Journal of Urology & Nephrology
Download PDF
Research Article
Utility of Fluorescent in situ Hybridization in Addition to Voided Urine Cytology in The Diagnostic Work Up of Bladder Cancer Patients: A Pilot Study from A South Indian Referral Laboratory
Ashok V1*, Vidya MN2, Anitha M2, Chander S1, Nivedha VK1 Shrivalli BS1, Ranganathan R1 and Sundareshan TS1
- 1Department of Cytogenetics, Anand Diagnostic Laboratory, India
- 2Department of Pathology, Anand Diagnostic Laboratory, India
*Address for Correspondence:Vishal Ashok, Department of Cytogenetics, Anand Diagnostic Laboratory, Bangalore, India, E-mail: vishal1988ashok@gmail.com
Citation: Ashok V, Vidya MN, Anitha M, Chander S, Nivedha VK, et al. Utility of Fluorescent In Situ Hybridization in Addition to Voided Urine Cytology in The Diagnostic Work Up of Bladder Cancer Patients: A Pilot Study from A South Indian Referral Laboratory. J Urol Nephrol. 2018;5(1): 5.
Submission: 15 March, 2018| Accepted:10 April, 2018 | Published: 16 April, 2018
Abstract
Background: Bladder cancer (BC) is amongst the most prevalent cancers associated with urinary tract. Although voided urine cytology (VUC) is currently an important urine-based laboratory test, it falls short due to high rate of false negative and equivocal diagnosis. Inter observer, intra observer and institutional variability have been few of the technical facets of cytology. Fluorescent In Situ Hybridization (FISH) has proven to be more sensitive and specific than VUC, with gradual improvement in higher grade tumours. This pilot study was conducted to evaluate the diagnostic yield of FISH on voided urine samples in correlation with cytology from patients suspected of and under surveillance for BC in an Indian population.
Materials and methods: VUC and Bladder cancer FISH (Aquariusprobes, Cytocell, UK) was performed on 24 urine samples from patients suspected of and under surveillance for BC. Findings were reported as per Paris System of Urinary Cytology and International System of Cytogenomic Nomenclature.
Results: FISH was positive in 8 patients (33%) while cytology was positive only in 4 patients (16%). Positivity increased to 41% (10 cases) when both techniques were combined. In our study, positive cases included 4 cases of polysomy, 3 cases of homozygous deletion of P16 and an isolated case of trisomy 3.
Conclusion: Interphase FISH is a fast, easy, and reliable test in identifying specific genetic aberrations in BC. When coupled with VUC, it increases the diagnostic yield in a routine diagnostic setup. It is important to implement molecular tests in the diagnostic work up of BC patients for effective diagnosis and management.
Keywords
Bladder cancer; FISH; Genetic abnormality; Urine cytology
Introduction
Bladder cancer (BC) is one of the most common cancers in the world with a male predominance [1,2]. In the Indian scenario, it is amongst the most prevalent cancers associated with urinary tract and accounts for 3.9% of total cancer cases diagnosed according to the Indian cancer registry [3,4]. 3 males and 1 female out of 1,00,000 individuals develop BC each year in India [4-6]. Microscopic and macroscopic haematuria are the most common clinical manifestation of this disease [7]. Currently, the established techniques for diagnosing and monitoring BCs are cystoscopy and Voided Urine Cytology (VUC). Cystoscopy is regarded as the gold standard for detection of BC. VUC has been the front runner in urine-based assays for detection of BC’s for more than 50 years, owing to its low false positive rate (high specificity) and simplicity in testing. The sensitivity and specificity in detecting BC’s increases significantly when these two tests are coupled. Although cystoscopy is considered as gold standard, its sensitivity in detecting flat lesions is relatively low. VUC falls short due to high rate of false negative and equivocal diagnosis or Atypical Urine Cytology (AUC) [8]. Inter observer, intra observer and institutional variability have been serious technical facets of cytology [9]. As a disease characterised by long follow up surveillance with multiple diagnostic procedures, BC has significant financial implication while repetitive invasive procedures cause undue anxiety to patients.
