Journal of Toxins
Download PDF
Research Article
Preliminary Studies of the Toxicity of Agaricus Bisporous Aqueous Enzymatic Extracts (AbAEE) In Rats
Carbonero-Aguilar P1, Falcón-García G1 Gallego-Yerga P1, del Campo JA2, Isabel Moreno N 3* and Bautista J
- 1Department of Biochemistry and Molecular Biology, University of Sevilla, Spain
- 2UCM Digestive Diseases & CIBERehd, Valme University Hospital, Spain
- 3Area of Toxicology, University of Sevilla, Spain
*Address for Correspondence: Isabel Moreno N, Department of Toxicology, Faculty of Pharmacy, University of Sevilla, C/ Profesor García González n° 2, 41012, Sevilla, Spain; Tel: +34954 556762; Fax: +34 954 233765; E-mail: imoreno@us.es
Citation: Carbonero-Aguilar P, Falcón-García G, Gallego-Yerga P, del Campo JA, Isabel Moreno N, et al. Preliminary Studies of the Toxicity of Agaricus Bisporous Aqueous Enzymatic Extracts (AbAEE) In Rats. J Toxins. 2018;5(1): 7
Copyright: © 2018 Carbonero-Aguilar P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 5, Issue: 1
Submission: 21 February, 2017| Accepted: 26 March, 2017 | Published: 30 March, 2017
Submission: 21 February, 2017| Accepted: 26 March, 2017 | Published: 30 March, 2017
Abstract
The health benefits of extracts from Agaricus bisporus greatly extend its use as components of new functional foods for the treatment and prevention of diseases, rather than restricting it to a good food with high nutritional value and good flavor. A. bisporus aqueous enzymatic extracts (AbAEE) have unique flavor, good taste, and health benefits which makes it a good candidate for its incorporation in different matrices for development of new functional foods and nutraceuticals. The potential use of AbAEE as nutraceutical or incorporated as a part of new functional foods requires its characterization and a subsequent food safety study. The focus of this paper was to carry out a preliminary toxicological study on experimental animals (rats in this case) by acute and sub-chronic oral administration. To evaluate potential adverse effects of AbAEE at high doses the acute toxic class method was used. After administration of the preset doses, behavior changes, toxic symptoms, and deaths were observed continuously for 1 h after treatment and then intermittently at 4th, 8th and 24th h. After these initial observations, the rats were further observed for up to 14 consecutive days for any signs of toxicity and/or death. Because of the likelihood that the results obtained in this preliminary acute toxicity test have a direct relevance for protecting human health with respect to the use of AbAEE as a functional food or nutraceutial, an additional upper dose level of 5000 mg/kg b.wt. was used. Since no death was observed, the LD50 could not be estimated but is expected to exceed 5000 mg/kg and this extract could be classified as hazard category 5 (in Globally Harmonized Classification System for Chemical Substances and Mixtures.) or even unclassified for its acute toxicity by the oral route. The sub-chronic oral toxicity study of AbAEE (250 and 500 mg/kg b.wt. day) did not induce significant alterations in almost all hematological and biochemical parameters in rats. Therefore, the overall results of the present study provide supportive data on the use of AbAEE and provide a promising first step for their use as component of new functional foods or as nutraceutical. Though, further studies in both, animals and humans are needed for better evaluation of the food safety of this extract.
Keywords
Agaricus bisporous; AbAEE; Acute toxicity; Chronic toxicity; Rats
Introduction
Traditionally, mushrooms have been used to improve human’s health [1], among other uses. In this respect, attention has been focused mainly on exotic mushrooms rather than on edible mushrooms. Recently, health benefits of extracts from Agaricus bisporus (one of the most consumed edible mushroom worldwide), such as antioxidant activity, immunoprotection, anticholesterolitic and antiviral activity, have been described [2-5]. These properties greatly extend the use of A. bisporus and its extracts as components of new functional foods for the treatment and prevention of diseases, rather than restricting its use to a good food with high nutritional value and good flavor [6].
