Journal of Toxins
Download PDF
Research Article
Insecticide Reproductive Toxicity Profile: Organophosphate, Carbamate and Pyrethroids
Jose Martin-Reina1, José A. Duarte1, Lucas Cerrillos 2, Juan D. Bautista3 and Mohamed Mostafa Soliman1*
- 1Area of Toxicology, Faculty of Pharmacy, University of Sevilla, Sevilla, Spain
- 2Area of Obstetrics, Virgen del Rocío Universitary Hospital
- 3Biochemistry Department, Faculty of Pharmacy, University of Sevilla
*Address for Correspondence: Isabel Moreno, Area of Toxicology, Faculty of Pharmacy, University of Sevilla, Sevilla, Spain, E-mail: imoreno@us.es
Citation: Martin-Reina J, Duarte JA, Cerrillos L, Bautista JD, Moreno I. Insecticide Reproductive Toxicity Profile: Organophosphate, Carbamate andPyrethroids. J Toxins. 2017;4(1): 7
Copyright: © 2017 Martin-Reina J, et al. This is an open access articledistributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal ofToxins | ISSN: 2328-1723 | Volume: 4, Issue: 1
Submission: 18 April, 2017| Accepted: 10 May, 2017 | Published: 15 May, 2017
Submission: 18 April, 2017| Accepted: 10 May, 2017 | Published: 15 May, 2017
Abstract
Exposure to pesticides is very common world-wide, and is broadly known the acute toxic effects to humans of pesticides following a high dose exposure; however, knowledge about chronic low-dose adverse effects to specific pesticides is more limited. Reproductive functions can be affected, with birth defects, impaired fecund ability, infertility and altered growth. This paper will focus on the deleterious effects that may appear in the offspring, during early and later stages of life, after prenatal exposure to insecticides, not only on women with direct exposure but also on subjects with indirect exposure such as consumers or residents of rural communities. Prenatal exposure to pesticides could alter normal fetal development and could threaten future welfare. The main changes observed in prenatal exposure to organophosphates are alterations in the central nervous system, in the metabolic and hormonal system as endocrine disruptor and over the birth outcomes. Carbamates may cause developmental delay when the applications of carbamates during pregnancy were nearby the home. Pyrethroids are among the most frequently used pesticides and account for morethan one-third of the insecticides currently marketed in the world. For this reason the prenatal exposition used to be for long periods causing clinical, biochemical and neurological changes.
Keywords
Organophosphate; Carbamate; Pyrethroids; Prenatal exposure; Toxic effects
Introduction
Pesticides are defined as “chemical substances used to prevent, destroy, repel or mitigate any pest ranging from insects, rodents and weeds to microorganisms” [1]. The use of these chemicals in modern agriculture has significantly increased productivity. But it has also significantly increased the concentration of pesticides in food and in our environment, with associated negative effects on human health [2]. Exposure to pesticides is very common world-wide and is broadly known the acute toxic effects to humans of pesticides following a high dose exposure; however, knowledge about chronic low-dose adverse effects to specific pesticides is more limited [3]. Acute and delayed health effects, range from simple irritation of the skin and eyes to general malaise and chronic and long term severe effects on the nervous system. Reproductive functions can also be affected, with birth defects, impaired fecund ability, infertility and altered growth [4]. Currently, only two pesticides, arsenical insecticides and TCOD (a dioxin) have been designated by IARC as known human carcinogens, but many others with world-wide use are suspected human carcinogens [1].
This paper will focus on the deleterious effects that may appear in the offspring, during early and later stages of life, after prenatal exposure to insecticides, not only on women with direct exposure but also on subjects with indirect exposure such as consumers or residents of rural communities.
Methodology
A systematic literature search was undertaken to locate and review research concerning the prenatal effects of pesticides. The review was designed to answer the following questions:
Which are the main toxic effects of the most pesticides used around the world?
How pesticides can produce these toxic effects? Which are their modes of action?
Search strategy
The Medline, Scopus and Web of knowledge citation databases were searched for relevant articles published between 2000-2017 using combinations of the following terms:
Table
Full-length experimental articles related to pesticides and prenatal toxic effects were retrieved. A total number of 286 records were obtained when combinations of all the keywords were made, and after excluding the duplicates, the remaining articles were classified according the main focus of each article. About literature reviews, a total number of 390 focused on organophosphates, carbamates, pyrethroids and toxicity were found. The abstracts of all articles were carefully studied, and the articles reporting the mode of action and/ or prenatal toxic effects (animals and humans) of the different types of pesticides were included. A final number of 53 studies were found relevant that constituted the main structure of the present review.