Analysis of urine for abnormal cells has been a priority resulting in development of FDA approved non-invasive urine-based assays detecting multiple targets such as NMP22 [quantitative or qualitative NUMA1(nuclear mitotic apparatus protein 1)], BTA stat/TRAK [qualitative or quantitative BTA (Bladder tumour associated antigen)], Urovysion (FISH), and ImmunoCyt/uCyt+ [1,10]. Fluorescent In Situ Hybridization (FISH) has proven to be more sensitive and specific than VUC, with gradual improvement in higher grade tumours in multiple comparative studies [11-15]. In addition, studies using centromeric specific DNA probes offers rapid detection of aneuploid changes within a malignant cell in bladder cancer [16-22]. Previous studies have identified multiple chromosomes to be structurally and numerically altered which are both specific and nonspecific for disease subtype and tumour progression [23,24].
To our knowledge, the utility of FISH as a diagnostic or screening tool in patients presenting with haematuria is not well characterised in India. We sought to evaluate the diagnostic yield of FISH on voided urine samples in correlation with cytology from patients suspected of and under surveillance for BC.
Materials and Methods
Patient population and samples
A total of 24 voided urine samples, obtained between November 2015 and December 2016, were included in this study. These comprised of 16 urine samples from patients who presented with primary haematuria suspected of, but with no prior history of BC (Group 1) and 8 urine samples from patients under surveillance for BC (Group 2). The urine samples were collected primarily for cytology and subsequently used for FISH within 2-3 hours of collection.
VUC Processing and slide preparation
The urine samples for cytology were processed immediately and centrifuged at 1500 rpm for 10 minutes. Slides were prepared and stained with Haematoxylin/Eosin and PAP stains. The slides were then observed under bright field microscope for presence of atypical cells.
Microscopy
Whenever atypical cells were seen they were subtyped as urothelial cells or squamous cells. The cells were graded into low and high grade wherever possible according to the cell morphology. The atypical cells which did not fall confidently into high grade or low grade were categorised as suspicious. Additional comments on whether the atypia was due to malignancy or inflammation were included. The Paris System of reporting was supplemented in the report (Table 1) [25].
FISH processing and slide preparation
30-40 ml of urine sample was centrifuged at 3000 rpm to obtain a visible pellet. The pellet was suspended in Phosphate-buffered Saline for preliminary rinsing. The cells were then re-suspended in 2-3 ml of 10X Trypsin-EDTA and incubated for 20 mins at 37 °C followed by centrifugation at 3000 rpm. Then, the sample was incubated for 15 mins in hypotonic solution of potassium chloride, KCl (0.075 M) followed by centrifugation at 3000 rpm. Sample was fixed with Cornoy’s fixative (2 parts methanol: 1 part acetic acid). Subsequently, 2-3 washes were given with Cornoy’s Fixative (3 parts methanol: 1 part acetic acid). Cell pellets were stored at 2-8 °C until FISH was performed.
A multi-targeted FISH probe labelled to the peri-centromeric regions of chromosomes 3, 7 and 17 for detecting aneuploidies and a locus specific probe for P16 gene for detecting deletion (Aquarius probes, Cytocell, UK) was used in the study. Chromosome 3, 7, 17 and P16 were labelled with red, green, blue and orange filters, respectively.
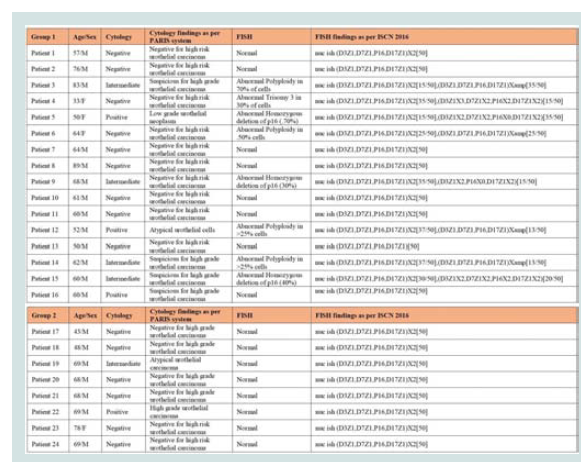
Table 1: Patient population considered in the study with VUC and FISH results categorized as Group1, presenting primary haematuria with no history of BC and Group 2, under surveillance of BC.
Slide preparation
The cell suspension was dropped on clean slides and observed under 10X and 25X bright field microscopy to evaluate critical parameters such as cell concentration and presence of contaminants (microbial and/or crystals). In case of contamination, the pellet was further diluted such that target cells were not juxtaposed with contaminants. After the optimal concentration was achieved, the slides were air dried and aged at 65 °C for 20 mins. 10 μl of the probe was added within the marked area, cover slipped, and sealed using rubber cement. The sealed slides were then co-denatured at 75 °C for 30 seconds followed by overnight hybridisation at 37 °C (Thermobrite). Post-Hybridisation, 2 mins wash with 0.4xSSC at 72 °C was performed, followed by a high stringency wash with 2xSSC, 0.05% Tween-20 at room temperature. The slides were drained and 10 μl of DAPI counter-stain was added, cover slipped and sealed.
