Journal of Vaccine & Immunotechnology
Download PDF
Research Article
Efficacy and safety of Specific Conjugate Particle (SCP)- Doxorubicin in Patients with Soft Tissue Sarcoma, a Randomized Clinical Study
Timothy Allen1*, Nepton Sheik Khoni2, Bruce Dunphy3, Abdul Rahman El Kinge4 and Naveed Basha Court5
- 1Global Allied Pharmaceuticals, Center for Excellence in Research & Development, USA
- 2Global Allied Pharmaceutical, Center for Excellence in Research & Development, USA
- 3School of Public Health and Social Work, Australia
- 4Division of Oncology, Riyadh, KSA
- 5Global Allied Pharmaceuticals, Center for Excellence in Research & Development, USA
*Address for Correspondence: Timothy Allen, Global Allied Pharmaceuticals, Center for Excellence in Research & Development, 160 Vista Oak Dr. Longwood, FL 32779,USA, Tel: 1-321-945-4283; E-mail: timothy.allen@gapsos.com
Citation: Allen T, Khoni NS, Dunphy B, Kinge ARE, Court NB. Efficacy and safety of Specific Conjugate Particle (SCP)-Doxorubicin in Patients with Soft Tissue Sarcoma, a Randomized Clinical Study. J Vaccine Immunotechnology. 2017;3(1): 5.
Copyright: © 2017 Allen T, et al, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Vaccines& Immunotechnology | ISSN: 2377-6668 | Volume: 3, Issue: 1
Submission: 1 March, 2017| Accepted: 03 April, 2017 | Published: 10 April, 2017
Submission: 1 March, 2017| Accepted: 03 April, 2017 | Published: 10 April, 2017
Abstract
Objective: Doxorubicin has been the mainstay of treatment foradvanced STS. However, the conventional formulation of doxorubicinhas not been used clinically, due to poor penetration of the naturalphysiologic barriers, poor water solubility and high side effects profile. To overcome these obstacles authors compared the use of Specific conjugate particle doxorubicin (Group 1), a proven efficacious agent with advanced technical drug, and PEGylated liposomal Doxorubicin (Group 2) in a randomized prospective Phase III trial involving patients with advanced soft tissue sarcomas.
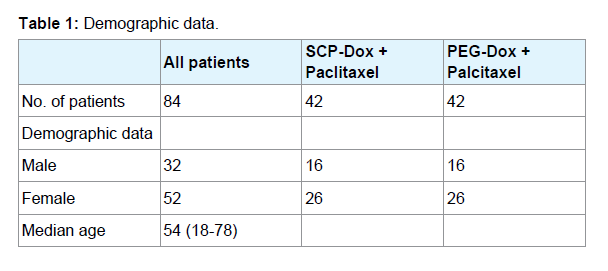
Methods: We recruited eighty-four patients (32 males and 52 females) with histologically confirmed locally advanced or metastatic Soft Tissue Sarcoma (STS). The participants were between the ages of 18 and 70 years. Patients were randomized to receive either Specific Conjugate Particle Doxorubicin (SCP-Doxorubicin) and paclitaxel or the conventionally accepted PEGylated Liposomal Doxorubicin (PEGDoxorubicin) and paclitaxel. Patients received 45 mg/m2 of either PEG-Doxorubicin or SCP-Doxorubicin by an intravenous infusion over 30 minutes on day 1, followed by paclitaxel 150 mg/m2 as an intravenous infusion over three hours on day 1. We repeated treatment cycles every 28 days. Patients received a total of six cycles unless disease progression or unacceptable toxicity occurred.