Types of Pesticides
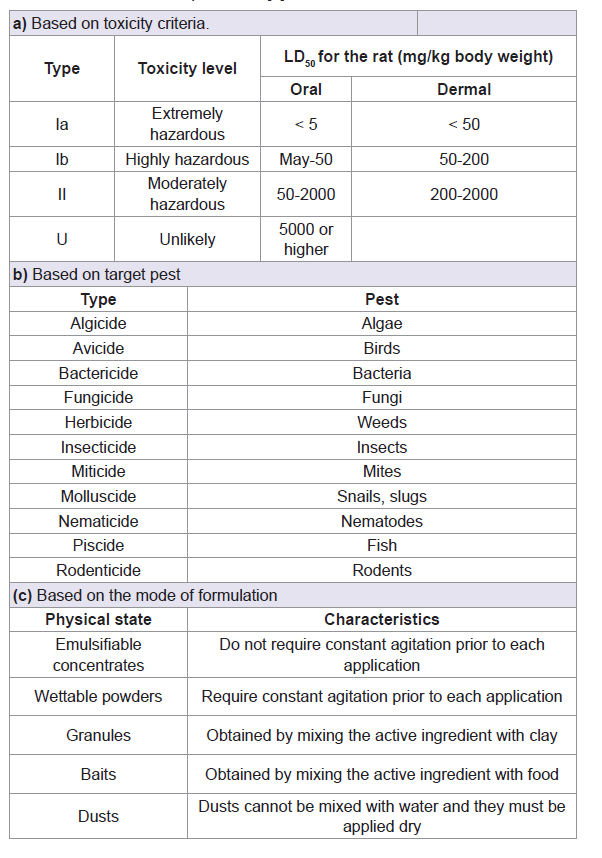
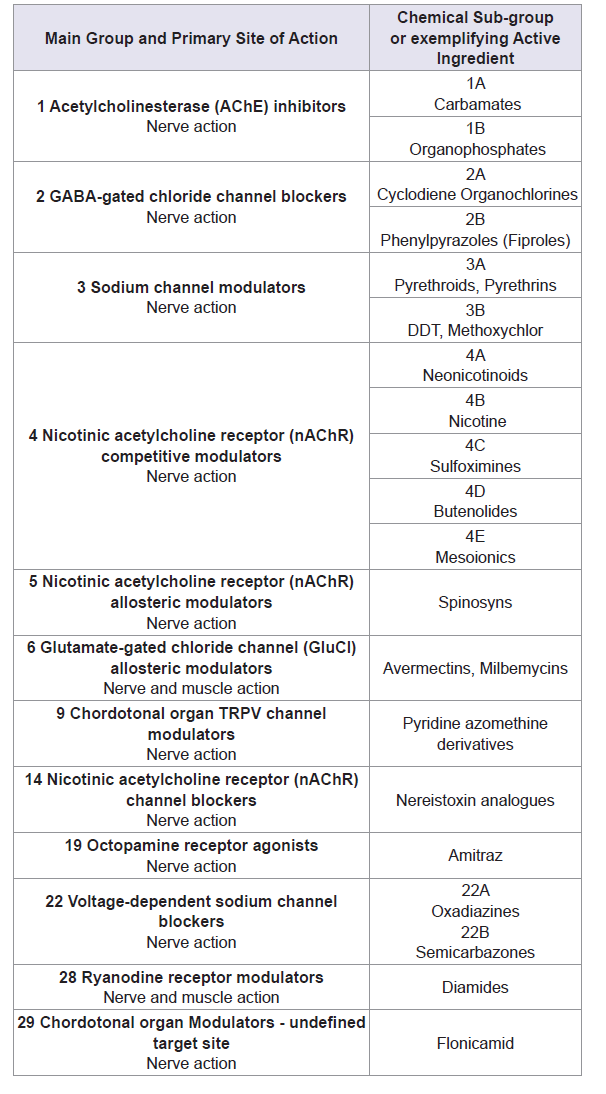
Pesticides can be classified by various criteria such as chemical classes, functional groups, mode of action, and toxicity (Table 1) [5]. Sometimes are classified by the type of target pest for which they are applied. The four major classes are those of fungicides, herbicides, rodenticides and insecticides [6]. Furthermore, within each class, several potentially toxic subclasses exist, for example among insecticides; one can find organochlorines, organophosphorus compounds, carbamates, pyrethroids, and many other chemicals (Table 2) [7].
Table 1: Classification of pesticides [5].
Insecticides are capable of killing insects by penetrating into their bodies via direct contact (dermal entry), oral, and/or respiratory entry [5]. Most of the chemical insecticide in use today are neurotoxicants, and act by poisoning the nervous system of the target organism (Table 2) [8].
