Journal of Toxins
Download PDF
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology and Exp Pharmacodynamics, Department of Physiology, University of Calcutta, 92 A P C Road, Kolkata 700 009, India, Tel: 91-33-23508386/ (M) 09433139031; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Das R, Ghosh S, Gomes A. Indian Black Scorpion (Heterometrus bengalensis) Venom Action Neutralization by Indian Medicinal Plants in Experimental Animals. J Toxins. 2016;3(2): 7
Copyright © 2016 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 2
Submission: 08 September, 2016 | Accepted: 28 September, 2016 | Published: 06 October, 2016
Treatments are commonly symptomatic since specific anti venoms are not available, especially in India. In some developed countries, like South Africa, Middle East, America, etc. antivenoms are available but due to their various side effects, their use is controversial. Thus, to emphasize has been given on ancillary treatment. Prophylactic immunisation against scorpion envenoming has also been advocated, but acceptable experimental evidences are lacking [12]. Various alternative/folk and traditional treatments are available against scorpion envenomation, among which the most common one is the usage of herbal products [13]. Plants like, Mangifera indica.L, Aristolochia indica, Solanum indicum L, Andrographis paniculata Nees, Barringtonia acutangula L, Hemidesmus indicus, Pluchea indica and Aristolochia indica etc. [14-16], have been mentioned in traditional medicine and used by village folks for the treatment against scorpion sting. These plant products are cheap and easily available; therefore based on traditional knowledge a few of these plants were selected for their scientific effectiveness. The present investigation explored the ethnopharmacological value of the plant root extracts of Hemidesmus indicus, Pluchea indica and Aristolochia indica, in experimental scorpion envenomation.
Neutralization of SV induced pro-inflammatory action
Scorpion venom neutralization studies (in vitro)
Neutralization of SV induced plasma recalcification
Neutralization of neurotoxic changes
Indian Black Scorpion (Heterometrus bengalensis) Venom Action Neutralization by Indian Medicinal Plants in Experimental Animals
Antony Gomes*, Rinku Das and Sourav Ghosh
- Department of Physiology, University of Calcutta, India
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology and Exp Pharmacodynamics, Department of Physiology, University of Calcutta, 92 A P C Road, Kolkata 700 009, India, Tel: 91-33-23508386/ (M) 09433139031; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Das R, Ghosh S, Gomes A. Indian Black Scorpion (Heterometrus bengalensis) Venom Action Neutralization by Indian Medicinal Plants in Experimental Animals. J Toxins. 2016;3(2): 7
Copyright © 2016 Gomes A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 3, Issue: 2
Submission: 08 September, 2016 | Accepted: 28 September, 2016 | Published: 06 October, 2016
Abstract
The anti scorpion venom activity of the Indian medicinal plant (Hemidesmus indicus, Pluchea indica and Aristolochia indica) root extracts (aqueous and methanol) was established in experimental animal models. Adult black scorpions (Heterometrous bengalensis) of both sexes were collected and the Scorpion Venom (SV) was collected by electrical stimulation, pooled, lyophilized and stored at 4 °C. Scorpion venom was expressed in terms of dry weight. Plant root extracts of Hemidesmus indicus (H.I) Pluchea indica (P.I) and Aristolochia indica (A.I) were (aqueous/methanol) prepared and expressed in terms of dry weight. Anti scorpion venom activity was evaluated using various in vivo and in vitro models (lethality, edema, urinary changes, plasma recalcification, cardiotoxicity, neurotoxicity, etc.). The aqueous/ methanol plant root extracts (H.I, P.I and A.I) could neutralize scorpion venom induced lethality, edema, urinary changes, plasma recalcification, cardiotoxicity, neurotoxicity, in experimental animal models. The aqueous root extracts were more effective in neutralizing scorpion venom actions as compared to methanol plant root extracts. These observations confirmed the ethnopharmacological value of the Indian medicinal plants active against scorpion venom and warrants further detailed studies.Keywords
Scorpion; Scorpion venom; Heterometrus Bengalensis; Venom neutralization; Herbal antagonistIntroduction
