Journal of Urology & Nephrology
Download PDF
Research Article
Incidence and Risk Factors for Hemorrhagic Complications after Percutaneous Renal Biopsy using a 16-Gauge Needle Followed by 6 Hours of Bed Rest
Takeshi Maehana1*, Shintaro Miyamoto,, Koji Ichihara,, Toshiaki Tanaka and Naoya Masumori
- 1Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan
*Address for Correspondence: Takeshi Maehana, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan, Tel: 81-11-611-2111; E-mail: maehana@sapmed.ac.jp
Citation: Maehana T, Miyamoto S, Ichihara K, Tanaka T, Masumori N. Incidence and Risk Factors for Hemorrhagic Complications after PercutaneousRenal Biopsy using a 16-Gauge Needle Followed by 6 Hours of Bed Rest. J Urol Nephrol. 2017;4(1): 5.
Journal of Urology & Nephrology | ISSN: 2380-0585 | Volume: 4, Issue: 1
Submission: 25 April, 2017| Accepted: 30 May, 2017 | Published: 12 June, 2017
Submission: 25 April, 2017| Accepted: 30 May, 2017 | Published: 12 June, 2017
Abstract
Background: The procedures and management protocols forpercutaneous renal biopsy (PRB) are different among institutions. Herewe present the outcomes of our protocol and identify risk factors forhemorrhagic complications (HCs).
Methods: Thirty From 2007 through 2016, 175 patients received PRB usinga 16-gauge biopsy needle with real-time ultrasound guidance. In asingle session, three specimen cores were obtained. Post operatively,all patients were monitored with strict bed rest without sandbagcompression for six hours. Adverse events were graded according tothe Clavien-Dindo classification..
Results: Ninety-seven patients (55.4%) were female and themedian age was 48.0 years (IQR 37.0-64.0). The median estimatedglomerular filtration rate based on serum creatinine (eGFRcreat) was57.8 mL/min/1.73 m2 (IQR 28.1-87.8). The median number of glomeruliassessable in each specimen was 19 (IQR 12-29). Symptomatic HCsdeveloped in 9 patients (5.1%). All the events occurred within 6 hoursafter PRB. Selective transcatheter arterial embolization and bloodtransfusion were required in 1 and 7 patients, respectively, but therewas no grade IIIb to V event. In univariate analysis, predictive factorsfor symptomatic HCs were being female (OR 1.102, p = 0.006), aplatelet count < 15.0 x 104/μl (OR 7.405, p = 0.004) and eGFRcreat < 30mL/min/1.73 m2 (OR 6.300, p = 0.005).
Conclusion: PRB using a 16-gauge needle followed by 6 hours ofstrict bed rest secures sufficient samples for histological diagnosis witha low incidence of severe HCs. Furthermore, monitoring with six hoursof bed rest contributes to early detection and management of severeHCs.
Keywords
Percutaneous renal biopsy; Bed rest; Hemorrhagic complication
Introduction
Renal biopsy of native kidneys is an essential examination for diagnosis and management of patients with renal disease. The Percutaneous renal Biopsy (PRB) technique using an automated biopsy gun in combination with real-time ultrasound guidance is widely used and has become the gold standard[1] . With technological advancements, PRB has become safer, and the incidence of lifethreatening complications has decreased [2]. However, renal hemorrhage remains one of the most worrisome complications associated with PRB. Therefore, postoperative strict bed rest and careful monitoring are considered mandatory. The incidence of macroscopic hematuria and requirement for erythrocyte transfusion after PRB were reported in a systematic review to be 3.5% and 0.9%, respectively [3]. In general, most severe complications become evident within 12 hours after the biopsy [4], but no consensus has been established for the postoperative management protocol.