Given the increasing utilization of phytonutrients with health benefits in functional foods formulation and as nutraceuticals, the safety of these products has become a major concern. A. bisporus aqueous enzymatic extracts (AbAEE) have unique flavor, good taste, and health benefits which makes it a good candidate for its incorporation in different matrices for development of new functional foods and nutraceuticals. AbAEE contains a high number of bioactive components which could be toxic for humans at high concentrations [7]. However, to the best of our knowledge, the toxicological evaluation of AbAEE had not been studied yet.
The potential use of AbAEE as nutraceutical or incorporated as a part of new functional foods requires its characterization and a subsequent food safety study. Characterization of the main components of AbAEE was addressed by the authors -using Liquid Chromatography-Quadrupole Time-of-Flight-Tandem Mass Spectrometry (LC-QTOF MS/MS) - and recently published [8]. A logical subsequent step was a preliminary toxicological study on experimental animals (rats in this case) by acute and sub-chronic oral administration, looking for establishing a range of allowed concentration, as a first step for application to humans.
Materials and Methods
Mushroom and AbAEE preparation
A. bisporus was supplied by the Grupo Riberebro (Alfaro, La Rioja, Spain), and the AbAEE were obtained according to the process described by Cremades et al. [2]. Briefly, after homogenization, and enzymatic digestion, the hydrolyzate was centrifuged at 8000 × g, resulting in a supernatant and a solid residue. The residue (insoluble material) was discharged, and the supernatant was collected and used as raw extract, which was ultra filtered through a 50 kDa ultra filtration membrane (Sartorius Gmbh, Germany) and concentrated by vacuum at 75 °C 10-fold to obtain the AbAEE.
Experimental animals
Wistar rats weighting 150±20 g for male and 121±18 g for female were used for acute toxicity studies, and rats weighting 103±12 g for male and 94±8 g for female were used for sub-chronic toxicity studies. The rats were supplied by the Central Animal Facility of the University of Seville (Espartinas, Seville, Spain).The animals were allowed to acclimate to the housing conditions for one week and healthy animals were selected for the study.
Four animals per stainless steel cage were housed throughout the study period. Tap water and pellet food for rodent (Panlab, Barcelona) were provided ad libitum. The housing conditions were: temperature 23±2 °C, relative humidity 50-70%, air ventilation 10-20 times/h and light intensity 150-300 lux with 12 h light-dark cycles. All experiments were performed in accordance with the guidelines of the ethic committee of the University of Seville (Spain).
Acute toxicity study
Acute toxicity test was carried out to evaluate any possible adverse effects occurring following oral administration of a single dose of AbAEE. For this study the acute toxic class method was used. This procedure is reproducible and uses very few animals. This method is based in biometric evaluations with fixed doses, adequately separated to enable a substance to be ranked for classification purposes and hazard assessment [2]. Twenty four rats were randomly divided into four groups: one control group (ACT-0) and three treated groups (ACT I-to-III). Each group was composed by 6 rats, 3 per sex (Table 1). After fasting for 12 h, the rats were orally administrated (by gavages) only once with AbAEE dissolved in 2-3 ml of distilled water, at doses 300, 2000 and 5000 mg/kg body weight (b.w.); and the control group was treated with distilled water (10 ml/kg b.w.). After administration of the preset doses, the rats were allowed access to food and water ad libitum, and behavior changes, toxic symptoms, and deaths were observed continuously for 1 h after treatment and then intermittently at 4th, 8th and 24th h. After these initial observations, the rats were further observed for up to 14 consecutive days for any signs of toxicity and/or death. Any adverse effects, such as hypoactivity, fur-erection, salivation, and syncope were evaluated immediately after administration of the different doses of AbAEE. Body weights were measured on day 1, 7 and 14, food and water intake were registered every two days and anorexia and weight loss were recorded. All test animals were subjected to gross necropsy and microscopic examination.
Sub-chronic toxicity study
The 90-day study provides information on the possible health hazards likely to arise from repeated exposure over a prolonged period of time covering post-weaning maturation and growth well into adulthood. The study will provide information on the major toxic effects, indicate target organs and the possibility of accumulation, and can provide an estimate of a no-observed-adverse-effect level of exposure which can be used in selecting dose levels for chronic studies and for establishing safety criteria for human exposure [10].
