Journal of Vaccine & Immunotechnology
Download PDF
Review Article
Elevated Immune Responses an Evidence of Protection against Malaria Infection in Sickle Hemoglobin Individuals
Bwire GM1*, Majigo MV2, Nkinda L2
1Department of Pharmaceutical Microbiology, Muhimbili University of Health and Allied Sciences, Tanzania
1Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Tanzania
*Address for Correspondence: Bwire GM, Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Box 65013, Dar es Salaam, Tanzania; E-mail: gbwire@muhas.ac.tz
Submission: 14 May, 2019;
Accepted: 09 August, 2019;
Published: 20 August 2019
Copyright: © 2019 Bwire GM, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
The development of malaria is partially inhibited in sickle
hemoglobin (HbS) individuals especially those with heterozygote trait
(HbAS) living in malaria endemic region. Factors such as immune
responses are associated with this protection. Therefore, this review
was conducted to describe the immunological mechanisms involved
in protecting HbS individuals against malaria infection. In summary,
immune factors contribute significantly in malaria protection; naturally,
HbS individuals are prevented from the parasites density through an
enhanced phagocytosis of the infected red blood cells in the spleen
as a result of an improved antigen presentation. Moreover, humoral
responses such as increased levels of Immunoglobulin G (IgG) and
cellular mediated responses i.e. cytokines (interleukin 6,8,10,12 and
tumor necrotic factors) protect HbS individuals from clinical malaria.
This review recommends that, sickle hemoglobin should be considered
when designing malaria vaccine trial.
Keywords
Immune responses; Malaria endemic region; Sickle cell individuals
Introduction
In the past two decades malaria especially caused by Plasmodium
falciparum continued to be a disease of public health consequences
in sub-Saharan Africa [1]. Furthermore, malaria is the evolutionary
driving force behind erythrocyte defects that comprise the most
common Mendelian diseases of humankind [2]. This evolutionary
mutations on the human genome has selected multiple genetic
polymorphisms such as sickle hemoglobin (HbS) to confers protection
against malaria [3,4].
On the other hand, HbS occurs as a single point mutation
(Glutamate→Valine) on the sixth codon of the beta globin gene
responsible for the production of hemoglobin [2,4]. Individuals with
either HbAS (heterozygous with normal hemoglobin A and sickle
hemoglobin S) or HbSS (homozygous with sickle hemoglobin S) are
considered to have sickle cell trait or sickle cell anemia respectively
while those with HbAA (homozygous hemoglobin A) are considered
to be normal [5-7]. Therefore, this review discusses the immunological
mechanisms associated with the protection against malaria in HbS
individuals.
Malaria immunity
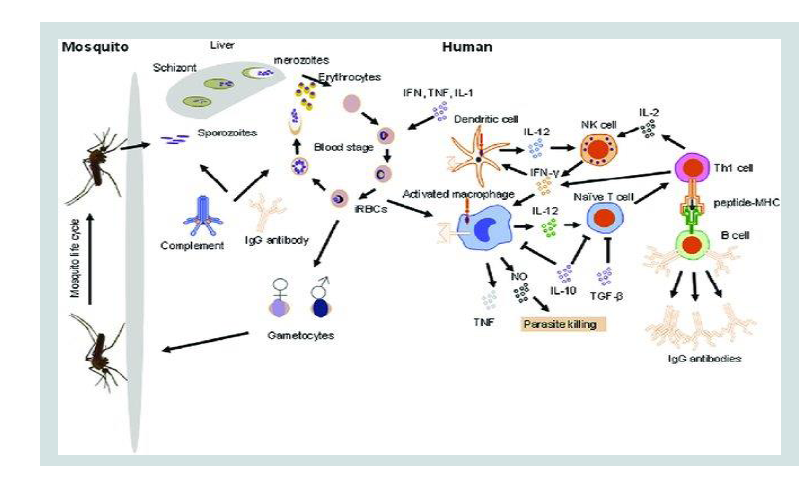
Plasmodium infection involves two hosts where some of
developmental stages occur in humans and other stages occurring
in Anopheles female mosquitos as the vector for the parasite [8].
Immune responses to malaria parasites in human stage begins when
the infected female Anopheles mosquito bites human skin for sucking
blood [9]. The saliva of infected mosquitos contains sporozoites which when injected into the patients’ skin they may remain for hours or
days [10]. This prompts the eliciting of innate immune system against
the invading parasites where factors such as complement system
and dendritic cells are involved. If uncontrolled the sporozoites then
cross the endothelium of the capillaries of the skin and moves to
infect hepatocytes in the liver. Sporozoites within liver cell replicate
and differentiate into blood stage parasites called merozoites [8].