Microscopy and scoring: FISH slides were assessed using Zesis Axio Imager fluorescent microscope with ISIS software (MetaSystems GmbH, Germany) under Texas Red (CEP3), FITC (CEP7), TRITC (P16) and Aqua (CEP17) filters. Priority was given to cells enclosing a nucleus with abnormal morphology such as irregular shape and relative large size. A positive FISH result was defined after 25 morphologically abnormal cells had been analysed, four or more nuclei showing gain for 2 or more chromosomes (3, 7, or 17) or 12 nuclei showing no 9p21 signals as formulated by Halling et al. [26]. Overlapping cells, cells with indistinct or blurry signals were not scored. Multinucleated “umbrella” cells were not included. Care was taken not to interpret split signals as two signals. The analysis was performed by two independent cytogeneticists and reported as per ISCN 2016 [27].
Results
Total of 24 patients were included in this pilot study. Of these, 20 (83%) were males and 4 (16%) were females. Median age was 60 years (range 33-89). Total of 16 and 8 samples belonged to Group 1 and 2, respectively.
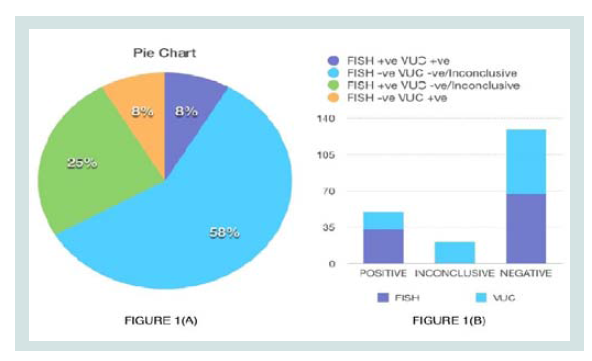
Group 1: Suspected BC Patients. A total of 16 patients were included in this group, of which 8 samples were positive on FISH and 3 samples were positive on VUC. 4 samples were “Inconclusive” by cytology as represented by (Figure 1).
Figure 1: (A) Pie Chart representing samples where FISH and VUC were concordant (BLUE/PURPLE), FISH was advantageous (GREEN) and VUC was advantageous (ORANGE). (B) Comparison of diagnostic yield of FISH and VUC.
Group 2: Patients under surveillance. A total of 8 patients were included in this group. All were negative on FISH. Only 1 sample had an “Inconclusive” VUC as represented by (Figure 1).
2 cases (Patient 5 and 14) in Group 1 had follow up biopsies, post cytology which confirmed presence of low grade urothelial carcinoma, non myoinvasive. The rest of the cases were lost on follow up and hence could not be correlated with biopsy report. 5 cases in group 2 were known cases of urothelial carcinoma which included 2 cases (Patient 17 and 18) of low grade and 3 cases (Patient 22, 23 and 24) of high grade. Remaining cases did not have the corresponding biopsy reports but were mentioned as bladder cancer patients on surveillance.
Diagnostic yield of cytology and FISH
FISH was positive in 8 patients (33%) while cytology was positive only in 4 patients (16%). Positive pick up increased to 41% (10 cases) when both techniques were combined.
Concordant results: Total of 16 samples (72%) had concordant results (13 negative and 3 positive) for both tests as indicated in Figure 1b.
Positive FISH and Negative/Intermediate VUC: Total of 4 cases benefitted from FISH testing where the corresponding VUC were either intermediate (Patient 3 and 9) or had negative reports (Patient 4 and 6).
Negative FISH and Positive VUC: 2 samples (Patient 16 and 22) benefitted from VUC where the corresponding FISH results were negative.
Discussion
BC is a genetically heterogeneous disease that accumulates specific, recurrent genetic alterations at various stages [28]. It has a high potential for recurrence and progression to more invasive stages. Although, cystoscopy is still considered as the gold standard for diagnostic investigations for BC, its non-concordance with VUC is a significant clinical problem [29].