Scorpions are one of the venomous arthropods, widely found all over the world, especially in tropical countries, like South America, Mexico, India, etc. There are 1500 species available worldwide, and approximately 25 species are dangerous to humans [1,2] especially in children and the elderly [3]. In India, 90 species of scorpions are available like Heterometrus swammardami, Buthus tamulus, Lycus leavifrons, etc. among which Heterometrus bengalensis is commonly found in West Bengal Figure 1 [4]. There are 33 species of genus Heterometrus around the world [5]. Envenomation by scorpion remains a serious health problem, causing child mortality and elderly morbidity [6]. Their venoms constitute a complex mixture of polypeptides exhibiting different pharmacological activities [7] along with other components, such as enzymes and cytotoxic agents [8,9]. Although only hundreds die annually from harmful species, not less than one million people around the world are predicted to suffer from symptoms at the stung site and are associated with the induction of systematic symptoms [10,11].Treatments are commonly symptomatic since specific anti venoms are not available, especially in India. In some developed countries, like South Africa, Middle East, America, etc. antivenoms are available but due to their various side effects, their use is controversial. Thus, to emphasize has been given on ancillary treatment. Prophylactic immunisation against scorpion envenoming has also been advocated, but acceptable experimental evidences are lacking [12]. Various alternative/folk and traditional treatments are available against scorpion envenomation, among which the most common one is the usage of herbal products [13]. Plants like, Mangifera indica.L, Aristolochia indica, Solanum indicum L, Andrographis paniculata Nees, Barringtonia acutangula L, Hemidesmus indicus, Pluchea indica and Aristolochia indica etc. [14-16], have been mentioned in traditional medicine and used by village folks for the treatment against scorpion sting. These plant products are cheap and easily available; therefore based on traditional knowledge a few of these plants were selected for their scientific effectiveness. The present investigation explored the ethnopharmacological value of the plant root extracts of Hemidesmus indicus, Pluchea indica and Aristolochia indica, in experimental scorpion envenomation.
Materials and Methods
ChemicalsAll chemicals and solvents used were of Analytical Grade, unless otherwise mentioned. Petroleum ether, Chloroform, Methanol (Spectrochem, India), Potassium Chloride (SRL, India), Calcium Chloride (Merck, India), Magnesium Chloride (Qualigen, India), Sodium bicarbonate (SRL, India), Sodium biphosphate (SRL, India), Glucose (Qualigen, India).
Collection of scorpion and scorpion venom
Adult black scorpions (Heterometrous bengalensis) of both sexes were collected from Burdwan district of West Bengal, India, during the rainy seasons (June-September) and were kept in a wire mesh cage Figure 1. They were provided with food (live cockroach, an egg), water ad libitum. The Scorpion Venom (SV) was collected once in a month by applying square wave electrical stimulation (15 V, 1 ms) to the telson. The venom was pooled, lyophilized and stored at 4 °C in amber colour bottle, until further use. Before use, SV was weighed, dissolve in 0.9% saline/ phosphate buffer 0.01 M, pH 7.2 and was expressed in terms of dry weight.
Collection of plant materials
The plant (Hemidesmus indicus, Pluchea indica and Aristolochia indica) roots were collected commercially from M/s United Chemicals and Allied Products, Kolkata and were identified by Prof N. Paria, Department of Botany, University of Calcutta. A voucher specimen (AG100699, AG100799, AG100) has been deposited at the Department of Botany, University of Calcutta, Kolkata, India.
Preparation of plant root extract
a) Aqueous plant extract: The air dried grounded plant roots (2 gm) were soaked in distilled water overnight at 8 °C. It was filtered and the filtrate was centrifuged at 2000 rpm X 10 mins. The supernatant was used for further testing and kept at 8 °C. The plant extract was expressed in terms of dry weight.
b) Methanol plant extract: Air dried plant roots were grounded and extracted by refluxing with petroleum ether (60-80 °C), chloroform (60-65 °C) and methanol (64-65 °C) in a soxhlet apparatus. The materials were then dried in a desiccators at room temperature for further use. Before use, the extracted material was dissolved in 0.9% saline and centrifuged at 2000 rpm X 10 mins at room temperature. Supernatant was used for further investigation and kept at 4 °C. The plant extract was expressed in terms of dry weight.