According to the Japanese guideline for PRB published in 2005,the recommendations for standard care after PRB include sandbagcompression at the puncture site for 2-8 hours and strict bed restfor 6-12 hours, with ambulation permitted after 18-24 hours [5].Although longer strict bed rest potentially contributes to hemostasisafter PRB, it may cause discomfort. A recently published paperreported that shortening strict bed rest from 7 to 2 hours couldreduce the patient’s discomfort, without an increase in the incidenceof bleeding or other biopsy-related complications [6]. On the otherhand, Simard-Meilleur et al. reported that 94% of complications wereidentified within 24 hours after PRB, and that an observation periodof less than 8 hours might miss 28% of complications [7].
The size of the biopsy needle may affect the extent of renalhemorrhage. Although 14- to 18-gauge biopsy needles are usuallyused for RPB, the selection is dependent on the operator’s preferenceor institutional policy. In general, a larger needle provides higherqualitysamples for diagnosis, but is considered to cause a higherincidence of hemorrhagic complications[3].
In 2007, we determined our standard institutional protocol forPRB, including: (1) using a 16-gauge biopsy needle, (2) monitoringwith bed rest in the supine position for 6 hours after PRB, (3) nosandbag compression at the puncture site, and (4) ambulationpermitted after 6 hours of bed rest if the vital signs are stable. In thisstudy, we verified the validity and safety of our protocol and identifiedthe risk factors for hemorrhagic complications.
Materials and Methods
Inclusion criteria and PRB procedure
This single-center retrospective study enrolled 175 consecutivepatients who received PRB of a native kidney at Sapporo MedicalUniversity Hospital from April 2007 through March 2016. This seriesdid not contain patients with a solitary kidney, and patients whounderwent PRB for a renal tumor were excluded from this study.
All patients were hospitalized and PRB was performed by urologicresidents under the supervision of attending urologists. Anti plateletand anticoagulant drugs were discontinued before biopsy accordingto the institutional requirement and, in some cases, continuousintravenous heparinization was performed until 6 hours prior to PRB.
The PRB procedure was performed as follows. First, the patientwas placed in the prone position. Then the bilateral kidneys werechecked by ultrasound (Hawk 2102 EXL; B-K Medical, Denmark). Akidney with easy visualization was selected as the target of PRB andthe puncture site to access the lower pole was determined. Next, thepuncture site was anesthetized with using local anesthesia with 1%xylocaine after disinfection of the skin with 10% povidone iodine anda 5-mm skin incision was made there. PRB was performed using a16-gauge automated biopsy needle (ACECUT®, 16G × 115 mm, TSKLABORATORY, Japan). The puncture was done three times or moreto obtain three sufficient cores of renal tissue. After the procedure,the puncture site was compressed manually for several minutes tocontrol active bleeding from the incision, and then the patient wasplaced in the supine position.
Care protocol after PRB
Strict bed rest in the supine position without sandbag compressionwas required for 6 hours. Routine in dwelling bladder catheterizationand administration of a hemostatic agent were not performed. Vitalsigns were checked at the time just after the biopsy, 1 and 6 hourslater, and when patients complained any kind of symptoms. After6 hours passed, patients without symptoms were allowed to turnover, elevate the head of the bed, and could walk for urination ordefecation. The next morning (after about 18-20 hours), patients werefree from restrictions except for heavy exercise and lifting of heavyweights if there were no problems with blood tests and vital signs.Routine ultrasound and computed tomography were not performedafter PRB. Anti platelet and anticoagulant drugs were resumed after2 or 3 days.
Definition of complications
Medical charts of enrolled patients were retrospectively reviewed,and all adverse events within 30 days after PRB were investigated. Theseverity of adverse events was graded according to the Clavien-Dindo(CD) classification [8]. In this system, any deviation from a normalpostoperative course without the need for intervention was classifiedas Grade I. Therefore, events/symptoms that required extendedbed rest for over 6 hours were defined as Grade I. In addition,any situation requiring blood transfusion, so-called hemorrhagiccomplications, was classified as Grade II. Grade III was for thoserequiring surgical, endoscopic or radiological intervention and IIIawas not under general anesthesia. In the present study, the onsettime of a hemorrhagic complication was defined as the time whenthe patient showed the following symptoms or signs: gross hematuria,severe back pain, nausea or vomiting, hypotension, and tachycardia.