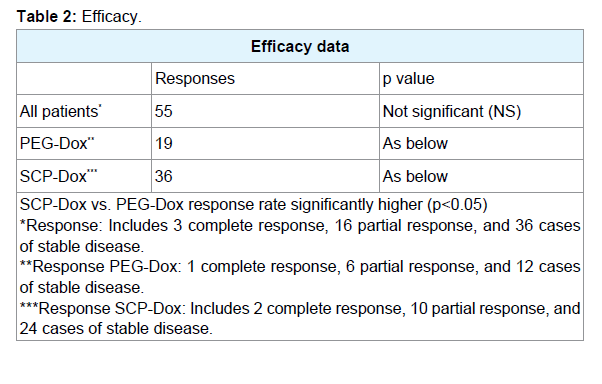
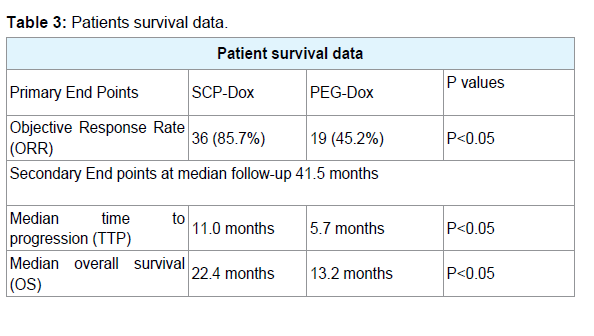
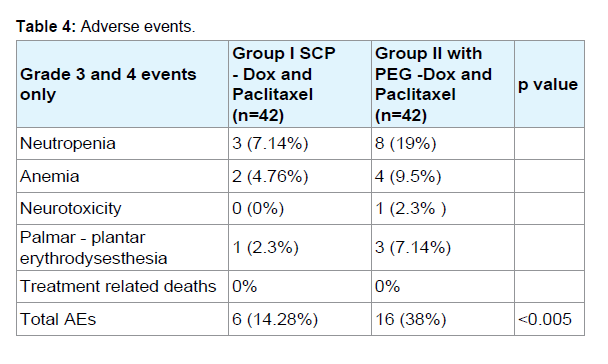
Results: Patients receiving SCP-Dox had a significantly better response to therapy (more CR, PR and SD) than those receiving PEG-Dox (p<0.05). Out of eighty four patients, 3 complete responses (CRs) and 16 Partial Responses (PRs) were observed. Stable Disease (SD) lasting longer than 16 weeks was noted in 36 patients (yielding an aggregate over all response rates of 65.5%). Patients receiving SCP-Dox had better ORR, PFS, and OS than those receiving PEGDox. Sixteen adverse events occurred in patients receiving PEG-Dox, whereas there were only six adverse events in patients who received SCP-Dox. Neutropenia was the most common adverse event in both the groups (p<0.05).
Conclusion: SCP-Dox was found to have superior efficacy to PEG-Dox in the management of soft tissue sarcoma irrespective of the primary disease sites. In addition, SCP-Dox proved to be a comparatively safer treatment regimen, with no major side effects when compared to PEG-Dox.
Abbrevations
SCP: Specific Conjugate Particle; PEG-Dox: Pegylated Liposomal Doxorubicin; SCP-DOX: Specific Conjugate Particle Doxorubicin; PFS: Progression Free Survival; OS: Overall Survival; TTP: Time To Progression; CR: Complete Repsonse; PR: Partial Response; SD: Stable Disease; MRI: Magnetic Resonance Imaging; WHO: World Health Organization
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of solid tumors, arising from mesenchymal or connective tissue. Sarcomas account for about 1% of all adult malignancies and 15% of pediatric malignancies [1]. Soft tissue sarcomas can occur at any age and in any part of the body [1]. The most commonly involved sites are lower and upper extremities (50%), the retro-peritoneum and the abdominal viscera (30%), the thorax (10%), and head and neck (10%). Exposure to ionizing radiation, chronic inflammation and inherited genetic alterations represents the known etiological factors [2].
The most important treatment for all localized STS is radical surgery whenever possible. For orthopedic sites, pre- or postoperative radiotherapy is demonstrated to decrease local recurrence [3,4]. Chemotherapy has widely used for decades in different situations in Soft Tissue Sarcomas: (i) as palliative treatment in advanced cases; (ii) for down staging, i.e. decreasing size to facilitate radical surgery of the primary tumor, lung metastases or, occasionally, metastases in other sites; and (iii) as adjuvant or neoadjuvant treatment in highgrade localized disease in combination with the local treatment of the primary tumor [5].
STSs remain a challenging malignancy to treat, not only because of their high clinic pathological heterogeneity but also because of their limited responsiveness to most conventional chemotherapeutic agents. Patients with STS, even after complete local disease control, often relapse locally or with distant metastases. Almost 50% of patients where there is good local control will eventually present with metastatic disease, and patients with advanced or metastatic STS have a dismal prognosis, with median survival less than one year. Therefore, novel therapies are urgently needed [5]. According to National Comprehensive Cancer Network (NCCN) current guidelines for the treatment of advanced unresectable STS are singleagent or combination regimens as options for the first-line treatment [6]. Doxorubicin is the single most active agent in the treatment of metastatic STS, producing Objective Response Rates of 16% to 27% in clinical trials [7,8]. Doxorubicin combination regimens are MAID (Mesna, doxorubicin, ifosfamide, and dacarbazine), Doxorubicin and Dacarbazine (AD) and Doxorubicin and Ifosfamide. There is no difference in overall survival rate between single agent regimen and combination regimens [9]. Regardless if the type of tumor is chemosensitive or not, regardless of its line, doxorubicin may be considered and needed as a key component [10].