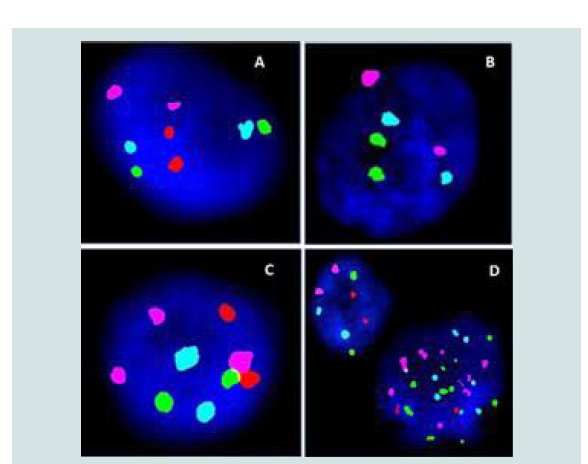
Interphase FISH is an established, adjunct tool used in diagnosis and surveillance of haematological malignancies and solid tumours [30]. FISH studies’ using centromeric probes has proven to be of immense value for precise and rapid detection of aneuploidies within malignant cells, especially in BC [16-19]. This cocktail consists of centromeric enumeration probes for chromosomes 3, 7 and 17 and locus-specific indicator (LSI) probe for P16 gene at the 9p21 band. The assay is being increasingly used as a valuable adjunct in detecting and monitoring BC [31,32]. The types of genetic aberrations observed in FISH are polysomic, trisomic, tetrasomic patterns for chromosome 3, 7, 17 and mono/biallelic loss of P16 located at 9p21. Polysomy is the most common aberration observed in BC which usually correlates with high tumour grade [26]. In our study, 4 cases (50%) of total positive cases exhibited polysomic patterns in a range of 25% to 70% of cells analysed. Aberrations on P16 gene in bladder cancer indicate progression of disease. It is either represented by monosomy or partial deletions [33]. Earlier studies using centromeric FISH and locus specific FISH have suggested that monosomy of chromosome 9 [34,35], especially deletion of tumour suppressor gene CDKN2A/P16 is a predictive marker for early tumour recurrence. We identified homozygous deletion of P16 in 3 cases in a range of 30% to 70% of cells analysed. Finally, an isolated case (Patient 4) of trisomy 3 was observed in a patient belonging to Group 1 with inflammatorycytology as represented in (Figure 2).
Figure 2:(A) Normal signal pattern (B) Homozygous loss of P16 gene (2 Orange signals absent) (C) Trisomy 3 represented by 3 Texas Red signals (pink colour) (D) Abnormal cell showing polysomic pattern and an adjacent cell presenting normal signal pattern.
Renu K et al. recommend FISH as an adjunct tool in risk stratification of BC patients with AUC. Although FISH provides additional information in cases where cytology is inconclusive, false positivity in FISH is a legitimate issue. Tetrasomic signal pattern in FISH should be cautiously classified as abnormal as studies have indicated that they may be more common in benign conditions or certain cell types. Reasons for false positive results in FISH test include genetic alterations of uerothelial cells in conditions such as polyomavirus infections, urolithiasis-related changes and changes induced by chemotherapy and radiation [36,37]. Unless tetrasomy is identified by specific FISH protocols such as “Target FISH” which assists in identifying true tetrasomic cells or if the population is significantly prominent, they should be reported as a non-specific finding. In our study, 3 cases were observed to have polysomic signal pattern in a high percentage (>40%) of atypical cells analysed. In the present study, 2 cases presented a positive VUC and a negative FISH result. One of the reasons could be the aberration driving the malignancy can be on a chromosome not studied in the FISH panel [38].
Although, the number of cases included in the present pilot study was small and lacking a thorough correlation with follow up of the patients including the cystoscopic findings, it sheds enough light on the diagnostic yield of aberrations in voided urine samples by FISH. Of the 8 positive FISH cases, 6 had inconclusive or negative cytology report. Interpretation of these aberrations is critical in establishing FISH as an effective adjunct diagnostic test in BC. Gopalkrishna A et al. described FISH as an anticipatory positive test capable of picking up evidence of the disease earlier to other established tests [29. Studies till date have provided strong evidence that FISH has greater sensitivity compared to VUC [11-13]. The specificity of the test is debatable owing to the mediocre performance of FISH in comparison with VUC in recent studies [39]. This can be countered as demonstrated by NA Moatamed et al. where the specificity and Positive Predictive Value (PPV) significantly increased by excluding uniform tetraploid cells from the analysis [40]. Utility of FISH as a screening or surveillance tool has proven to be a reliable non-invasive diagnostic test for BC patients [1,10].