Definition of complications
Ethical approval
All procedures performed in studies involving human participantswere in accordance with the ethical standards of the institutionalresearch committee at which the studies were conducted (Sapporo Medical University no. 282-44) and with the 1964 Helsinki declarationand its later amendments or comparable ethical standards.
Informed consent
Since this was an observational, but not prospective, interventionstudy, the Ethics Committee provided a waiver of written informedconsent. We announced the commencement of this study on ourwebsite (http://web.sapmed.ac.jp/uro/) with the proviso that thepatients who participated in this study could withdraw later.
Statistical Analysis
The patients’ characteristics are presented using the median andinterquartile range (IQR) for continuous variables. The estimatedglomerular filtration rate based on serum creatinine (eGFRcreat)was calculated as follows: eGFRcreat (mL/min/1.73 m2) = 194 ×serum creatinine (sCr)-1.094 × age-0.287 (in female: × 0.739) [9].Comparisons between two groups were performed using Fisher’sexact test and the chi-square test. P values of less than 0.05 wereconsidered statistically significant. All statistical analyses wereperformed with EZR statistical software (Saitama Medical Center,Jichi Medical University) [10], which is a graphical user interface forR (The R Foundation for Statistical Computing, version 2.13.0). Moreprecisely, it is a modified version of R Commander (version 1.6-3)that includes the statistical functions frequently used in biostatistics.
Results
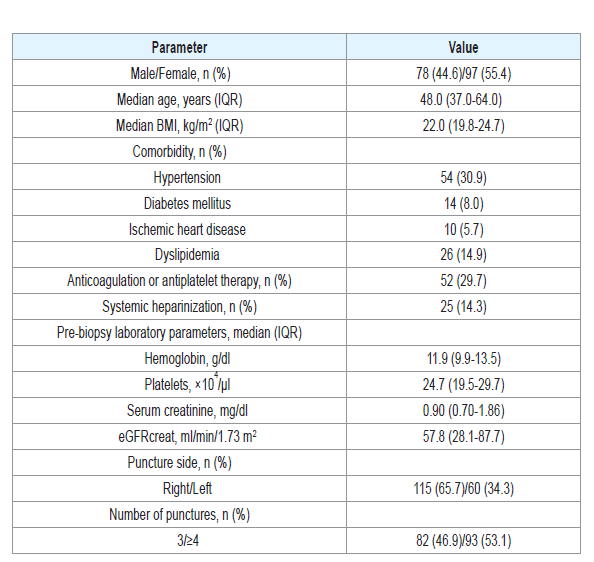
The characteristics of the patients are shown in (Table1). Themedian age of the 174 patients was 48.0 years (IQR 37.0-64.0), and55.4% were female. The median eGFRcreat was 57.8 mL/min/1.73m2 (IQR 28.1-87.8). The numbers of punctures in a single session ofPRB to obtain three sufficient cores for evaluation were 3, 4, 5 and 6or more in 46.6%, 31.0%, 10.3% and 12.0%, respectively (median 4,range 3-12). A median of 19 glomeruli (IQR 12-29) were present and assessable in each core specimen.
