, Calabrese V, Calvani M, Butterfield DA (2006) Proteomics analyses of specific protein oxidation and protein expression in aged rat brain and its modulation by L-acetylcarnitine: insights into the mechanisms of action of this proposed therapeutic agent for CNS disorders associated with oxidative stress. Antioxid Redox Signal 8: 381-394.
- Iwamoto M, Miura Y, Tsumoto H, Tanaka Y, Morisawa H, et al. (2014) Antioxidant effects of carnitine supplementation on 14-3-3 protein isoforms in the aged rat hippocampus detected using fully automated two-dimensional chip gel electrophoresis. Free Radic Res 48: 1409-1416.
- Mingorance C, Rodriguez-Rodriguez R, Justo ML, Herrera MD, de Sotomayor MA (2011) Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr Rev 69: 279-290.
- Lee BJ, Lin JS, Lin YC, Lin PT (2015) Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition 31: 475-479.
- Lee BJ, Lin JS, Lin YC, Lin PT (2014) Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr J 13: 79.
- Gürlek A, Tutar E, Akçil E, Dinçer I, Erol C, et al. (2000) The effects of L-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. Eur J Heart Fail 2: 189-193.
- Veselá E, Racek J, Trefil L, Jankovy'ch V, Pojer M (2001) Effect of L-carnitine supplementation in hemodialysis patients. Nephron 88: 218-223.
- Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, et al. (2010) Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med Sci Sports Exerc 42: 1809-1818.
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield DA, et al. (2003) Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res 28: 1321-1328.
- Ames BN, Liu J (2004) Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci 1033: 108-116.
- Ates O, Alp HH, Mumcu U, Azizi S, Cinici E, et al. (2008) The effect of L-carnitine treatment on levels of malondialdehyde and glutathione in patients with age related macular degeneration. Eurasian J Med 40: 1-5.
- Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, et al. (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of L-carnitine supplementation. Int J Dev Neurosci 28: 127-132.
- Pignatelli P, Tellan G, Marandola M, Carnevale R, Loffredo L, et al. (2011) Effect of L-carnitine on oxidative stress and platelet activation after major surgery. Acta Anaesthesiol Scand 55: 1022-1028.
- Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, et al. (2010) L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis--a randomized and controlled clinical trial. Am J Gastroenterol 105: 1338-1345.
- Sitta A, Barschak AG, Deon M, de Mari JF, Barden AT, et al. (2009) L-carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Neurobiol 29: 211-218.
- Sitta A, Vanzin CS, Biancini GB, Manfredini V, de Oliveira AB, et al. (2011) Evidence that L-carnitine and selenium supplementation reduces oxidative stress in phenylketonuric patients. Cell Mol Neurobiol 31: 429-436.
- Guerreiro G, Mescka CP, Sitta A, Donida B, Marchetti D, et al. (2015) Urinary biomarkers of oxidative damage in Maple syrup urine disease: the L-carnitine role. Int J Dev Neurosci 42: 10-14.
- Mescka CP, Guerreiro G, Donida B, Marchetti D, Wayhs CA, et al. (2015) Investigation of inflammatory profile in MSUD patients: benefit of L-carnitine supplementation. Metab Brain Dis 30: 1167-74
- Mescka CP, Guerreiro G, Hammerschmidt T, Faverzani J, de Moura Coelho D, et al. (2015) L-Carnitine supplementation decreases DNA damage in treated MSUD patients. Mutat Res 775: 43-47.
- Pagano G, Aiello Talamanca A, Castello G, Cordero MD, d'Ischia M, et al. (2014) Current experience in testing mitochondrial nutrients in disorders featuring oxidative stress and mitochondrial dysfunction: rational design of chemoprevention trials. Int J Mol Sci1 5: 20169-20208.
- Cao Y, Qu HJ, Li P, Wang CB, Wang LX, et al. (2011) Single dose administration of L-carnitine improves antioxidant activities in healthy subjects. Tohoku J Exp Med 224: 209-213.