In conclusion, Interphase FISH is an effective and rapid diagnostic tool in BC. Combined with the need for specialized infrastructure, instruments and personnel, implementation of FISH faces various challenges in developing countries. Therefore, it is imperative that a larger study be undertaken to understand the statistical significance of FISH against VUC, to effectively include it in the diagnostic work up of BC patients.
References
- Goodison S, Rosser CJ, Urquidi V (2013) Bladder cancer detection and monitoring: assessment of urine- and blood-based marker tests. Mol Diagn Ther 17: 71-84.
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9-29.
- Tiwana MS, Ni LH, Saini S, Verma SK, Doddamani D, et al. (2016) Radiation therapy outcomes in muscle invasive urinary bladder cancer: A single institution experience. Indian J Cancer 53: 143-146.
- Gupta P, Jain M, Kapoor R, Muruganandham K, Srivastava A, et al. (2009) Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian J Urol 25: 207-210.
- Verma A, Kapoor R, Mittal RD (2017) Cluster of differentiation 44 (CD44) gene variants: A putative cancer stem cell marker in risk prediction of bladder cancer in North Indian population. Indian J Clin Biochem 32: 74-83.
- Murthy NS, Nandakumar BS, Pruthvish S, George PS, Mathew A (2010) Disability adjusted life years for cancer patients in India. Asian Pac J Cancer Prev 11: 633-640.
- Ke Z, Lai Y, Ma X, Lil S, Huang W (2014) Diagnosis of bladder cancer from the voided urine specimens using multi-target fluorescence In Situ hybridisation. Oncol Lett 7: 325-330.
- Virk RK, Abro S, de Ubago JM, Pambuccian SE, Quek ML, et al. (2017) The value of the UroVysion® FISH assay in the risk-stratification of patients with “atypical urothelial cells” in urinary cytology specimens. Diagn Cytopathol 45: 481-500.
- Lotan Y, O’Sullivan P, Raman JD, Shariat SF, Kavalieris L, et al. (2017) Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol Oncol 35: 531.
- Chou R, Gore JL, Buckley D, Fu R, Gustafson K, et al. (2015) Urinary biomarkers for diagnosis of bladder cancer: A systematic review and metaanalysis. Ann Intern Med 163: 922-931.
- Caraway NP, Khanna A, Fernandez RL, Payne L, Bassett RL Jr, et al. (2010) Fluorescence in situ hybridisation for detecting urothelial carcinoma: A clinicopathologic study. Cancer Cytopathol 118: 259-268.
- Halling KC, King W, Sokolova IA, Meyer RG, Burkhardt HM, et al. (2000) A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol 164: 1768-1775.
- Sarosdy MF, Khan PR, Ziffer MD, Love WR, Barkin J, et al. (2006) Use of a multi target fluorescence in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J Urol 176: 44-47.
- Laudadio J, Keane TE, Reeves HM, Savage SJ, Hoda RS, et al. (2005) Fluorescence in situ hybridization for detecting transitional cell carcinoma: Implications for clinical practice. BJU Int 96: 1280-1285.
- Chen AA, Grasso M (2008) Is there a role for FISH in the management and surveillance of patients with upper tract transitional-cell carcinoma? J Endourol 22: 137-144.
- Sandberg AA (2002) Cytogenetics and molecular genetics of bladder cancer: A personal view. Am J Med Genet 115: 173-182.
- Fadl-Elmula I, Kytola S, Pan Y, Lui WO, Derienzo G, et al. (2001) Characterization of chromosomal abnormalities in uroepithelial carcinomas by G-banding, spectral karyotyping and FISH analysis. Int J Cancer 92: 824-831.
- Hoglund M, Sall T, Heim S, Mitelman F, Mandahl N, et al. (2001) Identification of cytogenetic subgroups and karyotypic pathways in transitional cell carcinoma. Cancer Res 61: 8241-8246.
- Yu Ds, Chen Hl, Chang SY (2002) Chromosomal aberrations in transitional cell carcinoma: Its correlation with tumour behaviour. Urol Int 69: 129-135.
- Ribal MJ, Alcaraz A, Mengual L, Carrio A, Lopez-Guillerno A, et al. (2004) Chromosomal high-polysomies predict tumour progression in T1 transitional cell carcinoma of the bladder. Eur Urol 45: 593-599.