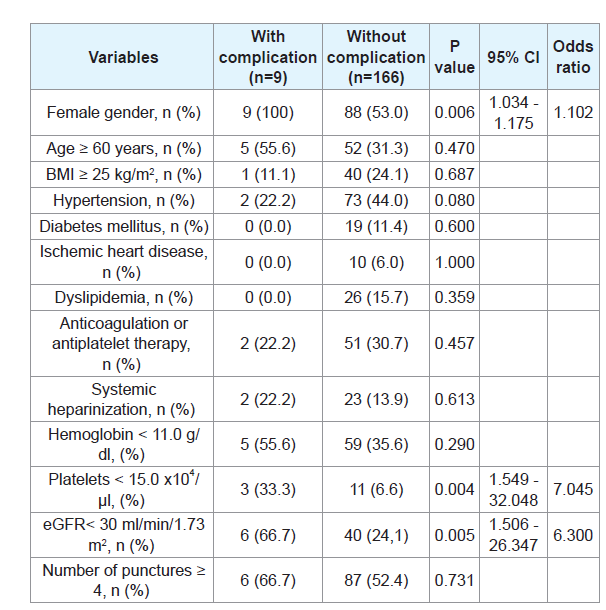
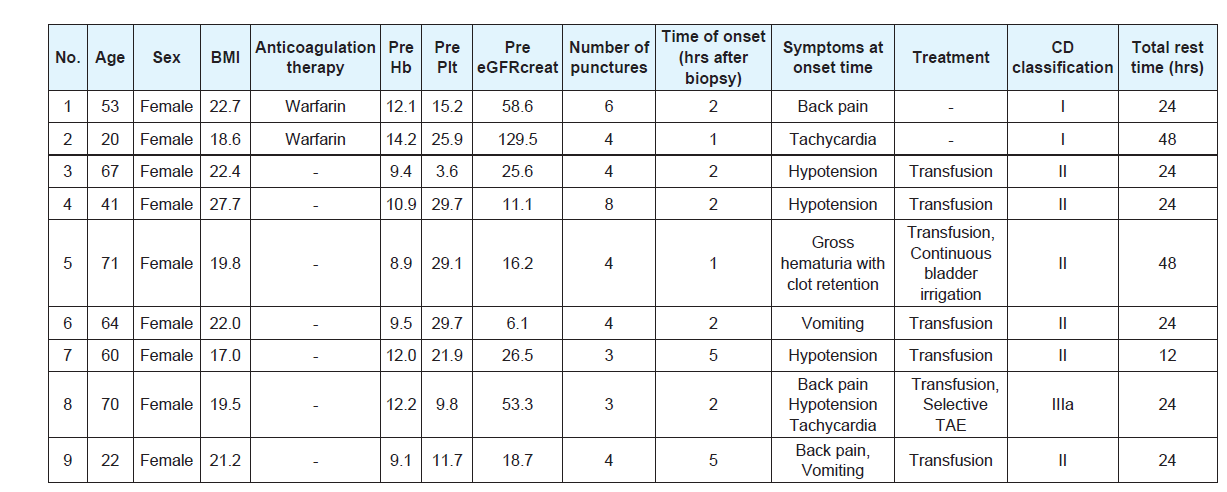
There were no grade IIIb or more complications associated withPRB according to the CD classification in this cohort. In addition,there was no complication other than hemorrhagic complications.Hemorrhagic complications requiring extended bed rest over 6 hourswere observed in 9patients (5.1%). Six patients (3.4%) had grade II(requiring transfusion) and 1 patient (0.6%) had grade IIIa (treatedwith percutaneous selective transcatheter arterial embolization). Inunivariate analysis, female gender, a platelet count < 15.0 × 104/μl andeGFRcreat < 30 mL/min/1.73 m2 were significantly associated with the development of hemorrhagic complications (Table 2). Details ofthe patients with hemorrhagic complications are shown in (Table 3). Inall patients these events were diagnosed within 6 hours after PRB. Thetotal times of bed rest after PRB were 12, 24 and 48 hours for 1, 6, and2 patients, respectively.