The Organochlorine Pesticides (OCPs) are considered as persistent organic pollutants, once released in the environment, they break down very slowly in air, water, soil and in living organisms. As OCPs bio-magnify through the food chain, consumers of food of animal origin such as fish, meat, milk and dairy products end up with high levels of exposure and due to slow biodegradation, OCPs accumulate in the body [9]. For this reason, insecticides suchas organophosphates, pyrethroids, and carbamates have becomeattractive alternatives to OCPs because they do not persist in the environment. Organophosphate pesticides are currently the most heavily used insecticide in US agriculture whereas pyrethroids are the most common class of pesticide used in homes [10]. However, extensive use of these products has culminated in constant human exposure to pesticides via domestic use or the food chain [11]. In this review, we will provide an overview of the toxic effects ofthese most employed insecticides over one of the most sensiblepopulation, pregnant women and the newborn. First of all, we willdiscuss mechanisms by which organophosphates, carbamates, andpyrethroids may elicit the toxic response after the prenatal exposure and the potential consequences of this exposition.
Impacts of Insecticides on Reproduction
Organophosphates
Organophosphorus (OP) is the general name for organic derivatives of phosphorus. They are the most commonly used insecticides in the world because their unstable chemical structure leads to rapid hydrolysis and little long-term accumulation in the environment [12].
Toxicity mechanism
a) Inhibition of acetylcholinesterase (AChE)
The well known OP acute toxicity is initiated by inhibition of acetylcholinesterase (AChE). This enzyme is classed as a B esterase whose major role is the hydrolysis of acetylcholine (ACh). ACh is a major neurotransmitter in the peripheral and central nerve system. This inhibition disrupts the ability of the enzyme to bind to its normal substrate with the subsequent accumulation of ACh at nerve endings [13,14]. Consequently, an overstimulation followed by desensitization of muscarinic and nicotinic ACh receptors occurs. Inhibition of AChE occurs after phosphorylation of hydroxyl group at serine at the active site of the enzyme. Depending on the degree of AChE inhibition, cholinergic stimulation may lead to hyperactivity of excitable tissues, causing fasciculation, seizures, convulsions, severe muscle paralysis, hypersecretion from secretory glands, respiratory failure, coma, and death [15,16].
Based on systematic investigations of the relationship between chemical structure and inhibition of AChE, it is apparent that the single most important property required in an organophosphate for anticholinesterase activity is chemical reactivity. Structure-activity studies have revealed a direct relationship between anticholinesterase activity and reactivity of the phosphorus atom [17]. Thus the most important properties required for anticholinesterase activity are (1) the steric properties to diffuse into the active site gorge and form a stable enzyme-inhibitor complex and (2) sufficient reactivity of the reaction center, phosphorus, to accept a nucleophilic attack from the serine hydroxyl in the active site [18].
Aside from the inhibition of AChE, certain OPs can cause another type of toxicity called Organophosphate Induced Delayed Polyneuropathy (OPIDP). It is characterized by degeneration of long axons in the central and peripheral nervous system and consequent ataxia and paralysis that appear about 2-3 weeks after exposure or later [19]. OPIDP is initiated by phosphorylation and subsequent aging of >70% of the functional Neuropathy Target Esterase (NTE). NTE may have important functions during brain development through involvement in cell-signaling pathways between neurons and glial cells [16].
b) Oxidative stress and apoptosis
As it has been said above, the main target of action for OP is the inhibition of AChE; but it has also postulated that both acute and chronic exposures to these compounds alter the redox processes and thus induce oxidative stress [20].
Pesticides are known to disturb oxidative homeostasis through direct or indirect pathways, including mitochondrial or extramitochondrial production of free radicals, thiol oxidation, and depletion of cellular antioxidant reservoirs [21]. Pesticide damage is generated by the imbalance between Reactive Oxygen Species (ROS) production and elimination [22]. ROS may be produced as the result of the metabolism of organophosphates by cytochrome P450s by addition of one atom of molecular oxygen into the molecule by an electron transport pathway. The OP change normal antioxidant homeostasis resulting in antioxidant depletion [23].
Additionally, disrupting effects of organophosphates on glucose homeostasis have been reportedly linked to oxidative [21]. Glucose is the source of reducing equivalents (NADH, NADPH, and GSH etc.) which are involved in the recycling of oxidized cellular antioxidants. On the other hand, hyperglycemia increases non-enzymatic glycation leading to the formation of advanced glycation end products (AGEs), which alter structure and functions of proteins, which activate specific membrane receptors called receptors of advanced glycation end products (RAGE) and induce an intracellular oxidative stress. When ROS levels exceed the scavenging capacity of the body, mitochondria will swell with permeability changes. It has been reported that exposure to OPs causes mitochondrial damages and the cytochrome c is released into the cytoplasm, and the caspase cascade is activated, which lead to apoptosis [22].