Animals used
Male Swiss albino mice (20±2 gm), male guineapig (180±10 gm) and Wister albino rats (100±10 gm) were obtained from enlisted supplier of Calcutta University and were maintained in standard laboratory conditions, diet and water ad libitum. All animal experiments were approved by the University Animal Ethics Committee, Department of Physiology, University of Calcutta, Kolkata, India and were in accordance with the guidelines of the committee for the purpose of Control and Supervision of Experiments on Animal (CPCSEA), Government of India (Ref. No.: 820/04/ac/ CPCSEA dated 06.08.2004).
Scorpion venom neutralization (in vivo)
Neutrlization of lethal activity: MLD (Minimum Lethal Dose) of SV was defined as the minimum dose of SV, which when injected in tail vein (i.v) of male albino mice (20±2 g), caused death of the animal within 24 hrs [17]. Antilethal activity of plant root extracts was studied by the neutralization method [18] using incubate of certain amount of plant (aqueous and methanol) and venom, incubated at 37 °C for 30 mins and then centrifuging at 2000 rpm X 10 mins. The supernatant was injected (i.v) into male albino mice and mortality was recorded.
Neutralization of pro-inflammatory activity: The Minimum Edema Dose (MED) of SV was defined as the least amount of SV which when injected into male albino mice (20±2 g) produces edema after 2 hrs, in mice paw. Pro- inflammatory activity of plant extract was studied by using incubate of certain amount of plant (aqueous and methanol) and venom, incubated at 37 °C for 30 mins and then centrifuging at 2000 rpm X 10 mins. The supernatant was injected in the Subplantor (s.p) region of the left hind paw. Equal amount of saline was injected in the right hind paw (s.p) as control. The diameter of both hind paws of each mouse was measured with the help of a digital micro caliper (Mitutua, Japan) [19].
Neutralization of urinary changes
The Minimum Dose of SV Affecting Urinary Constituents (MDAUC) was defined as the amount of SV, which when injected (i.v) in male albino mice, produces significant changes in urine, in 24 hrs. Urine analysis was done by injecting SV (i.v) into male albino mice (20±2 g) followed by a saline load of 2 ml (i.p) and their urine was collected after 24 hrs. The urinary constituents were measured using standard reagent strips (Multistrix SG, Bayer, Germany). Neutralization was done using a certain amount of plant (aqueous and methanol) and venom, incubated at 37 °C for 30 mins and centrifuged at 2000 rpm X 10 min. The supernatant was injected (i.v) for the urine analysis as stated above.
Scorpion venom neutralization (in vitro)
Neutralization of PLA2 activity: Phospholipase A2 activity was estimated by the egg yolk coagulation method [20]. One unit of PLA2 activity was defined as the amount of SV which increases the coagulation time of egg yolk suspension by 1 min. Inhibition of enzyme activity was estimated by mixing fixed amount of plant (aqueous and methanolic) and venom and then incubating at 37 °C for 30 mins and centrifuged at 2000 rpm X 10 mins. The supernatant was used for testing PLA2 activity [21].
Neutralization of plasma recalcification
Fresh goat´s blood was collected in 3.8% trisodium citrate (1:9 v/v) and centrifuged at 3000 rpm X 15 mins. 0.1 ml of plasma, 0.1 ml of 0.5% CaCl2 and 1ml of saline (control)/SV (treated) was added in a test tube and placed in a water bath at 37 °C. Clotting time of plasma was recorded with the help of a stop watch at 15 secs interval [15]. Minimum Clotting Dose of Plasma (MCPD) is defined as the minimum amount of SV, which increased the clotting time of plasma by 1 min compared to saline control. Neutralization was done using certain amount of plant root extract (aqueous and methanol) and venom incubated at 37 °C for 30 mins and centrifuged at 2000 rpm X 10 mins. The supernatant was used for plasma recalcification activity testing [21].