Sixty rats were randomly divided into three groups: one control group (SCT-0) and two treated groups (SCT I and SCTII), each group integrated by 20 rats, ten per sex (Table 2). Rats in treatment groups were orally administrated with AbAEE at doses 0,250, and 500 mg/kg day for 90 days (13 weeks). Rats in the control group were administrated orally with only water, which was used as a vehicle. According to Wang et al. [11], the higher dose (500 mg/kg) should be estimated as 10% of LD50. In our case, and because estimation of the LD50 was unable, the highest dose assayed for acute toxicity (5000 mg/kg) was used as reference for estimation. Thirty six rats (18 males and 18 females), six per group and sex, were sacrificed at the end of day 90 (13 weeks). The remaining rats in each group were continuously observed during the days following day 90 for four weeks; then sacrificed at the end of day 119 (17 weeks). Blood samples were collected for hematological and biochemical analyses. Body weight, food and water consumption were recorded once a week during the experimental period.
Measurement of blood parameters
The rats were anesthetized with sodium pentobarbital by intraperitoneal injection, and the blood samples were obtained through abdominal aorta puncture and collected into vials containing sodium citrate for complete blood analysis. Hematological and serum biochemical analyses were performed for 12 rats/group, 6 rats/sex at the end of treatment period (90 days), and 8 rats/group, 4 rats/sex at the end of the recovery period (21 days).
Hematological analysis: The following hematological parameters were analyzed: White Blood Cell (WBC), Red Blood Cell (RBC), Hematocrit (HCT), Hemoglobin (HGB), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Lymphocytes (LYM), Platelet (PLT), Monocytes (MON), Neutrophil Granulocytes (NEUT), Basophils (BAS), Eosinophils (EOS), and Reticulocyte (Ret) were measured using a fully automatic hematology analyzer (Roche Cobas 6000, USA). The count of Ret was calculated by examining the stained blood smears under a light-microscope (Leica DC-300, Germany).
Coagulation parameters, including Activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT), were analyzed using a semiautomatic blood coagulation-analyzer (Maccura BCS-04).
Biochemical analysis: Biochemical analyses were carried out in serum. Serum samples were obtained by centrifugation at 1.500 x g for 15 min, and stored at -20 °C until analysis. The biochemical parameters of serum, including Alanine Transaminase (ALT), Aspartate Transaminase (AST), Alkaline Phosphatase (ALP), Albumin (ALB), Globulin (GLB), Albumin-Globulin Ratio (A/G), Total Protein (TP), Total Bilirubin (TBIL), Creatinine (CREA), Uric Acid (UA), Urea (UREA), Creatine-Kinase (CK), Triglyceride (TG), Total Cholesterol (TCHO), Lactate Dehydrogenase (LDH), and Glucose (GLU), were determined by a fully automatic biochemistry analyzer (Beckman Coulter-AU480, USA). Na+, K+, Ca2+, and Cl- were determined by an electrolyte analyzer (Roche-AVL9181, USA).
Organ weight and histopathology analysis
Animals were sacrificed after blood collection. Necropsies were performed on all animals at the end of the treatment and recovery periods. The major organs, including heart, spleen, liver, lungs, kidneys, brain, thymus, adrenal gland, uterus, ovary, and testis, were isolated, and absolute organ weights were determined.
For histopathology analysis, only organs of the high dose group (0.75 g/kg) and control group of both sexes were examined, providing no abnormal changes appeared in the treatment group after macroscopic examination. All organs samples (4 μm thick) were fixed in 10% neutral formalin buffer and stained with hematoxylineosin dye. The pathological changes were observed under a lightmicroscope (Leica DC-300, Germany), and the histopathological analysis was conducted by a pathologist who was blind to provenance of the samples.
Statistical analysis
The results are presented as mean value ± standard deviation (SD). Statistical analyses were performed using a 2-way Analysis of Variance (ANOVA) with a subsequent Bonferroni post hoc test for pair wise comparisons between various combinations of two groups. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using the SPSS 16.0 software.
Results and Discussion
AbAEE could be considered as a functional food or nutraceutial per se or as component of formulations thanks to its health benefits. This extract not only provides nutrients (carbohydrates, proteins, vitamins and minerals) but also it has a unique profile of bioactive components with health benefits, especially those with antioxidant and antiviral activity [2-4].