Discussion
The purposes of strict bed rest after PRB are: (1) to avoidexpansion of renal hemorrhage and (2) to monitor symptomsand signs such as gross hematuria, flank pain, hypotension andtachycardia, which are suggestive of active renal hemorrhage. Inthose with unstable hemorrhage, further careful monitoring withstrict bed rest is needed and blood transfusion and interventionmust be considered. Conventional standard management after RPBincludes 24 hours of bed rest after the biopsy in both the inpatientand outpatient settings [4]. In Japan, PRB is commonly performed inthe inpatient setting. The standard post-PRB care recommendationincludes compression of the biopsy site with sandbags or a bandagefor 2-8 hours, and strict bed rest for 6-12 hours. Moreover, patientsremain in bed for an additional 12 hours and are finally allowed tostand and walk 18-24 hours after RPB [5]. However, controversyhas emerged regarding the optimal duration of strict bed rest andmonitoring. Several studies showed that outpatient observation for6-8 hours was safe enough [7,11,12]. On the other hand, prolongedbed rest after the procedure is not always significantly safe [13]. In2009, Ishikawa et al. reported that no significant differences wereobserved between 2 and 7 hours of strict rest with respect to severebleeding complications or progression of anemia after PRB [6]. Intheir series, only 2 of 94 patients (2.1%) who were put on strict restin the decubitus position for 2 hours after PRB needed transfusionor hemostatic intervention. Therefore, strict rest for a long durationafter PRB may not be required to avoid expansion of hemorrhage.In our series, the incidence of transfusion or intervention was 4.0%,which is comparable with other reports [7,14]. Moreover, all of theevents were detected within 6 hours after the procedure. These resultssuggest that strict bed rest with monitoring for 6 hours after PRB is reasonable and that patients can be allowed to be out of bed after 6hours of bed rest safely if they have stable vital signs with no specificsymptoms.
Frequent, strict monitoring of vital signs and renal hemorrhageis employed in some institutions. Whittier et al. checked vitalsigns every 15 minutes for 2 hours, every hour for 4 hours, every2 hours for 6 hours, and then every 4 hours up to 24 hours post-PRB [14]. Moreover, single voided urine was visually inspected forgross hematuria, and hemoglobin levels were checked at 5-8, 10-13and 18-20 hours post-RPB. Routine ultrasound examination wasperformed at 1 hour after PRB. In contrast, our study adopted asimple protocol. Vital signs were routinely checked only at 1 and 6hours after PRB unless the patient complained of specific symptoms.Routine ultrasound examination was not done and a blood test wasonly performed the next morning. Even so, we did not overlookclinically significant hemorrhagic complications, suggesting that oursimple protocol was safe and sufficient for post-PRB management.Furthermore, discomfort associated with frequent blood drawing andexamination should be avoided as much as possible.
In recent review articles, hemorrhagic complications associatedwith PRB of native kidneys were reported to occur in 3.0-8.0% ofcases [3,15].In addition, the transfusion rates were said to be 0.9-6.3% [7,16]. However, the exact incidence and severity of the complicationswas unclear because the evaluation methods for complicationsassociated with PRB were different among the reports. To evaluatethe incidence and severity of postoperative complications, the CDclassification has generally been used [17,18]. This classification mayalso be applicable for evaluation of post-PRB complications.
Age, female gender, elevated blood pressure, a low baselinehemoglobin level and platelet count, elevated sCr (the same as loweGFRcreat), and prolonged bleeding time were reported to be riskfactors for hemorrhagic complications after PRB [1,15,19-21]. Ina meta-analysis by Corapi et al. age, female gender, hypertension,increased sCr and the use of a 14-gauge needle were independentlyassociated with hemorrhagic complications [3]. Although multivariateanalysis could not be performed due to the small number of eventsin the present study, our results are in accord with other reports.We should thus anticipate hemorrhagic complications needingtransfusion and interventions after PRB, especially in cases with suchrisk factors, and provide sufficient information to the patients beforePRB. Furthermore, we should also consider open or laparoscopicbiopsy that can secure certain hemostasis during the procedure insuch cases.
The transfusion rate in our series was comparable with those inother reports where a 16-gauge needle was mainly used [7,14,16].However, the rate might be slightly higher than that in a contemporaryreport, in which an 18-gauge needle was used [6]. The decision fortransfusion is dependent on physicians and the patients conditionsare not always uniform. Transfusion might have been over treatmentin some cases of our series. On the other hand, the size of the needlemight affect the incidence of hemorrhagic complications [3]. Weobtained a sufficient number of glomeruli in each specimen. Thequality of samples might be quite high as compared with other reports[7,22]. Renal biopsy is an essential examination for the diagnosis and management of patients with renal disease. Therefore, highqualitysamples should be secured as long as the procedure can bedone safely. Simard-Meilleur et al. reported that an 18-gauge needleprovided a significantly fewer glomeluri in a specimen, althoughthere was a trend toward a lower rate of symptomatic hematomas [7].Considering the above and our findings together, usage of 16-gaugeneedle may be beneficial.