The negative effect of compounds with low solubility include poor absorption and bioavailability, insufficient solubility for IV dosing, development challenges leading to an increase in cost and time, and the burden shifted to patient (frequent high-dose administration). Low aqueous solubility is a major problem encountering almost 40% of the new chemical entities in the pharmaceutical industry.
Selection of method for solubility enhancement depends upon drug characteristics like solubility, chemical nature, melting point, absorption site, physical nature, pharmacokinetic behavior and so forth, dosage form requirement, strength, immediate, or modified release and so forth, and regulatory requirements like maximum daily dose of any excipients and/or drug, approved excipients, analytical accuracy and so forth. Specific Conjugated Particle (SCP) provides a homogenous system which achieves the desired concentration of doxorubicin in systemic circulation to provide the anticipated pharmacological response. SCP’s intellectually protected design uses crystal engineering as well as complexation to overcome the solubilitychallenge of doxorubicin. It reduces frequency of dosing and better patient compliance combined with a low cost of production.
SCP technology is a promising candidate for effective delivery of deprived water-soluble moieties. SCP is a sub-micron colloidal dispersion of pure particles of moiety, which is stabilized by a naturally occurring hydrolyzing entity; unlike surfactants, whose use expands across topical, oral, parenteral or pulmonary administration. In particle-suspension, their size is usually in the range of 200 and 600 nm [21]. The SCP-Doxorubicin is 4-5 times more soluble in water and no precipitation or sedimentation was observed even after 24 hours of dissolving it in water at room temperature when compared to conventional doxorubicin. The solution remains stable over a wide pH range, with no aggregation at either acidic or neutral pH. The hybrid formulation is stable for two years in cold (8 °C) and for four weeks at 37 °C. There is no sign of interaction of serum proteins upon entry of the hybrid product into the body [11].
Simply put, our current indulgence of the molecular basis of cancer in addition to the advances in its discovery and treatment, and poor outcome is acute in seeking the right cure despite great advances that have been made in therapies. SCP-Dox is an attempt in proceeding with the current treatment regimens for cancer which have shown limited survival benefits when used for most advanced stage cancers. We generally target the treatments on tumor bulk but not its cancer stem cells [12,13]. Conventional therapies target cancercells which are highly proliferative and improve the patients’ survival if properly targeted [14].
The traditional cancer therapies; including surgery, hormonal therapy, anti-angiogenesis therapy, and/or immunotherapy show the lack of prolonged efficacy in its long-term outcome. This is deemed to the non-specific effects on normal cells. SCP-Dox may be deemed as an opening in answering this important element of our fight against cancer cells and/or its neoplastic tissues. We tackle the tumors specifically by utilizing leaky tumor phenomenon of the targeted malignancies and expose the stem cells in addition to the differentiating cells. This allows us to venture into a longer overall survival among the treated patients with the highly aggressive tumors such as STS, GBM, etc. We use the particle producing element of the SCP-Dox to mimic salinomycin, sulforaphane, a novel Gemini vitamin D analog (BXL0124). These naturally occurring compounds have the ability to target the stem cells which in turn relinquish the element of their refractory nature of the neoplasm [15-18].
We know that cancer stem cells possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample; however, it is often considered to be associated with chemo resistance and radio resistance that lead to the failure of traditional therapy [19]. Hence a new technology was invented to stabilize the Doxorubicin better called “Specific Conjugate Particle”(SCP) technology; we combined two different and independently acting compounds into one hybrid compound that can synergize. The potency of the new composite compound is greater than the sum of each moiety. The precedent hybrid compound comes from naturally occurring proteins and small molecules, such as botulinum toxin and bleomycin [20].
Given that mandate and the excellent results that we have observed in our other SCP therapy studies, we undertook a prospective Randomized Clinical Trial (RCT) in locally advanced or metastatic STS.