AbAEE is a complex extract that mainly contains polar compounds due to its aqueous nature [12], but also no polar compounds could be present. The composition of AbAEE as determined by Delgado-Povedano et al. [8] by high quality MS/MS spectra obtained by LCQTOF MS/MS allowed identification of one hundred twenty-three compounds which were divided into 21 groups, and among which fifty five were described for the first time, including alkaloids, amino acids and derivatives, carboxilic acids and derivatives, mono-and disaccharides, sugar acids and derivatives, fatty acids and conjugates, phospholipids and derivatives, and nucleotides and derivatives, among the most significant.
Most of the so far known compounds in AbAEE are safe; however, some of them have in the past been considered toxic at relatively low concentration, in particular agaritine, a naturally occurring phenylhydrazine derivative present in wild and cultivated Agaricus mushroom species, including the cultivated mushroom A. bisporus. Agaritine has been described in some studies as a potential carcinogen; however, the available evidence to date shows that agaritine of cultivated A. bisporus mushrooms poses no known toxicological risk to humans [13].
To evaluate the safety of AbAEE as a potential dietary supplement for human consumption (nutraceutical), data regarding acute (LD50) and chronic toxicity are needed to establish potential hazardous effects derived from repeated exposure over a long period of time.
Although in silico search on TOXNET Database for potential toxicity of the 95 compounds found in AbAEE reveals no potential toxicity [14], we decide to test the safety of AbAEE in experimental animals both exposed only once to a very high dose (acute toxicity), and to a relative high dose (sub-chronical toxicity) for long exposure (90 days).
Acute toxicity study of AbAEE
To evaluate potential adverse effects of AbAEE at high doses, an acute toxicity test was carried out. As there was no information on AbAEE about mortality, for animal welfare reasons it was recommended to use the starting dose of 300 mg/kg of bodyweight. In Table 3 is shown the effects and general behavioral changes of the rats following oral administration of AbAEE at the doses of 0.00 (controls), 300 and 2000 mg/kg. The results showed that AbAEE, at 2000 mg/kg b.wt., did not cause mortality or any sign of adverse effects during the 14 days of study, and no abnormal clinical symptoms in fur, eye color, asthenia, anorexia, salivation, piloerection, locomotors activity or diarrheal in all the treated animals, were observed. The body weight and food consumption also remain unchanged when compared to the untreated control group. The internal organs, including liver, kidney, brain, heart, lung, spleen and thymus, of control and treated groups do not show any unusual signs and were found normal in both size and color. It is necessary to take into account the likelihood that the results obtained in this preliminary acute toxicity test have a direct relevance for protecting human health with respect to the use of AbAEE as a functional food or nutraceutial. For this reason and exceptionally, an additional upper dose level of 5000 mg/kg b.wt., was used. Since no death was observed, the LD50 could not be estimated but is expected to exceed 5000 mg/kg. Therefore, it can be admitted that acute toxicity of AbAEE by the oral route can be classified as category 5 or even unclassified in the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). In conclusion, it can be said that the absence of signs of morbidity and mortality in the oral dose study is an evidence of the safety of the short-term oral administration of AbAEE.
Sub-chronic toxicity study of AbAEE
The assessment and evaluation of sub-chronic oral toxicity of AbAEE was carried out in an attempt to obtain information on the possible health hazards caused by repeated exposure over long periods of time. The study was carried out using animals covering postweaving maturation and growths well into adulthood. No treatment related abnormalities in clinical and behavioral signs or deaths, related with AbAEE treatment, were observed during treatment and recovery periods at any of the assayed doses (250 and 500 mg/kg b.wt.). All animals in the control and AbAEE treated groups survived until the scheduled necropsy on days 90 and 119.