Neutralization of neurotoxic activity: Minimum Neurotoxic Dose (MNTD) was defined as the amount of SV which blocks Toad Gastrocnemius Sciatic nerve muscle preparation (TGS) within 1 hour. Isolated Toad Sciatic nerve preparation (TGS) was suspended (6 ml bath) in oxygenated (95% O2 and 5% CO2) Frog Ringer’s solution (NaCl 154 mM, KCl 5.6 mM, CaCl2 2.2 mM, NaHCO3 6.0 mMand Glucose 5.55 mM) and maintained at room temperature. The preparation was stimulated with a square wave electronic stimulator (Grass, USA) at 12 V, stimulus duration 0.2 millisec and pulse at intervals of 10 sec. Contractions were recorded by Brodie´s lever on
Neutralization of cardiotoxic activity: Minimum Cardiotoxic Dose (MCTD) was defined as the least amount of SV, which inhibited amplitude of contraction and heart rate (50%) within 30 mins. The guinea pig (250±10 g) heart was prepared [22], perfused with oxygenated (95% O2 and 5% CO2) mammalian Tyrode´s solution (NaCl 137 mM, KCl 2.7 mM, CaCl2 2.6 mM, MgCl2 1.27 mM, NaHCO3 11.9 mM, NaH2PO4 0.4 mM and glucose 5.5 mM) containing double dextrose (2 g/l). The temperature was maintained at 37±1 °C. Contraction was recorded on a rotating drum using a spring heart lever. Neutralization of cardiotoxic activity was estimated with plant root extract (aqueous and methanol) and venom incubate kept at 37 °C X 10 mins and then centrifuged at 2000 rpm X 10 mins. The supernatant was used for neutralization of cardiotoxic activity stated as above.
Statistics
All results were expressed as mean±standard error of mean (n=6). The level of significance of difference between means was determined by Student´s t test and P
Results
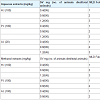
Scorpion venom neutralization studies (in vivo)Neutralization of SV induced lethal action: 1 MLD of SV was found to be 20 mg/kg (iv). SV (1-3 MLD) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 20 to 100 g/kg) and injected (i.v) in male albino mice. All extracts gave 200% protection Table 1.SV (1-3 MLD) was incubated at 37 °C X 15 mins with methanol plant root extracts (A.I, H.I and P.I at 100 mg/kg) and injected (i.v) in male albino mice. H.I gave 200% protection and P.I and A.I gave 100 % protection Table 1.
Neutralization of SV induced pro-inflammatory action
1 MED of SV was found to be 50 µg. SV (1-2 MED) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 20 to 100 mg) and injected (s.p) in male albino mice. All the plant aqueous extracts gave no protection.
SV (1-2 MED) was incubated at 37 °C X15 mins with methanol plant root extracts (A.I, H.I and P.I at 0.3 mg/kg) and injected (s.p) in male albino mice. H.I gave 100% protection and P.I and A.I gave no protection.
Neutralization of SV induced urinary changes
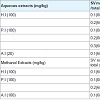
1 MDAUC (presence of blood and protein) of SV was found to be 5 mg/kg (i.v). SV (1-3 MDAUC) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 20 to 100 mg/kg) and injected (i.v) in male albino mice. P.I gave 200% protection, H.I gave 100% protection and no protection was given by A.I.
SV (1-2 MDAUC) was incubated at 37 °C X 15 mins with methanol plant root extracts (A.I, H.I and P.I at 100 mg/kg) and injected (i.v) in male albino mice, P.I gave 100% protection and no protection by P.I and A.I. Table 2.
Scorpion venom neutralization studies (in vitro)
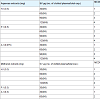
Neutralization of SV PLA2 activity: 1 unit of PLA2 activity of SV was found to be 5µg. SV (1-10 PLA2 unit) was incubated at 37 °C X15 mins with aqueous plant root extracts (A.I, H.I and P.I at 0.5 to 2.0 mg) and tested for PLA2 activity. All the three aqueous extracts gave 800% protection. The ED50 doses of plants were H.I- 1.98 mg, P.I- 1.98 mg and A.I- 0.49 mg respectively.
SV (1-10 PLA2 unit) was also incubated at 37 °C X15 mins with methanol plant root extracts (A.I, H.I and P.I at 0.2 mg) and tested for PLA2 activity, H.I gave 800% protection and 100% protection were given by P.I and A.I. The ED50 doses of plants were H.I- 0.19 mg, P.I- 0.18 mg and A.I- 0.19 mg, respectively Table 3.
Neutralization of SV induced plasma recalcification
1 MCDP of SV was found to be 30 µg. SV (1-4 MCDP) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 0.075 to 0.5 mg) and tested for plasma recalcification, where all the three aqueous extracts gave 300% protection. The ED50 doses of plants were H.I- 0.49, P.I- 0.49 and A.I- 0.07.