Merozoites are then released into the blood stream where they begin
48 h cycles of invasion of Red Blood Cells (RBCs) [8,10]. During the
blood stage different components are released when the infected RBCs
lyse. The release of these parasites’ components triggers the humoral
and cellular mediated responses as indicated in Figure 1 below.
Innate immunity
Natural (innate) immunity refers to non-specific or first line
of defense, which come into play within hours of an antigen’s
presentation in the body. Different mechanisms i.e. Phagocytosis and
complement activation involving factors such as skin, chemicals in
saliva and blood, mucosal, dendritic and epithelial cells are involved
in natural defense. Innate immune responses contribute to the control
of malaria infection and influence the nature and magnitude of the
adaptive immune response to malaria [9].
Moreover, dendritic cells (DCs) and Natural Killer T (NKT) cells
play a role in immunity to liver stage malaria parasites and contribute
to parasite clearance [11]. In addition to that NKT cells are frequently
the earliest source of interferon-γ during a blood-stage malaria
infection and have an essential role in controlling acute parasitaemia
[9,10].
DCs and NKT in individuals with sickle cell trait reduce the
number of parasites density by preventing the establishing of blood
stage infection through enhanced phagocytic mechanisms [14].
Furthermore, membrane-bound hemichromes, aggregated band
3, autologous and complement (C3c) fragments are reported to be
highly expressed in all mutants Red Blood Cells (RBCs) [12-14].
This results to an enhanced phagocytosis of ring-parasitized mutant RBCs by complement mediated and removal of senescent through
phagocytic recognition [14].
Adaptive immunity
Adaptive (acquired) immunity refers to antigen specific immune
response. This is more complex than innate but both innate and
adaptive immune significantly protect an individual from an infection.
Unlike innate, in adaptive immunity and antigen should first be
processed and recognized, the recognized antigen is attached by the
body immunity while keeping memory cells for future responses
against the antigen more efficient [4].
Children with HbAS have lower parasite densities, which result to
a decreased risk of progression of symptomatic malaria [9]. Findings
from research revealed that HbAS associated protection against
high-density parasitemia is mediated by an innate mechanism,
whereas HbAS-associated protection against acquisition of infection
and development of symptomatic malaria is mediated by acquired
mechanisms [4].
The hypothesis that HbAS accelerates the development of
acquired immunity to P falciparum, was proved from a crosssectional
study of children 9 months to 6 years of age which found
that HbAS were most protective in children between 2 and 6 years of age in addition to that another recent study also found a trend toward
increased protection with advancing age, from 20% at 2 years of age
to 56% at 10 years of age [4,15]. Enhanced acquisitions of acquired
immunity in HbAS individuals are suggested to be a result of innate
mechanisms of protection, with increased phagocytosis of infected
RBCs in the spleen resulting in improved antigen presentation [4].
Find the specific discussions on two types of adaptive responses i) the
cell-mediated immune response, which is carried out by T cells, and
ii) the humoral immune response, which is controlled by activated B
cells and antibodies.
Humoral immunity
Recently, investigators found that humoral responses are involved
in malaria protection of HbS individuals [4]. Higher levels of
Plasmodium falciparum Immunoglobulin G (IgG) responses directed
against specific antigens have been shown to correlate with clinical
protection in children with HbS [16]. More recently, IgG responses
against Plasmodium falciparum (Pf) erythrocytes binding antigens
(PfEBA-175), gametocyte Protein (Pfg27) and zygote and ookinete
protein (yPfs28C) were elevated in HbSS as compared to HbAA
children [17]. Another study, which tried to establish how genetic
factors including hemoglobinopathies, influence progression from
the initial infection with Plasmodium spp. to the development of the
infection through liver and blood-stage to clinical malaria attack, and
finally to severe malaria found sickle haemoglobin play a protective
effect [18].
Moreover, enhanced levels of cross-reactive anti-variant surface
antigens (VSA) responses in children with HbAS were intimately
associated with the protection they have against malaria [16].
Another evidence from children living in area with low malaria
transmission revealed an increased levels of antibodies toward
malaria parasites antigens apical membrane antigen (AMA1),
Erythrocytes Binding Antigen (EBA175), Merozoites Surface Protein
(MSP1, MSP2, MSP3), Circumsporozoite Protein (CSP), and Parasite
Schizont Extract (PSE) in HbAS when compared to Has children [19].