Materials and Methods
Patients and selection criteria
Entry criteria for the study included patients with histologically confirmed advanced STS, and be less than 78 years of age. No prior chemotherapy for advanced disease was allowed. However, previous adjuvant or neo-adjuvant treatment with an anthracycline-containing regimen was permitted provided that there was at least a 12-month treatment-free interval.
The trial design and treatment administration
Doxorubicin has been the mainstay of treatment for advanced STS and has consistently produced responses as monotherapy in more than 20% of previously untreated patients. Patients were randomized to receive either Specific Conjugate Particle Doxorubicin (SCP-Doxorubicin) and paclitaxel or the conventionally accepted PEGylatedLiposomal Doxorubicin (PEG-Doxorubicin) and paclitaxel. Patients received 45 mg/m2 of either PEG-Doxorubicin or SCP-Doxorubicin by an intravenous infusion over 30 minutes on day 1, followed by paclitaxel 150 mg/m2 as an intravenous infusion over three hours, on day 1. We repeated treatment cycles every 28 days. Patients received a total of six cycles unless disease progression or unacceptable toxicity occurred. All patients received standard premedication before paclitaxel administration to prevent hypersensitivity reactions.
Evaluation of response and toxicity
Clinical data, as well as Karnofsky performance status (KPS) and Mini-Mental Status, were assessed before each cycle. Standard laboratory parameters were measured each week or more frequently, if clinically necessary. Objective Response Rate (ORR) was the primary endpoint of the study; Time to progression (TTP) and Overall Survival (OS) were secondary endpoints. Established criteria were based on tumor response. Side effects were graded according to the National Cancer Institute Common Toxicity Criteria (Version 4.0) for chemotherapy-related side effects.
Statistics
The researchers based the current study on an adapted intentto- treat design. Cox’s Regression Model of life table analysis was utilized to assess outcomes and statistical significance was confirmed via Kaplan-Meier estimates. After four treatment courses (8 weeks), response status was determined for the first time. In clinical trials involving patients with high-grade glioma, it is common to count patients with Stable Disease (SD) as responders, as was done in the statistical analysis. Nonetheless, Complete Responses (CRs), Partial Responses (PRs), and cases of SD are reported separately in the text. Cox’s regression method was used to estimate the risk of occurrence of defined events, and significance was assessed using the Wilcoxon test.
Results
Patient characteristics
Eighty-four patients (32 males and 52 females) with locally advanced or metastatic Soft Tissue Sarcoma (STS) were enrolled into the study. Demographic information for study participants is summarized in (Table 1). Patient age ranged from 18 to 78 years, with a median age of 54 years. The primary disease site was visceral in 43% of cases (gastrointestinal tract, 12%; lung, 7%; uterus, 24%), in the extremities (19%), retroperitoneal (12%), and other locations (26%). Histological tumor types include dleiomyosarcoma (43%), malignantfibrous histiocytoma (14%) and liposarcoma (12%). Prior to study recruitment, 50% of patients had undergone complete surgical excision of the primary tumor, while 36% had received postsurgical radiotherapy and 21% had received adjuvant chemotherapy. Patients were randomized to receive either Specific Conjugate Particle Doxorubicin (SCP-Dox) and paclitaxel (Group 1) or Pegylated liposomal Doxorubicin (PEG-Dox) and Paclitaxel (Group 2).
Treatment schedule
Treatment was administered on an outpatient basis. PEG-Dox or SCP-DOX of 45 mg/m2 was administered by anintravenous infusion over 30 min on day 1, followed by paclitaxel 150 mg/m2 as an intravenous infusion over 3 h, on day 1. Cycles were repeated every 28 days. All patients received standard premedication prior to paclitaxel administration in order to prevent hypersensitivity reactions. Standard antiemetic premedication and treatment was also administered. Patients received total of six cycles unless disease progression or unacceptable toxicity occurred.
Efficacy of SCP-Dox and PEG-Dox
From a total of 42 patients randomized to receive PEG-Dox and paclitaxel, six (14%) patients were not evaluable for response but they were included as non-responders for the purposes of an Intention- To-Treat (ITT) analysis. In the 84 patients evaluated, 3 complete responses (CRs) and 16 Partial Responses (PRs) were observed. Stable Disease (SD) lasting longer than 16 weeks was noted in 36 patients (yielding an aggregate overall response rate of 65.5%). Patients receiving SCP-Dox had a significantly better response to therapy (more CR, PR and SD) than those receiving PEG-Dox (p<0.05).