SV (1-4 MCDP) was also incubated at 37 °C X 15 mins with methanol plant root extracts (A.I, H.I and P.I at 0.3 mg) and tested for plasma recalcification. H.I gave 300% protection and 100% protection was given by P.I and A.I. The ED50 doses of plants were H.I- 0.29 mg, P.I- 0.29 mg and A.I- 0.29 mg, respectively Table 4.
Neutralization of neurotoxic changes
1 MNTD of SV was found to be 2 mg/ml. SV (1-2 MNTD) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 0.25 to 2.0 mg/ml) and tested for neurotoxicity in isolated toad´s gastrocnemius sciatic nerve preparation. All the three aqueous extracts gave 100% protection.
SV (1-2 MNTD) was also incubated at 37 °C X 15 mins with methanol plant root extracts (A.I, H.I and P.I at 1mg/ml) and tested for neurotoxicity in isolated toad´s gastrocnemius sciatic nerve preparation. H.I gave 100% protection and no protection was given by P.I and A.I.
Neutralization of cardiotoxic changes
1/2 MCTD of SV was found to be 2 mg. SV (1/2 MCTD) was incubated at 37 °C X 15 mins with aqueous plant root extracts (A.I, H.I and P.I at 0.5 to 5.0 mg) and tested for cardiotoxicity in isolated guinea pigs heart. P.I gave 93% protection (significant, P<0.01) in amplitude (Control- 24.67±0.33 mm; SV- 8.83±0.4 mm; P.I- 23.50±0.22 mm) and 80% protection (significant, P<0.01) in heart rate (Control- 142±8.2 beats/min; SV- 203±7.4 beats/min; P.I- 154±7.6 beats/min). No protection was given by H.I and A.I.
SV (1/2 MCTD) was incubated at 37 °C X 15 mins with methanol plant root extracts (A.I, H.I and P.I at 4 mg) and tested for cardiotoxicity in isolated guinea pigs heart. P.I gave 99% protection (significant, P<0.01) in amplitude (Control- 24.67±0.33 mm; SV- 8.83±0.4 mm; P.I- 23.83±0.31 mm) and 76% protection (significant, P<0.01) in heart rate (Control- 142±8.2 beats/min; SV- 203±7.4 beats/ min; P.I- 157±8.1 beats/min). No protection was given by H.I and A.I.
Discussion
Till date only symptomatic treatments are available against scorpion envenomation. Countries like India, where the anti scorpion venoms are not available, people in rural areas basically rely on folk usage of herbal products either orally or as paste applied on the bitten parts. Plants like Mangifera indica L., Aristolochia indica, Solanum indicum L., Andrographis paniculata Nees, Barringtonia acutangula L., Calamus sp. etc have been used by village folks as a treatment for scorpion envenomation [14,15]. Andrographis paniculata have been reported to possess neutralizing activity against red scorpion venom [23]. Mimosa tinuiflora could effectively neutralize Tityus serrulatus venom induced inflammation [24]. But there is no scientific validation of neutralization activities of herbs against Heterometrus bengalensis scorpion venom. The present study identified three plant root extracts (aqueous/methanol) of Hemidesmus indicus (H.I), Pluchea indica (P.I) and Aristolochia indica (A.I) having neutralizing capacity against black scorpion (Heterometrus bengalensis) venom in experimental animal models. The maximum fold of neutralization was found with aqueous root extracts as compared to methanol extracts.H.I could neutralize scorpion venom induced lethality, inflammation, PLA2 change, etc. Further, crude extract of H.I has been already reported to possess antioxidant, hepatoprotective, antisnake venom activity [25-27]. A pure compound (lupeol acetate) isolated from H.I have been already reported to possess anti inflammatory activity induced by snake venom [21]. It shows antiinflammatory activity by suppressing the capacity of snake venom induced oxidative stress and pro-inflammatory cytokines release, the two important inflammatory mediators. Therefore scorpion venom induced inflammation might be neutralized by the same mechanism as that of snake venom. Compounds like lupeol acetate, 2-OH- 4- Methoxy benzoic acid, isolated from H.I neutralized snake venom induced in vivo and in vitro changes [25,28]. Similar compounds might be responsible in neutralizing scorpion venom induced in vivo and in vitro changes subject to further testing.