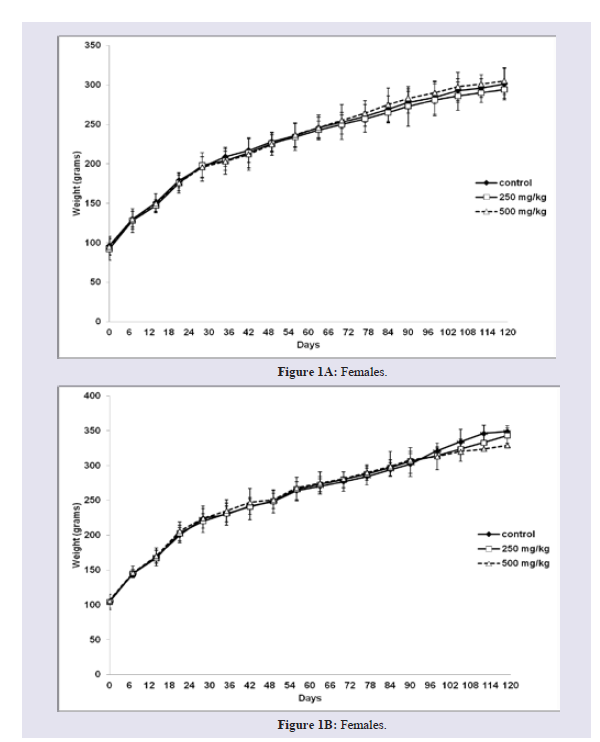
Effects of sub-chronic oral administration of AbAEE on the body weight, food and water consumption: As shown in Figure 1A, no significant difference (p>0.05) in the body weight of female rats was observed between AbAEE-treated groups and the control group at the end of both treatment and recovery periods. However, the body weight of male rats subjected to the higher dose (500 mg/ kg) was slightly lower than that of the control groups during the recovery period (29 days) 329±11 g and 349±8 g, respectively (Figure 1B). No statistically significant difference was observed in food and water consumption between AbAEE-treated groups and the control group at the end of both treatment and recovery periods (data not shown). The fact that body weight, water and food intakes were not altered during the treatment and recovery periods suggests no serious toxic effects of oral administration of AbAEE. As the changes in body weight have been used as an indicator of adverse effects of drugs and chemicals [15,16], the present results suggest that 500 mg/kg of AbAEE as oral doses are non-toxic in Wistar rats.
Figure 1: Body weight of rats (female and males) observed in AbAEE treated groups (250 and 500 mg/kg) and the control group at the end of both treatment (90 days) and recovery period (119 days).
Effects of sub-chronic oral administration of AbAEE on blood parameters: At the end of the treatment and recovery periods, blood samples were collected and hematological, coagulation and biochemical parameters of both control and treated groups were measured.
- Effects of AbAEE on hematological parameters
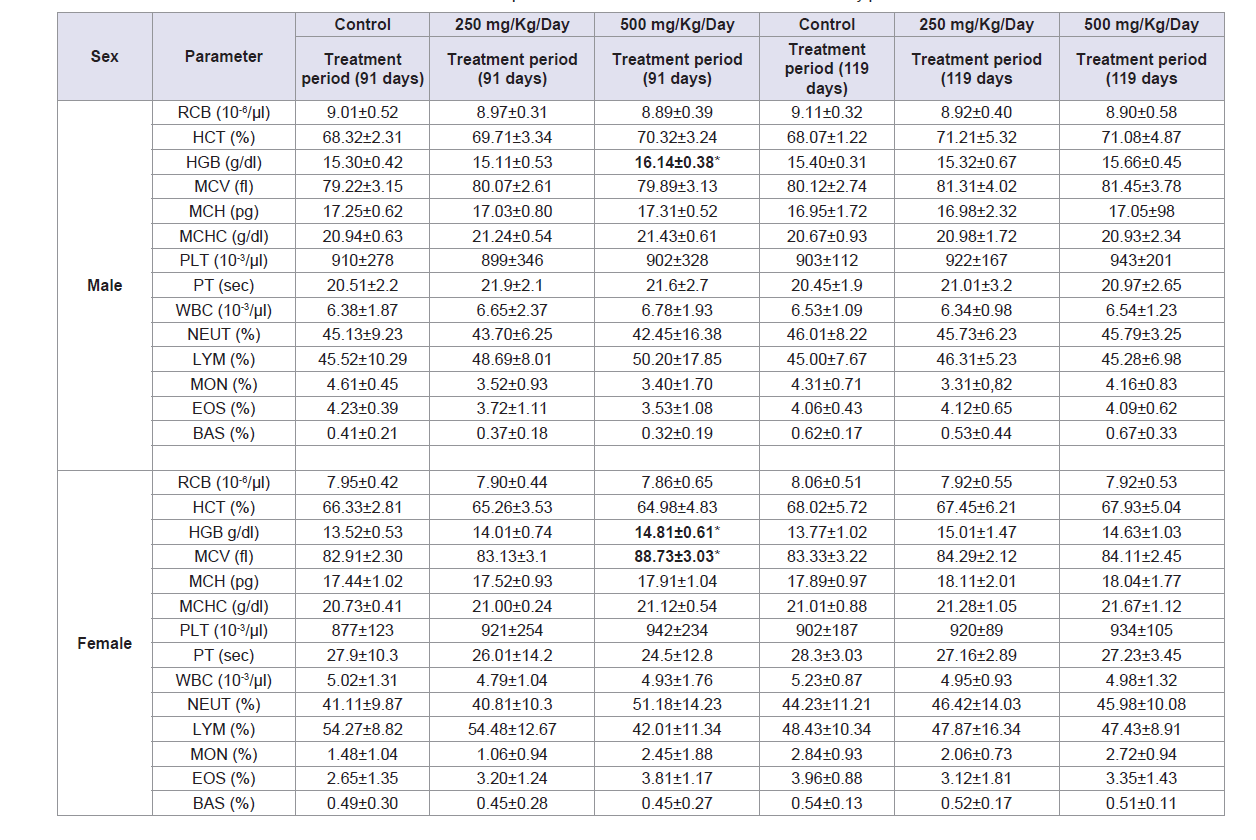