- Panani AD, Babanaraki A, Malianga E, Roussos Ch (2004) Numerical aberrations of chromosomes 9 and 11 detected by FISH in Greek bladder cancer patients. Anticancer Res 24: 3857-3861.
- Placer J, Espinet B, Salido M, Sole F, Gelabert-Mas A (2005) Correlation between histologic findings and cytogenetic abnormalities in bladder carcinoma: A FISH study. Urology 65: 913-918.
- Shackney SE, Berg G, Simon SR, Cohen J, Amina S, et al. (1995) Origins and clinical implications of aneuploidy in early bladder cancer. Cytometry 22: 307-316.
- Zhou AG, Liu Y, Cyr MS, Garver J, Woda BA, et al. (2016) Role of tetrasomy for the diagnosis of urothelial carcinoma using UroVysion® fluorescent in situ hybridization. Arch Pathol Lab Med 140: 552-559.
- Barkan GA, Wojcik EM, Nayar R, Savic-Prince S, Quek ML, et al. (2016) The Paris system for reporting urinary cytology: The quest to develop a standardized terminology. Adv Anat Pathol 23: 193-201.
- Halling KC, Kipp BR (2008) Bladder cancer detection using FISH (UroVysion® assay). Adv Anat Pathol 15: 279-286.
- McGowan-Jordan J, Simons A, Schmid M (2016) ISCN 2016: An international system for human cytogenomic nomenclature. Karger, New York, U S A.
- Sidransky D, Messing E (1992) Molecular genetics and biochemical mechanisms in bladder cancer. Oncogenes, tumor suppressor genes, and growth factors. Urol Clin North Am 19: 629-639.
- Gopalakrishna A, Fantony JJ, Longo TA, Owusu R, Foo WC, et al. (2017) Anticipatory positive urine tests for bladder cancer. Ann Surg Oncol 24: 1747-1753.
- King W, Proffitt J, Morrison L, Piper J, Lane D, et al. (2000) The role of fluorescence In Situ hybridization technologies in molecular diagnostics and disease management. Mol Diagn 5: 309-319.
- Sokolova IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG, et al. (2000) The development of a multitarget, multicolor fluorescence In Situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn 2: 116-123.
- Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, et al. (2002) Clinical evaluation of a multi-target fluorescent In Situ hybridization assay for detection of bladder cancer. J Urol 168: 1950-1954.
- Panani AD, Kozirakis D, Anastasiou J, Babanaraki A, Malovrouvas D, et al. (2006) Is aneusomy of chromosome 9 alone a valid biomarker for urinary bladder cancer screening? Anticancer Res 26: 1161-1165.
- Tsukamoto M, Matsuyama H, Oba K, Yoshihiro S, Takahashi M, et al. (2002) Numerical aberrations of chromosome 9 in bladder cancer. A possible prognostic marker for early tumor recurrence. Cancer Genet Cytogenet 134:41-45.
- Jung I, Reeder JE, Cox C, Siddiqui JF, O’Conell MJ, et al. (1999) Chromosome 9 monosomy by fluorescence In Situ hybridization of bladder irrigation specimens in predictive of tumour recurrence. J Urol 162: 1900-1903.
- Hossain D, Hull D, Kalantarpour F, Maitlen R, Qian J, et al. (2014) Does polyomavirus infection interfere with bladder cancer fluorescence In Situ hybridization? Diagn Cytopathol 42: 225-229.
- Tapia C, Glatz K, Obermann EC, Grilli B, Barascud A, et al. (2011) Evaluation of chromosomal aberrations in patients with benign conditions and reactive changes in urinary cytology. Cancer Cytopathol 119: 404-410.
- Tinawi-Aljundi R, King L, Knuth ST, Gildea M, Carrie Ng, et al. (2015) Oneyear monitoring of an oligonucleotide fluorescence In Situ hybridization probe panel laboratory-developed test for bladder cancer detection. Res Rep Urol 7: 49-55.
- Riesz P, Lotz G, Paska C, Szendroi A, Majoros A, et al. (2007) Detection of bladder cancer from the urine using fluorescence In Situ hybridization technique. Pathol Oncol Res 13: 187-194.
- Moatamed NA, Apple SK, Bennett CJ, Aronson WJ, Klisak I, et al. (2013) Exclusion of the uniform tetraploid cells significantly improves specificity of the urine FISH assay. Diagn Cytopathol 41: 218-225.