P.I has also been reported to possess anti-inflammatory, antioxidant and antisnake venom activity [29-31]. Earlier, compounds like beta-sitosterol and stigmasterol isolated from the root extract of P.I significantly neutralized viper venom-induced lethal, hemorrhagic, defibrinogenation, edema and PLA2 activity [32]. Therefore the scorpion venom induced lethality, inflammation, etc. could be neutralized by the same or homologous compounds present in P.I. In the Ethnobotanical survey of folk plants for the treatment of snake envenomation in Southern part of India, A.I and H.I both have been used to neutralize the toxic effects of snake venoms in in vitro and in vivo models [27]. Both the plants have been mentioned in the folk medicine against scorpion envenomation. Other effect induced by SV includes systemic effects is renal toxicity. The sign of renal toxicity is the presence of high protein and blood in urine of SV reated animals. The plant root extracts of H.I, P.I and A.I (aqueous/ methanol) could decrease the above mentioned parameters from the urine of SV treated animals.
Previous studies indicated the presence of alkaloids, steroids, terpenoids, flavonoids, phenolic compounds, tannin, lignin in Hemidesmus indicus root extract, flavonoids, phenolic compounds, tannin, saponin, proanthocyanidins in Pluchea indica root extract and essential oil, aristolochic acid, isoaristolochic acid, sesquiterpene, ishwarol, aristololactam, 12-nonacosenoic acid in Aristolochia indica root extract [33-35]. These bioactive compounds might be responsible against experimental scorpion envenomation. Till date, no specific anti-scorpion venom (against H bengalensis) is available in India. Use of Prazosin (α1 adrenergic blocker) against red scorpion envenomation has been documented [36]. Plant extracts along with synthetic drugs might be of great importance to develop scorpion venom antagonists against scorpion sting.
Conclusion
From the above study it may be concluded that Indian medicinal plants (Hemidesmus indicus, Pluchea indica and Aristolochia indica) has the potential to neutralize scorpion venom activity in animal models subject to further detail investigations at molecular levels.Acknowledgements
The authors are indebted to Indian Council of Medical Research (ICMR), New Delhi, India (Ref. No- 58/7/2002, dt. 20.07.2006) for partial funding and to Prof. N. Paria, Department of Botany, Calcutta University, Calcutta, India for identification of the plant.References
- Srinivasan KN, Gopalakrishnakone P, Tan PT, Chew KC, Cheng B, et al. (2002) Scorpion, a molecular database of scorpion toxins. Toxicon 40: 23-31.
- de Roodt AR, Garcia SI, Salomon OD, Segre L, Dolab JA, et al. (2003) Epidemiological and clinical aspects of scorpionism by Tityus trivittatus in Argentina. Toxicon 41: 971-977.
- Meki AR, Mohamed ZM, Mohey El-deen HM (2003) Significance of assessment of serum cardiac troponin I and interleukin-8 in scorpion envenomed children. Toxicon 41: 129-137.
- Tikader BK, Bastawade DB (1983) The fauna of India. Scorpions, Scorpionida: Arachnida. Volume III, Zoological Survey of India, Calcutta, India.
- Gomes A (2014) Scorpion venom research around the world: Heterometrus Species. Toxinology 4: 351-367.
- Theakston RD, Warrell DA, Griffiths E (2003) Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 41: 541-557.
- Legros C, Martin-Eauclaire MF (1997) Scorpion toxins. C R Seances Soc Biol Fil 191: 345-380.
- Osnaya-Romero N, de Jesus Medina-Hernández T, Flores-Hernández SS, León-Rojas G (2001) Clinical symptoms observed in children envenomated by scorpion stings, at the children´s hospital from the State of Morelos, Mexico. Toxicon 39: 781-785.
- Goudet C, Chi CW, TytgatJ (2002) An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 40: 1239-1258.
- Malhotra KK, Mirdehghan CM, Tandon HD (1978) Acute renal failure following scorpion sting. Am J Trop Med Hyg 27: 623-626.
- Ismail M (1995) The scorpion envenoming syndrome. Toxicon 33: 825-858.
- Calderon-Aranda ES, Olamendi-Portugal T, Possani LD (1995) The use of synthetic peptides can be a misleading approach to generate vaccines against scorpion toxins. Vaccine 13: 1198-1206.
- Dey A, Dey A, De JN (2013) Scorpion anti-venom activity of botanicals: a pharmacological approach. Pak J Biol Sci 16: 201-207.