There were some limitations in this study. First, the number ofevents was small due to the small sample size. Therefore, independentrisk factors for hemorrhagic complications could not be determined.Second, this study did not have a control group, so the superiority ornon-inferiority of our PRB management protocol could not be tested.Third, the retrospective single center nature of this study limits itsgeneralizability. Therefore, a multicenter prospective randomizedcontrolled study with larger populations may be required to confirmthe validity of the protocol.
Conclusion
In conclusion, the incidence of complications associated withPRB using a 16-gauge needle was 5.1%, and female gender, a lowplatelet count and low eGFRcreat were significant risk factors in ourseries. Hemorrhagic complications of grade II and III were observedin 3.4% and 0.6% of the cases, respectively, all of which were detectedwithin 6 hours post-RPB. All biopsy specimens contained a sufficientnumber of glomeruli. These results suggest that our 6-hour strictbed-rest protocol after 16-gauge needle PRB is safe and reasonable.
Conflicts of interest statement and funding
Koji Ichihara has received Grants from Kyowa Hakko Kirin Co.,Ltd. (KHKS20160517012).
Acknowledgements
The authors thank Mr. Kim Barrymore for Englishproofreading of this manuscript.
References
- Eiro M, Katoh T, Watanabe T (2005) Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol 9: 40-45.
- Kim D, Kim H, Shin G, Ku S, Ma K, et al. (1998) A randomized, prospective, comparative study of manual and automated renal biopsies. Am J Kidney Dis 32: 426-431.
- Corapi KM, Chen JL, Balk EM, Gordon CE (2012) Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 60: 62-73.
- Marwah DS, Korbet SM (1996) Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? Am J Kidney Dis 28: 47-52.
- Nishi S (2005) Prescription of bed-rest after renal biopsy. Nihon Jinzo Gakkai Shi 47: 491-496.
- Ishikawa E, Nomura S, Obe T, Katayama K, Oosugi K, et al. (2009) How long is strict bed rest necessary after renal biopsy? Clin Exp Nephrol 13: 594-597.
- Simard-Meilleur MC, Troyanov S, Roy L, Dalaire E, Brachemi S (2014) Risk factors and timing of native kidney biopsy complications. Nephron Extra 4: 42-49.
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213.
- Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992..
- Kanda Y (2012) Free statistical software: EZR (Easy R) on R commander.
- Lin WC, Yang Y, Wen YK, Chang CC (2006) Outpatient versus inpatient renal biopsy: a retrospective study. Clin Nephrol 66: 17-24.
- Maya ID, Allon M (2009) Percutaneous renal biopsy: outpatient observation without hospitalization is safe. Semin Dial 22: 458-461.
- Allen C, Glasziou P, Del Mar C (1999) Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 354:1229-1233.
- Whittier WL, Sayeed K, Korbet SM (2016) Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin Kidney J 9: 102-107.
- Tondel C, Vikse BE, Bostad L, Svarstad E (2012) Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 7: 1591-1597.
- Stratta P, Canavese C, Marengo M, Mesiano P, Besso L, et al. (2007) Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest 37: 954-963.
- Losa A, Gadda GM, Lazzeri M, Lughezzani G, Cardone G, et al. (2013) Complications and quality of life after template-assisted transperineal prostate biopsy in patients eligible for focal therapy. Urology 81: 1291-1296.
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, et al. (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250: 187-196..
- Korbet SM, Volpini KC, Whittier WL (2014) Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol 39: 153-162.
- Manno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, et al. (2004) Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66: 1570-1577.
- Whittier WL, Korbet SM (2004) Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15: 142-147.
- Torres Muñoz A, Valdez-Ortiz R, González-Parra C, Espinoza-Dávila E, Morales-Buenrostro LE, et al. (2011) Percutaneous renal biopsy of native kidneys: efficiency, safety and risk factors associated with major complications. Arch Med Sci 823-831.