In contrast to that, a study which was conducted in the endemic area
with high malaria transmission found a significant high total IgG,
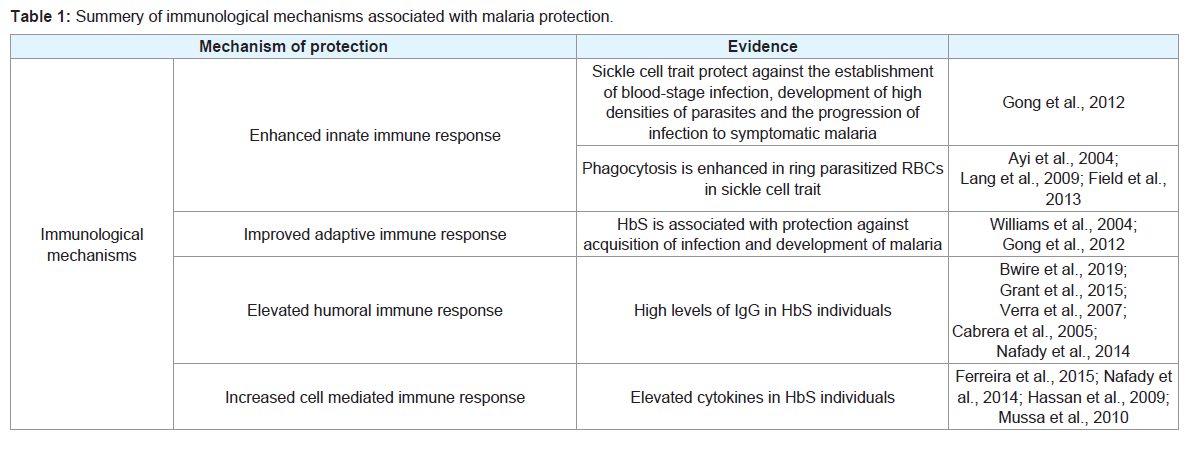
directed against malaria parasites [20] [Table 1].
Cell mediated immunity
The human cellular immune response to malaria parasites
antigens involves the release of cytokines that may contribute to the
control of the parasites’ replication. These cytokines are also involved
in the pathogenesis of the malaria that lead to the manifestation of
clinical symptoms [21].
Study on cytokines among malaria patients [Interleukin (IL);
IL 6,12,18] and uncomplicated clinical malaria were found to be
higher in children with HbS than those with normal hemoglobin
[20]. Research by Ferreira et al found sickle-conferring protection
against Plasmodium infection, HbS inhibits activation and/
or expansion of pathogenic CD8+ T cells recognizing antigens
expressed by Plasmodium, an immunoregulatory effect that does
not involve transcription factor NF-E2-related factor 2 (Nrf2) and/
or heme oxygenase-1 (HO-1 [22]. Furthermore, study by Hassan et
al on cellular immune responses on children with either non-HbS,
with severe malaria, mild malaria or no symptoms of malaria, or
asymptomatic HbS found that IL-12 was weakly expressed by all
the groups of children. When compared with the other groups, the
asymptomatic non-HbS had lower expression of all the cytokines
studied. In addition, the asymptomatic HbS had significantly lower
expression of tumour necrosis factor (TNF) than the non-HbS with
severe malaria, but these two groups showed similar expression
of interferon-c, IL-4 and IL-6. Gene expression of the regulatory
cytokine, IL-10, by the asymptomatic HbS was significantly lower
than that by the non-HbS with severe malaria but higher than that in
the non-HbS with mild malaria [21].
Another study found a coexistence of both high and low levels
of helper T cells (TH), TH1- and TH2-type cytokines, as well as
diminished levels of T-cell subsets in sickle cell disease children [23].
To summarize, these regulations of cytokine release appear to protect
HbS from clinical malaria.
Conclusion
This review concludes that, immune systems play roles in
protection against malaria in HbS individuals. HbS individuals are
prevented from increased number of parasites (parasites density)
through an enhanced phagocytosis of infected RBCs in the spleen as
a result of the improved antigen presentation. This finally enhance
the acquisitions of acquired immunity in HbS particularly HbAS
individuals. Humoral responses such as increased levels of IgG
and cellular mediated responses such as cytokines (IL-6,8,10,12 and
TNF) expression are the specific
In summary, this review recommends more studies to be
conducted to establish the effect of sickle hemoglobin and its
implication on malaria vaccine development. Moreover, investigators
should consider sickle hemoglobin when designing malaria vaccine
trial.
Acknowledgement
I wish to acknowledge the constant support and encouragement
from Muhimbili University of Health and Allied Sciences, Sickle
Cell Program particularly Prof. Julie Makani and the Department of
Pharmaceutical Microbiology