- Hutt MJ, Houghton PJ (1998) A survey from the literature of plants used to treat scorpion stings. J Ethnopharmacol 60: 97- 110.
- Uawonggul N, Chaveerach A, Thammasirirak S, Arkaravichien T, Chuachan C, et al (2006) Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J Ethnopharmacol 103: 201-207.
- Biswas K, Ghosh A (1973) Bharatiya Vanoushadhi (2 edn). Calcutta University, India.
- Theakston RDG, Reid HA (1983) Development of simple standard assay procedures for the characterization of snake venom. Bull World Health Organ 61: 949- 956.
- Ipsen J (1942) Systematische und zufallige Fehlerquellen bei Messung kleiner Antitoxin-mengen. I. Mitteilung: tetanusantitoxin. Zeit Immunitatsforsch Exper Ther 102: 347-368.
- Wang JP, Teng CMK (1988) Rat paw edema induced by trimucase II, a kinin releasing enzyme from Trimeresurus mycrosquatus venom. Eur J Pharmacol 157: 61-66.
- Habermann E, Neumann W (1954) Characterization of the effective components of snake venoms. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 223: 388- 398.
- Chatterjee I, Chakravarty AK, Gomes A (2006) Daboia russelli and Naja kauuthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R.Br. J Ethnopharmacol 106: 38-43.
- Langendorff O (1895) Untersuchungen am uberlebenden Saugethierherzen. Pflugers Arch 61: 291-332.
- Brahmane RI, Pathak SS, Wanmali VV, Salwe KJ, Premendran SJ, et al. (2011) Partial in vitro and in vivo red scorpion venom neutralization activity of Andrographis paniculata. Pharmacognosy Res 3: 44-48.
- Bitencourt MA, de Souza Lima MC, Torres-Rego M, Fernandes JM, da Silva Junior AA, et al. (2014) Neutralizing efects of Mimosa tenuiflora extracts against inflammation caused by Tityus serrulatus scorpion venom. Biomed Res Int 2014: 378235.
- Saravanan N, Rajasankar S, Nalini N (2007) Antioxidant effect of 2-hydroxy- 4-methoxy benzoic acid on ethanol-induced hepatotoxicity in rats. J Pharm Pharmacol 59: 445-453.
- SaravananN, Nalini N (2008) Hemidesmus indicus protects against ethanolinduced liver toxicity. Cell Mol Biol Lett 13: 20-37.
- Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S (2008) Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J Ethnopharmacol 115: 302-312.
- Alam MI, Gomes A (1998) Adjuvant effects and antiserum action potentiation by a (herbal) compound 2-hydroxy-4-methoxy benzoic acid isolated from the root extract of the Indian medicinal plant ´Sarsaparilla´ (Hemidesmus indicus R.Br.). Toxicon 36: 1423-1431.
- Sen T, Nag Chaudhuri AK (1991) Anti-inflammatory evaluation of Pluchea indica root extract. J Ethnopharmacol 33: 135-141.
- Sen T, Dhara AK, Bhattacharjee S, Pal S, Nag Chaudhuri AK (2002) Antioxidant activity of the methanol fraction of Pluchea indica root extract. Phytother Res 16: 331-335.
- Alam MI, Auddy B, Gomes A (1996) Viper venom neutralization by Indian medicinal plant (Hemidesmus indicus and Pluchea indica) root extracts. Phytother Res 10: 58-61.
- Gomes A, Saha A, Chatterjee I, Chakravarty AK (2007) Viper and cobra venom neutralization by beta-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae). Phytomedicine 14: 637-643.
- Rajan S, Shalini R, Bharathi C, Aruna V, Elgin A, et al. (2011) Pharmacognostical and phytochemical studies on Hemidesmus indicus root. Int J Pharmacogn Phytochem Res 3: 74-79.
- Cho JJ, Cho CL, Kao CL, Chen CM, Tseng CN, et al. (2012) Crude aqueous extracts of Pluchea indica (L.) less. inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death. BMC Complement Altern Med 12: 265.
- Dey A, De JN (2011) Aristolochia indica L.: A review. Asian J Plant Sci 10: 108-116.
- Bawaskar HS, Bawaskar PH (1986) Prazosin in the management of cardiovascular manifestations of scorpion sting. Lancet 1: 510-511.