Comparison of the two treatment regimens revealed significant differences for Progression Free Survival (PFS) and Overall Survival (OS) as documented in (Table 3). PFS for SCP-Dox and PEG-Dox at 6 months was 75% and 25% respectively, and at 12 months was 50% and 12.5% respectively.
Safety
PEG-Dox was discontinued in five patients. This was due to the development of a grade IV neutropenia in 4 patients and neurotoxicity in one patient. The most common adverse event observed was neutropenia, which occurred in 11 patients (3 receiving SCP-Dox and 8 receiving PEG- Dox). Three patients who received PEG-Dox developed bullous exanthema, which resulted in their treatment being delayed. Those subjects who developed palmar-plantar erythrodysesthesia, were treated with oral pyridoxine and methyl prednisolone as required. Eight patients on PEG-Dox developed Grade III/IV neutropenia and four developed super infection and so were discontinued from the study. No cardio-toxic side effects were observed in either group (including cases where the maximum cumulative dose was > 400 mg/m2. One patient in the PEG-Dox group developed a severe peripheral neuropathy. No fatalities occurred as a result of treatment related toxicity.
As shown in (Table 4), a total of 16 adverse events occurred in patients receiving PEG-Dox, whereas there were only 6 adverse events in patients who received SCP-Dox.
Discussion
Although this study was undertaken utilizing rigorous methodologies, there are some potential limitations. Firstly, the population of the experimental group is small, as it involved only eighty patients and so may not be representative of the general population. However, there was a statistically significant superior efficacy and reduced side effect profile associated with SCP-Dox. Secondly, patients were followed up for 12 months only. Future studies could undertake a longer period of follow-up based on the stage of carcinoma. These limitations would suggest further research may be required to confirm the promising results of this study, which include a larger number of patients over a longer period.
Solubility performance is the most challenging feature for various new chemical entities. Almost 60% of the new potential products retain solubility complications. This is the main cause for some of the New Drug Applications not successfully enter the market or reach their full clinical potential. There are many techniques in attempting to improve the drug solubility. A hybrid method in utilizing particle size reduction, nanosuspension, and the use of solid dispersion, are employed in producing SCP. Solid dispersion is an important approach for improvement of bioavailability of poor water-soluble drugs; however, our employed techniques of achieving SCPs are not unique to use of single hydrolyzing agent such as surfactants. A combination of the appropriate linking hydrophilic structure with the manufacturing techniques in achieving long shelf life of the produced structured SCPs is the key to its advantageous landscape. This is particularly important to combine the advanced technology of its production, with inexpensive product line, long shelf life, as well as its strong multi-aspect of IP protection. From technical and regulatory prospective as well as its legal front, SCP is a multi-edge advancement in drug delivery techniques.
The diverse kinetics of response detected with SCP-Dox mirror its exclusively latent mechanism of action. In some unambiguous presentation, it may take more time to build antitumor resistance in some patients as opposed to others. This may account for the deferred responses observed in some patients or the expansion in responses over time in other patients. Comparable to the kinetics of response, clinical studies with SCP-Dox in soft tissue sarcoma may indicate an overdue departure of Kaplan-Meier survival curves. The approaches presently used to analyze survival data from randomized clinical trials do not have an established structure for a delay in the parting of Kaplan-Meier curves; therefore, the alternate methods may be needed to compute the required number of responding events and the timing for final analysis of randomized trials where a delayed parting may be anticipated. This may avoid loss of statistical power due to biostatisticians’ exaggeration of required events.
Conclusions and Future Directions
SCP-Dox was found to have superior efficacy to PEG-Dox in the management ofsoft tissue sarcoma irrespective of the primary disease sites. In addition, SCP-Dox proved to be a comparatively safer treatment regimen, with no major side effects when compared to PEG-Dox. Non-responders were treated at considerably later stages of disease when compared to responders (54 weeks vs. 35 weeks). Thus, improved results might be expected if these patients had been recruited to the study earlier in the course of their disease.