c) Endocrine disruptors
Most endocrine disrupting pesticides mimic estrogen function by acting as a ligand for receptor, converting other steroids to active estrogen or increasing the expression of estrogen responsive genes as shown by some organochlorines, organophosphates, carbamates, and pyrethroids [21]. These pesticides can affect the endocrine system in any stage of hormonal regulation, from synthesis to hormone receptor binding, resulting in reproductive and developmental adverse effects [24,25]. Thus, results reported by Walsh et al. showed that dimethoateinhibited steroidogenesis in both a dose- and time-dependent manner by blocking transcription of the Steroidogenic Acute Regulatory (StAR) gene [26]. Other OPs are capable of interfering with the endocrine function by inhibiting the binding of thyroid hormones to their corresponding receptors. OPs such as chlorpyrifos are alsoable to inhibit adrenal steroidogenesis, thus affecting the hormonal status [21,24].
Prenatal exposure and its consequences for the new born
In utero exposure is the first point of contact with environmental xenobiotics that may affect the maternal-placental-fetal balance [27]. This is believed to be the critical exposure period to OP insect ides for human neurological development and is, by definition, the only relevant exposure period for birth outcomes [28].
a) Effects in the central nervous system
Prenatal exposure causes disruption in brain development, leading to behavioral deficits, impaired cognitive and motor functions, and alterations in the cholinergic system that affects learning and memory processes [29]. Thus, Young et al. assess the relationship between in utero and early postnatal OP exposure and neonatal neurobehavior in humans, as measured by seven clusters (habituation, orientation, motor performance, range of state, regulation of state, autonomic stability, and reflex) on the Brazelton Neonatal Behavioral Assessment Scale (BNBAS) [30]. Exposure to OP pesticides was determined by urinary levels of Dialkylphosphate (DAP) metabolites, including dimethyl and diethylphosphate metabolites. The relationship between exposure and BNBAS performance was examined by the median age at assessment, 3 days. Results are suggestive of a detrimental impact of in utero OP exposure, as measured by total DAP, on reflex functioning, particularly in those infants assessed after 3 days of life. Specifically, the most common primitive reflexes rated as abnormal included: rooting (23%), passive resistance-legs (39%), walking (25%), incurvation (40%), and Moro reflex (18%).
b) Metabolic and hormonal alterations
Pesticides can cause endocrine disruption, either by direct interaction with receptors or alteration of the enzymes involved in steroid hormone synthesis and metabolism. They can potentially alter hormone concentrations in blood and tissues [27]. Thus, Usmani et al. observed that OP pesticides were very potent inhibitors of the production of the primary metabolites of CYP3A4 and inhibited major testosterone metabolites noncompetitively and irreversibly [31]. Furthermore, OP components are to affect the male reproductive system by such mechanisms as reduction of sperm activities, inhibition of spermatogenesis, reduction of testis weights, damaging sperm DNA, and increasing abnormal sperm morphology [5]. An increased level of FSH accompanying a decreased level of inhibin B, testosterone, LH, and Free Androgen Index (FAI) in association with exposure to some OP insecticides [21].
c) Effects over the birth outcomes
It has been proved the association between OP exposure and birth outcomes, suggesting a decrease in birth weight and head circumference in newborns born from mothers with low PON1 activity that were exposed to OP pesticides [32]. Also has been observed a fairly consistent adverse association of in utero organophosphate pesticide exposure with gestational duration. These associations with gestational age may be biologically plausible given that organophosphate pesticides depress cholinesterase and acetylcholine stimulates contraction of the uterus [33].
Carbamates
Toxicity mechanism
The inhibition of AChE by a carbamate insecticide occurs by a mechanism virtually identical to that described earlier for an organophosphorus ester [17]. As with organophosphates, the signs and symptoms are based on excessive cholinergic stimulation. Unlike organophosphate poisoning, carbamate poisonings tend to be of shorter duration because the inhibition of nervous tissue acetylcholinesterase is reversible, and carbamates are more rapidly metabolized [34].
Prenatal exposure and its consequences for the new born
The study of the carbamate exposure in pregnant women is usually made in association with other pesticides more used as organophosphates and pyrethroids. And the results obtained are often due to these last more than to the carbamates. Thus, the carbamate exposure of the pregnant women was studied by Forde et al. [35]. The evaluation of this exposition was made by looking for the presence of two carbamate metabolites (propoxur metabolite, 2-Isopropoxyphenol (2-IPP) and carbofuranphenol). Results showed very low or no existent level of these metabolites throughout the ten Caribbean countries sampled in this study. On the other hand, Carmichael et al. studied the relationship between residential agricultural pesticide exposures (including carbamates) and risks of selected birth defects among offspring [36]. Results of this study did not indicate strong associations of the studied birth defects with residential proximity to agricultural pesticide applications, even though risk for birth defects within an area of high pesticide use was investigated. In a previous study, Sheldon et al. tried to relate neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides (organophophates, organochlorines, pyrethroids, and carbamates) [37]. The main relationships were detected between organophophates, organochlorines, and pyrethroids. Only when the applications of carbamates during pregnancy were nearby the home it was suggested an association between these pesticides and developmental delay.