The effect of sub-chronic oral administration of AbAEE on the hematological parameters at the end of treatment and recovery periods is summarized in Table 4. As compared with the control group, most of hematological parameters measured in the treated animal were not significantly different. Statistically significant differences were observed only for HGB in male rats and in HGB and MCV in female rats at the dose of 500 mg/kg day at the end of the treatment period. On the contrary, no significant difference was observed in AbAEE-treated groups for female and male rats compared with the control group at the end of the recovery period.
Table 4: Effect of sub-chronic oral administration of AbAEE on the hematological parameters in Wistar rats after 91 days of treatment and 28 days of recovery.
Hematological parameters, together with coagulation and biochemical parameters, are relevant to evaluate risks as changes in the hematological system. Also, they are of capital importance as the most sensitive targets for toxic chemicals and as key indices of physiological and pathological status in humans when the data are extrapolated from animal studies [17,18]. In this study with the exception of a short temporary increase in HGB in male rats and in HGB and MCV in female rats at the dose of 500 mg/kg day, no significant differences were found in hematological parameters at the end of the treatment period as compared with the control group; as also didn’t at the end of the recovery period; therefore, the results suggest that AbAEE has no influence on these parameters in rats.
- Effects of AbAEE on coagulation parameters
No statistically significant differences in coagulation parameters (APTT and PT) were observed between control and AbAEE-treated groups for female and male rats at the end of both treatment and recovery periods (data not shown).
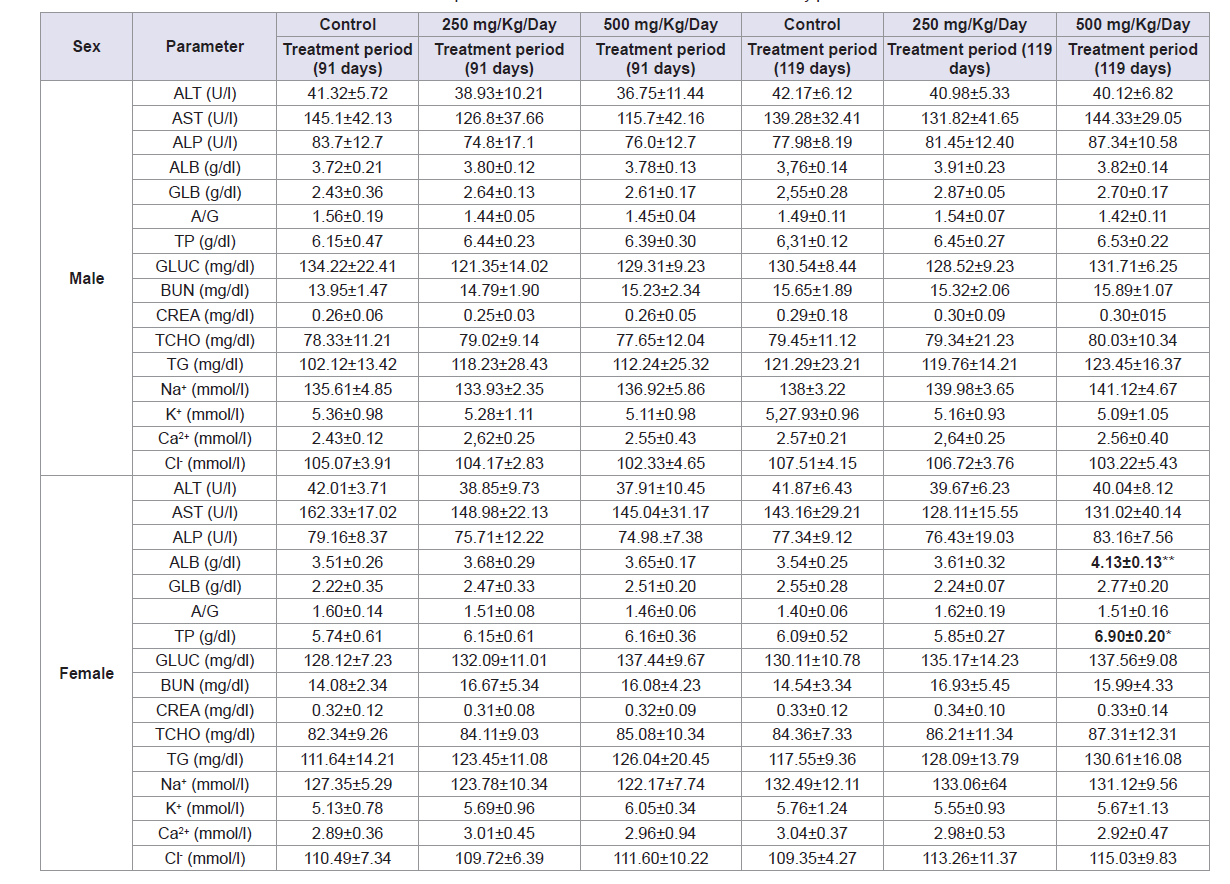
- Effects of AbAEE on biochemical parameters