The development of SCP-Dox may be accompanied by a number of important lessons for the agent and for cancer therapy as a whole. These lessons have led to a successful clinical trial program for SCP-Dox in Soft tissue sarcomas. SCP-Dox is the first modulated Doxorubicin to demonstrate a statistically significant improvement in overall survival in a trial in patients with Soft tissue sarcomas. The predicted adverse events associated with SCP-Dox therapy are well described and reflect its small conjugated particle mechanism of action. Clinical studies showed that most adverse events were reversible using product-specific treatment guidelines, including prolonged duration of treatment and/or early temporary discontinuation of treatment. These guidelines can reduce the incidence of life-threatening events. The nature of the adverse events observed with SCP-Dox, along with potential safety studies; support the ability of SCP-Dox to break peripheral cytotoxic tolerance and to potentiate an anti tumor chemotherapeutic response mediated by leaky tumor phenomenon. The kinetics of response and survival data for SCP-Dox in Soft tissue sarcomas are characterized based on new insights for small particle development and may have general applicability for similar therapies. Thus, the observations made during the development of SCP-Dox add to the growing evidence for the utility of the clinical paradigm for cancer cytotoxic therapies as may be defined by the Cancer Consortiums.
Disclaimer Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forwardlooking statements relate to Nexus Alliance Biopharmaceuticals’ (NAB) current expectations, beliefs, projections and similar expressions concerning matters that are not historical facts and are not guarantees of future performance. Forward-looking statements involve uncertainties, risks, assumptions and contingencies, many of which are outside NAB’s control that may cause actual results to differ materially from those described in or implied by any forwardlooking statements. All forward-looking statements are based on currently available information and speak only as of the date on which they are made. NAB assumes no obligation to update any forwardlooking statement made in this press release that becomes untrue because of subsequent events, new information or otherwise, except to the extent it is required to do so in connection with its ongoing requirements under Federal securities laws. For a further discussion of factors that could cause NAB’s future results to differ materially from any forward-looking statements, see the section entitled “Risk Factors” in NAB’s Annual Report on Form 10-K for the year ended June 30, 2017 and other risks described in documents filed by NAB from time to time with the Securities and Exchange Commission or other notified financial bodies.
References
- Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, et al. (2016) American Brain Tumor Association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol 18: i1-i50
- National Comprehensive Cancer network (2006) Central nervous system cancers. NCCN Clinical Practice Guidelines in Oncology, Version 2.
- Glioma Meta-analysis Trialists (GMT) Group (2002) Chemotherapy for high-grade glioma. Cochrane Database Syst Rev 4: CD003913.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10: 459-466.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. ( 2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
- Wolff JE, Trilling T, Molenkamp G, Egeler RM, Jurgens H (1999) Chemosensitivity of glioma cells in vitro: a meta-analysis. J Cancer Res Clin Oncol 125: 481-486.
- Siegal T, Horowitz A, Gabizon A (1995) Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of brain tumor model: biodistribution and therapeutic efficacy. J Neurosurg 83: 1029-1037.
- Cabanes A, Tzemach D, Goren D, Horowitz AT, Gabizon A (1998) Comparative study of the antitumor activity of free doxorubicin and polyethylene glycol-coated liposomal doxorubicin in a mouse lymphoma model. Clin Cancer Res 4: 499-505.
- Twentyman PR (1992) MDR1 (P-glycoprotein) gene expression--implications for resistance modifier trials. J Natl Cancer Inst 84: 1458-1460.
- Cole SP, Deeley RG (1998) Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays 20: 931-940.
- Bredel M, Zentner J (2002) Brain-tumour drug resistance: the bare essentials. Lancet Oncol 3: 397-406.
- Abe T, Mori T, Wakabayashi Y, Nakagawa M, Cole SP, et al. (1998) Expression of multidrug resistance protein gene in patients with glioma after chemotherapy. J Neurooncol 40: 11-18.
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105-111.
- Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275-284.
- Jones RJ, Matsui WH, Smith BD (2004) Cancer stem cells: are we missing the target? J Natl Cancer Inst 96: 583-595.
- Wang Y (2011) Effects of salinomycin on cancer stem cell in human lung adenocarcinoma A549 cells. Med Chem 7: 106-111.
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, et al. (2010) Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122: 777-785.
- Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, Xiao D, et al. (2007) D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis 28: 151-162.
- Jeong WS, Kim IW, Hu R, Kong AN (2004) Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 21: 661-670.
- Moltzahn FR, Volkmer JP, Rottke D, Ackermann R (2008) "Cancer stem cells"-lessons from Hercules to fight the Hydra. Urol Oncol 26: 581-589.
- Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277-1280.
- O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, et al. (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15: 440-449.