In spite of the lack of results about the reproductive toxicity of carbamates in human, there are authors that have studied the main toxic effects in offsprings when pregnant rats were exposed to the most used carbamates pesticides. Thus, Mishra et al. studied the effects of carbofuran, a carbamate pesticide, in rat offspring, after a chronic gestational exposure [38]. Results showed a decreased neurogenesis, altered neuronal and glial differentiation, increased cell death in the hippocampus, and cognitive impairments in rat offspring. The observed decrease in neurogenesis in the present study can be a result of reduction in the proliferation rate, decreased survival and maturation of newborn neurons, altered cellular fate specification, or a combination of all three effects. A later study with carbosulfan showed that this carbamate also affect the neurogenesis and synaptic development in rat offspring after exposure during the embryonic period. A dose dependent reduction in body weight was observed in pups exposed to carbosulfan. Also showed a decrease in their surface righting reflex compared with the control. In the negative geotaxis paradigm, control animals turned the 45° slope rapidly. However, pups exhibited a statistically significant delay in re-orientation on exposure to increasing doses of carbosulfan. Moreover, levels of MDA and protein carbonyl were elevated in the carbosulfan exposed pups compared with the control and while exposure to this carbamate dose dependently produced a decline in the levels of AChE [39].
Pyrethroids
Owing to their relatively low toxicity to mammals in contrast to organophosphorus insecticides, synthetic pyrethroids are among the most frequently used pesticides and account for more than one-third of the insecticides currently marketed in the world [40]. The first pyrethroid pesticide, allethrin, was identified in 1949. Its structure and those of other pyrethroids with the basic cyclopropane carboxylic ester structure were called type I pyrethroids. The insecticidal activity of synthetic pyrethroids was enhanced further by the addition of a cyano group at the benzylic carbon atom to give α-cyano (type II) pyrethroids such as cyphenothrin and cypermethrin [41].
The reported toxic effects of pyrethroids include neurotoxicity, skin contact toxicity, respiratory toxicity and reproductive system toxicity [40]. Type I pyrethroids effects typically include rapid onset of aggressive behavior and increased sensitivity to external stimuli, followed by fine tremor, prostration with coarse whole body tremor, elevated body temperature, coma, and death. The term T-syndrome (from tremor) has been applied to Type I responses. Type II effects are usually characterized by pawing and burrowing behavior, followed by profuse salivation, increased startle response, abnormal hindlimb movements, and coarse whole body tremors that progress to sinuous writhing (choreoathetosis). Clonic seizures may be observed prior to death. Body temperature usually is not increased, but may decrease. The term CS-syndrome (from choreoathetosis and salivation) has been applied to Type II responses [42].
Mechanisms of action
a) Effects on sodium channels
The mechanisms by which pyrethroids alone are toxic are complex and become more complicated when they are coformulated with piperonyl butoxide, an organophosphorus insecticide, or both, as these compounds inhibit pyrethroid metabolism.
The main effects of pyrethroids are on sodium and chloride channels. As a result, excitable (nerve and muscle) cells are the principal targets of pyrethroid toxicity, which is manifest as disordered function rather than structural damage [41]. Pyrethroids slowed Voltage-Gated Sodium Channels (VGSC) activation leading to a decrease in peak Na+ current. Pyrethroids slowed VGSC inactivation and deactivation leading to a prolonged VGSC open time. Type II pyrethroids prolonged channel open time more than type I pyrethroids. The longer channel open time results in more Na+ entering the cell leading to hyperexcitability with type I pyrethroids, membrane depolarization and conduction block with type II pyrethroids. Even though not every VGSC is altered by pyrethroids, modification of a small percentage of VGSCs can increase Na+ current substantially [43,44]. This is so-called sodium ‘tail current’. If a sodium tail current is sufficient to hold the cell membrane potential above threshold, an abnormally early second action potential will occur and a repetitive strain of action potentials can be triggered. This is the likely mechanism underlying pyrethroid- induced paraesthesiae. Despite the presence of tail currents, cells may continue to function, albeit at an abnormally high level of excitation. However, there may come a point when they cannot sustain function at this level and what has been termed ‘conduction block’ results. Conduction block may be induced by exposure to high concentrations of either type II pyrethroids (when the sodium cannel is kept open unduly long) or type I pyrethroids (when a large amplitude tail current is produced) [41].