Table 5 shows the values of the biochemical parameters at the end of the treatment period, at which all biochemical parameters tested were not significantly different between the control and AbAEE treated groups for female and male rats. Same results were observed at the end of the recovery period with the exception of a statistically significant decrease in female rats between the control group and the 500 mg/kg AbAEE-treated group in two parameters: ALB: 3.54±0.25 and 4.13±0.13 g/dL, respectively, (p <0.01) and TP: 6.09±0.52 and 6.90±0.20 g/dL, respectively, (p <0.05).
- Effects of AbAEE on blood electrolytic parameters
No significant differences were observed between the control and AbAEE-treated groups for female and male rats in blood electrolytic parameters at the end of both treatment and recovery periods (Table 5).
Table 5: Effect of sub-chronic oral administration of AbAEE on biochemical parameters at the end of treatment and recovery periods.
Effects of sub-chronic oral administration of AbAEE on organ weight: Statistically significant decreases between the control and 500 mg/Kg AbAEE-treated group were observed in female rats for absolute brain weight (2.04±0.11 g and 1.91±1.63 g, respectively, p < 0.05) and relative thymus weight (0.28±0.07 g and 0.25±0.03 g, respectively, p < 0.05), at the end of the treatment period. For male rats, a statistically significant decrease between control and 500 mg/Kg AbAEE-treated group was observed for thymus weight (0.40±0.08 g and 0.30±0.07 g, respectively, p < 0.05), whereas the adrenal gland weight was statistically significant increased (0.05±0.01 g and 0.06±0.03 g, respectively, p < 0.05). At the end of the recovery period, no significant difference was observed in AbAEE-treated groups for female and male rats in organ weights as compared with the control group.
Effects of sub-chronic oral administration of AbAEE on histopathological analysis: For the histopathological analysis, the organs, including heart, spleen, liver, kidneys, lungs, brain, thymus, testis, uterus, and ovary, were examined. No pathological changes were observed in both AbAEE-treated groups and the control group for rats at the end of both treatment and recovery periods (data no shown).