Thus, it can be said that for the Type I and Type II structural subclasses of pyrethroids, qualitative differences in sodium channel modification are generally correlated with the production of different intoxication syndromes, suggesting that actions on sodium channels are sufficient to account for the acute toxicity of this insecticide class [45].
b) Effects on chloride channel
One additional target of type II pyrethroids is the membrane chloride ion cannel. The voltage-gated chloride channel was then proposed as a target and for deltamethrin at least, this does appear to be sufficiently sensitive. Indeed it is possible to antagonize both the salivation and choreoathetosis, which are the prominent characteristics of type II pyrethroid poisoning, with chloride channel agonists [46]. At relatively high concentrations, pyrethroids can also act on GABA-gated chloride channels, which may contribute to the seizures seen in severe type II poisoning [41].
c) Endocrine disruptor
In spite of several epidemiological studies have linked environmental exposure to pyrethroids to alterations of the reproductive health of adult male subjects, Saillenfait et al. showed in a systematic review that several in vitro screen studies suggest that certain pyrethroids may have the potential to affect the estrogenic and androgenic pathways, but available data do not provide evidence for strong interactions. It has been proposed that the biphenyl ether moieties shared by several pyrethroids (e.g. cypermethrin, deltamethrin, esfenvalerate, permethrin) may play a role in their interaction with the hormone receptors [47].
Prenatal exposure and its consequences for the new born
New born babies and children are often exposed to pyrethroids for long periods by the use of liquid vaporizers. Occupational and experimental studies indicate that pyrethroids can cause clinical, biochemical and neurological changes, and that exposure to pyrethroids during organogenesis and early developmental period is especially harmful [48].
a) Effects in the central nervous system
The effect of exposure of rat pups during early developmental stages to pyrethroids on Blood-Brain Barrier (BBB) permeability was investigated by Sinha et al. [48]. Results indicated, that inhalation of pyrethroids during early life may lead to adverse effect on infants causing significant abnormalities affecting the CNS by breaching the BBB. The damage was more during postnatal and perinatal exposures than during the prenatal exposure. In the latter case, the bloodplacental barrier is formed/developed which limits the availability of the pyrethroid. A later study, showed the effects of prenatal exposure to permethrin on the development of cerebral arteries in fetal brains, neurotransmitter in neonatal brains, and locomotor activities in offspring mice. This exposition in utero caused fetal brain vascular malformations and changes in motor behavior in adult mice [49].
b) Epidemiological studies
Several epidemiologic studies have been performed. Thus, Qi et al. showed that prenatal exposure to elevated levels of pyrethroid pesticides was associated with reduced neurodevelopment of infants from somewhere of Jiangsu Province (China) [50]. Other authors have been measured pyrethroid metabolites in biological samples, thus, Dewailly et al. demonstrated an extensive use of pyrethroid compounds such as permethrin and cypermethrin in Caribbean households measuring its metabolites concentrations in Caribbean pregnant women urine [51]. Berton et al. analyzed 171 meconium amples collected in the Picardie region of northern France looking for pesticides and its metabolites [52]. The pyrethroids cypermethrin and cyfluthrin were detected in only 11 and 3 samples, respectively, but were present at high concentrations than the other studied pesticides. The high concentrations observed in some samples may suggest direct exposure of the mother through use at home or in the workplace.
Prenatal exposure to pyrethroid insecticides and birth outcomes were studied in Rural Northern China by Ding et al. [53]. No associations were found between individual or total metabolite levels and birth length, head circumference, or gestational duration, however, an adverse association of prenatal exposure to pyrethroids as measured by urinary metabolites with birth weight was reported. On the other hand, associations of birth defects with residential proximity to commercial agricultural pesticide applications in California were made. Most of the individual pesticides were not associated with increased risk. Pyrethroids only were associated with craniosynostosis, but this result should be interpreted with caution given the novelty of this investigation and potential false positive due to multiple testing [36].
Conclusion
In summary, organophosphates, carbamates and pyrethroids are less persistent in nature than organochlorine pesticides but even though they are not safe for humans exposed to small concentrations but along their life. Nowadays there is a growing trend for reducing the use of organophosphates pesticides and replaced by carbamates and pyrethroids, due its toxic effects. The main route of exposure is through diet but this exposition increase when people are living in rural areas near of cultures. Pregnant women are risk population and it has been proved the toxic effects of organophosphates, carbamates and pyrethroids in offsprings. Thus, organophosphates, causes disruption in brain development, leading to behavioral deficits, impaired cognitive and motor functions, and alterations in the cholinergic system that affects learning and memory processes. The carbamate exposure in pregnant women is usually made in association with other pesticides. There is a lack of results about the reproductive toxicity of carbamates in human. The epidemiological studies made only associate adverse effects in offspring when the applications of carbamates during pregnancy were nearby the home. New born babies and children are often exposed to pyrethroids for long periods by the use of liquid vaporizers at home. This exposition can cause clinical, biochemical and neurological changes, being especially harmful if that exposure to pyrethroids is during organogenesis and early developmental period.
References
- Alavanja MCR (2009) Pesticides Use and Exposure Extensive Worldwide. Rev Environ Health 24: 303-309.
- Tago D, Andersson H, Treich N (2014) Pesticides and health: A review of evidence on health effects, valuation of risks, and benefit-cost analysis. Adv Health Econ Health Serv Res 24: 203-295.
- Alavanja MC, Bonner MR (2012) Occupational pesticide exposures and cancer risk: a review. J Toxicol Environ Health B Crit Rev 15: 238-263.
- Collotta M, Bertazzi PA, Bollati V (2013) Epigenetics and pesticides. Toxicology 307: 35-41.
- Kim KH, Kabir E, Jahan SA (2017) Exposure to pesticides and the associated human health effects. Sci Total Environ 575: 525-535.
- Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2: 1-12.
- Holstege CP, Baer AB (2004) Insecticides. Curr Treat Options Neurol 6:17-23.
- Casida JE (2009) Pest toxicology: the primary mechanisms of pesticide actions. Chem Res Toxicol 22: 609-619.
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, et al. (2013) Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307: 74-88.
- Rosas LG, Eskenazi B (2008) Pesticides and child neurodevelopment. Curr Opin Pediatr 20:191-197.
- Pereira LC, de Souza AO, Franco Bernardes MF, Pazin M, Tasso MJ, et al. (2015) A perspective on the potential risks of emerging contaminants to human and environmental health. Environ Sci Pollut Res Int 22: 13800-13823.
- Soltaninejad K, Shadnia S (2014) History of the Use and Epidemiology of Organophosphorus Poisoning. In: Balali-Mood M, Abdollahi M (Eds). Basic and Clinical Toxicology of Organophosphorus Compounds pp. 25-43.
- Jokanovic M (2001) Biotransformation of organophosphorus compounds. Toxicology 166: 139-160.
- Remington SE (2010) Cellular response to DNA damage after exposure to organophosphates in vitro. BSc (Hons) Medical Biochemistry (Birmingham University). MSc Toxicology (Birmingham University).
- Milatovic D, Gupta RC, Aschner M (2006) Anticholinesterase toxicity and oxidative stress. Sci World J. 6: 295-310.
- Jokanović M, Kosanović M (2010) Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ Toxicol Pharmacol 29: 195-201.
- Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87: 245-254.
- Lee S, Barron MG (2016) A mechanism-based 3D-QSAR approach for classification and prediction of acetylcholinesterase inhibitory potency of organophosphate and carbamate analogs. J Comput Aided Mol Des 30: 347-363.
- Jokanović M, Kosanović M, Brkić D, Vukomanović P (2011) Organophosphate induced delayed polyneuropathy in man: An overview. Clin Neurol Neurosurg 113: 7-10.
- Ramirez-Vargas MA, Huerta-Beristain G, Guzman-Guzman IP, Alarcon-Romero LD, Flores-Alfaro E, et al. (2017) Methamidophos induces cytotoxicity and oxidative stress in human peripheral blood mononuclear cells. Environ Toxicol 32: 147-155.
- Mostafalou S, Abdollahi M (2017) Pesticides: an update of human exposure and toxicity. Arch Toxicol 91: 549-599.
- Li F, Xu K, Ni M, Wang B, Gu Z, et al. (2017) Effect of oxidative phosphorylation signaling pathway on silkworm midgut following exposure to phoxim. Environ Toxicol 32: 167-175.
- Lukaszewicz-Hussain A (2010) Role of oxidative stress in organophosphate insecticide toxicity-Short review. Pestic Biochem Phys 98: 145-150.
- Androutsopoulos VP, Hernandez AF, Liesivuori J, Tsatsakis AM (2013) A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology 307: 89-94.
- Senthilkumaran B (2015) Pesticide- and sex steroid analogue-induced endocrine disruption differentially targets hypothalamo-hypophyseal-gonadal system during gametogenesis in teleosts - A review. Gen Comp Endocrinol 219: 136-142.
- Walsh LP, Webster DR, Stocco DM (2000) Dimethoate inhibits steroidogenesis by disrupting transcription of the steroidogenic acute regulatory (StAR) gene. J Endocrinol 167: 253-263.
- Cecchi A, Rovedatti MG, Sabino G, Magnarelli GG (2012) Environmental exposure to organophosphate pesticides : Assessment of endocrine disruption and hepatotoxicity in pregnant women. Ecotoxicol Environ Saf 80: 280-287.