Conclusion
In conclusion, AbAEE cannot be classified for its acute toxicity, as they did not cause either lethality or adverse changes in the general behavior when the highest dose (5000 mg/kg) was administrated orally. Thus, this substance could be considered without acute toxicity. The sub-chronic oral toxicity study of AbAEE (250 and 500 mg/kg b.wt. day) only induced significant alterations in some hematological and biochemical parameters in rats. Therefore, the overall results of the present study provide supportive data on the use of AbAEE and provide a promising first step for their use as component of new functional foods or as nutraceutical. However, this is a preliminary study and further studies in both animals and humans are needed for better evaluation of this finding.
Acknowledgements
This work was supported by funds from the Spanish Ministerio de Economía y Competitividad and Fondos FEDER (Project: IPT-2011-1418-060000; Project: RTC-2015-4039-2). The authors thank Paula Bautista for assistance in the preparation of the manuscript.
JA Del Campo supported by Nicolás Monardes Program from Servicio Andaluz de Salud (SAS)
References
- Lee KH, Morris-Natschke SL, Yang X, Huang R, Zhou T, et al. (2012) Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional Chinese medicine. J Tradit Complement Med 2: 84-95.
- Cremades O, Diaz-Herrero MM, Carbonero-Aguilar P, Gutierrez-Gil JF, Fontiveros E, et al. (2012) Preparation and characterization of selenium-enriched mushroom aqueous enzymatic extracts (MAEE) obtained from the white button mushroom (Agaricus bisporus). Food Chem 133: 1538-1543.
- Cremades O, Diaz-Herrero MM, Carbonero-Aguilar P, Gutierrez-Gil JF, Fontiveros E, et al. (2015) White button mushroom ergothioneine aqueous extracts obtained by the application of enzymes and membrane technology. Food Biosci 10: 42-47.
- Gallego-Yerga P (2015) Study of the inhibition of the NS3 protease involved in the propagation of hepatitis C virus. Final Degree Project. Pharmacy faculty. Sevilla University.
- Gil-Ramírez A, Ruiz-Rodríguez Á, Marín FR, Reglero G, Soler-Rivas C (2014) Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J Funct Foods 11: 589-597.
- Mattila P, Konko K, Eurola M, Pihlava JM, Astola J, et al. (2001) Contents of vitamins, mineral elements, sand some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49: 2343-2348.
- Shu-YaoT, Tsai-Ping W, Shih-Jeng H, Jeng-Leun M (2007) Nonvolatile taste components of Agaricus bisporus harvested at different stages of maturity. Food Chem 103: 1457-1464.
- Delgado-Povedano MM, de Medina VS, Bautista J, Priego-Capote F, de Castro MD (2016) Tentative identification of the composition of Agaricus bisporus aqueous enzymatic extracts with antiviral activity against HCV: a study by liquid chromatography-tandem mass spectrometry in high resolution mode. J Funct Foods 24: 403-419.
- OCDE (2002) Test No. 423: Acute oral toxicity - acute toxic class method. In: OECD guidelines for the testing of chemicals, Section 4. Health Effects pp. 14.
- OCDE (1998) Test No. 408: Repeated dose 90-day oral toxicity study in rodents. In: OECD guidelines for the testing of chemicals, Section 4. Health Effects pp. 10.
- Wang QL, Li H, Li XX, Cui CY, Wang R, et al. (2012) Acute and 30-day oral toxicity studies of administered carnosic acid. Food Chem Toxicol 50: 4348-4355.
- Kalac P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113: 9-16.
- Roupas P, Keogh J, Noakes M, Margetts C, Taylor P (2010) Mushrooms and agaritine: A mini-review. J Funct Foods 2: 91-98.
- TOXNET Database. U.S. National Library of Medicine. National Institutes of Health, USA.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm 70: 135-145.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, et al. (2002) A 90- day oral gavage toxicity study of d-methylphenidate and d,l-methylphenidate in Sprague-Dawley rats. Toxicology 179: 183-196.
- Carrillo JC, Adenuga MD, McKee RH (2014) The sub-chronic toxicity of regular White Spirit in rats. Regul Toxicol Pharmacol 70: 222-230.
- Olson H, Betton G, Robinson D, Thomas K, Monro A, et al. (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32: 56-67.