- Reiss R, Chang ET, Richardson RJ, Goodman M (2015) A review of epidemiologic studies of low-level exposures to organophosphorus insecticides in non-occupational populations. Crit Rev Toxicol 45: 531-641.
- Handal AJ, Harlow SD, Breilh J, Lozoff B (2008) Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology 19: 851-859.
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Johnson C, et al. (2005) Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology 26: 199-209.
- Usmani KA, Rose RL, Hodgson E (2003) Inhibition and activation of the human liver microsomal and human cytochome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metab Dispos 31: 384-391.
- Naksen W, Prapamontol T, Mangklabruks A, Ryan PB, Riederer AM, et al. (2015) Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort. Environ Res 142: 288-296.
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, et al. (2004) Children’s Health Article Association of in Utero Organophosphate Pesticide Exposure and Fetal Growth and Length of Gestation in an Agricultural Population. Environ Health Perspect 10: 1116-1124.
- Fishel FM (2011) Pesticide Toxicity Profile: Chlorinated Hydrocarbon Pesticides. pp. 1-3.
- Forde MS, Robertson L, Laouan Sidi EA, Côté S, Gaudreau E, et al. (2015) Evaluation of exposure to organophosphate, carbamate, phenoxy acid, and chlorophenol pesticides in pregnant women from 10 Caribbean countries. Environ Sci Process Impacts 17: 1661-1671.
- Carmichael SL, Yang W, Roberts E, Kegley SE, Brown TJ, et al. (2016) Residential agricultural pesticide exposures and risks of selected birth defects among offspring in the San Joaquin Valley of California. Birth Defects Res A Clin Mol Teratol 106: 27-35.
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, et al. (2014) Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Persp 122: 1103-1109.
- Mishra D, Tiwari SK, Agarwal S, Sharma VP, Chaturvedi RK (2012) Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol Sci 127: 84-100.
- Banji D, Banji OJ, Ragini M, Annamalai AR (2014) Carbosulfan exposure during embryonic period can cause developmental disability in rats. Environ Toxicol Pharmacol 38: 230-238.
- Lei W, Wang DD, Dou TY, Hou J, Feng L, et al. (2017) Assessment of the inhibitory effects of pyrethroids against human carboxylesterases. Toxicol Appl Pharmacol 321: 48-56.
- Bradberry SM, Cage SA, Proudfoot AT, Vale JA (2005) Poisoning due to pyrethroids. Toxicol Rev 24: 93-106.
- U.S Department of Health and Human Services (2003) Toxicological Profile For Pyrethrins And Pyrethroids.
- Shafer TJ, Meyer DA, Crofton KM (2005) Developmental neurotoxicity of pyrethroid insecticides : critical review and future research needs. Environ Health Perspect 113: 123-136.
- Wakeling EN, Neal AP, Atchison WD (2012) Pyrethroids and Their Effects on Ion Channels. Pesticides- Advances in Chemical and Botanical Pesticides.
- Soderlund DM (2012) Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol 86: 165-181.
- Burr SA, Ray DE (2004) Structure-Activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol Sci 346: 341-346.
- Saillenfait AM, Ndiaye D, Sabaté JP (2016) The estrogenic and androgenic potential of pyrethroids in vitro. Review. Toxicol In Vitro 34: 321-332.
- Sinha C, Seth K, Islam F, Kumar R, Shukla S, et al. (2006) Behavioral and neurochemical effects induced by pyrethroid-based mosquito repellent exposure in rat offsprings during prenatal and early postnatal period. Neurotoxicol Teratol 28: 472-481.
- Imanishi S, Okura M, Zaha H, Yamamoto T, Akanuma H, et al. (2013) Prenatal exposure to permethrin influences vascular development of fetal brain and adult behavior in mice offspring. Environ Toxicol 28: 617-629.
- Qi X, Zheng M, Wu C, Chang X, Wang G, et al. (2011) Impact of prenatal pyrethroid exposure on neurodevelopment of one-year old infants. Wei Sheng Yan Jiu 40: 693-697.
- Dewailly E, Forde M, Robertson L, Kaddar N, Laouan Sidi EA, et al. (2014) Evaluation of pyrethroid exposures in pregnant women from 10 Caribbean countries. Environ Int 63: 201-206.
- Berton T, Mayhoub F, Chardon K, Duca RC, Lestremau F, et al. (2014) Development of an analytical strategy based on LC-MS/MS for the measurement of different classes of pesticides and theirs metabolites in meconium: Application and characterisation of foetal exposure in France. Environ Res 132: 311-320.
- Ding G, Cui C, Chen L, Gao Y, Zhou Y, et al. (2015) Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J Expo Sci Environ Epidemiol 25: 264-270.