Journal of Toxins
Download PDF
Research Article
*Address for Correspondence: D Gonzalez Weller, Department of Obstetrics and Gynaecology, Pediatrics, Preventive Medicine and Public Health, Toxicology, Forensic and Legal Medicine and Parasitology, University of La Laguna, 38071, La Laguna, Tenerife, Canary Islands, Spain, Tel: 616-992-801; Fax: 922-626-497; E-mail: dgonzal@ull.es
Citation: Gonzalez Weller D, Caballero A, Karlsson L, Hernandez F, Gutierrez AJ, et al. Determination of Iron, Copper, Zinc and Manganese in Sausage, Poultry-Rabbit Meat, Viscera and Red Meats Consumed by the Population in The Canary Islands, Spain. J Toxins. 2014;1(1): 7.
Copyright © 2014 Weller et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 1, Issue: 1
Submission: 27 May 2014 | Accepted: 20 June 2014 | Published: 23 June 2014
Reviewed & Approved by: Dr. Guangming Xiong, Head of the Pharmacology and Toxicology, University Kiel, Germany.
Data homogeneity was determined by Kolmogorov-Smirnov test, and Levene’s test was used for analysis of variance. As data were not distributed normally, the non-parametric tests Kruskal-Wallis and Mann Whitney U-test were performed.
The following food groups are listed in decreasing order of iron contents: viscera, red meat, cold meats, and poultry-rabbit. Decreasing concentrations of copper were found from viscera to cold meat, red meat, and meat from the poultry-rabbit group. Zinc concentrations from high to low were detected in viscera, red meat, cold meat, and poultry-rabbit. Finally, manganese contents decreased from viscera to cold meat, poultry-rabbit, and red meat.
Iron intake in the Canary Islands stemming from the consumption of cold meats, poultry-rabbit, viscera, and red meat was set at 1.1658 mg/day, with the highest intake in the island of Tenerife (1.3078 mg/ day) and the lowest in La Gomera (1.0105 mg/day). Among the four food groups studied, red meat provided the most (0.5935 mg/day) and viscera the least (0.0523 mg/day) iron intake to the overall population of the Canary Islands. These food groups provide a total copper intake of 0.1460 mg/day. The greatest intake of copper was detected on the island of Fuerteventura (0.2465 mg/day) and the lowest in Lanzarote (0.0867 mg day). In the Canary Islands, the group that contributes most to the intake of this element is that of viscera, with an intake of 0.0598 mg/day; the smallest contribution stems from poultry-rabbit meat with 0.0170 mg/day.
Determination of Iron, Copper, Zinc and Manganese in Sausage, Poultry-Rabbit Meat, Viscera and Red Meats Consumed by the Population in The Canary Islands, Spain
D Gonzalez Weller1*, A Caballero2, L Karlsson3, F Hernandez3, AJ Gutierrez1, C Rubio1, C Revert 1, JM Troyano1 and A Hardisson1
- 1Department of Obstetrics and Gynaecology, Pediatrics, Preventive Medicine and Public Health, Toxicology, Forensic and Legal Medicine and Parasitology, University of La Laguna, 38071, La Laguna, Tenerife, Canary Islands, Spain
- 2Health Inspection and Laboratory Service, Canary Health Service, 38006, Santa Cruz de Tenerife, Tenerife, Canary Islands, Spain
- 3Medical Physics and Environmental Radioactivity Laboratory, University of La Laguna, 38320, La Laguna, Tenerife, Canary Islands, Spain
*Address for Correspondence: D Gonzalez Weller, Department of Obstetrics and Gynaecology, Pediatrics, Preventive Medicine and Public Health, Toxicology, Forensic and Legal Medicine and Parasitology, University of La Laguna, 38071, La Laguna, Tenerife, Canary Islands, Spain, Tel: 616-992-801; Fax: 922-626-497; E-mail: dgonzal@ull.es
Citation: Gonzalez Weller D, Caballero A, Karlsson L, Hernandez F, Gutierrez AJ, et al. Determination of Iron, Copper, Zinc and Manganese in Sausage, Poultry-Rabbit Meat, Viscera and Red Meats Consumed by the Population in The Canary Islands, Spain. J Toxins. 2014;1(1): 7.
Copyright © 2014 Weller et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 1, Issue: 1
Submission: 27 May 2014 | Accepted: 20 June 2014 | Published: 23 June 2014
Reviewed & Approved by: Dr. Guangming Xiong, Head of the Pharmacology and Toxicology, University Kiel, Germany.
Abstract
The aim of this work was to determine the levels of Fe, Cu, Zn, and Mn in samples of sausage, bird poultry-rabbit meat, viscera and red meats, collected in the Canary Islands (Spain). Fe, Cu, Zn, and Mn were analysed by Inductively Coupled Plasma Optical Emission Spectrophotometry (ICP-OES). Mean levels of Fe, Cu, Zn, and Mn in meat samples were as follows: 12.53 mg/Kg, 1.13 mg/Kg, 20.54 mg/Kg, and 0.56 mg/Kg, respectively for sausage, 6.09 mg/Kg, 0.53 mg/Kg, 13.23 mg/Kg, and 0.23 mg/Kg, respectively for bird poultry-rabbit meat, 43.61 mg/Kg, 49.81 mg/Kg, 36.35 mg/Kg, and 2.12 mg/Kg, respectively for viscera, and 12.93 mg/Kg, 0.87 mg/Kg, 33.15 mg/Kg and 0.11 mg/ Kg, respectively for red meats. The contribution percentage of the analysed metals to Dietary Reference Intakes (DRI) and statistical analysis of the Fe, Cu, Zn, and Mn contents were carried out.Keywords
Iron; Copper; Zinc; Manganese; Meat; Meat products; ICP-OESIntroduction
Although metals are perhaps the longest known toxic agents, they are still of scientific interest. Hence, knowledge regarding their potential toxic effects and mechanisms of action has increased in recent years [1]. Metals as, e.g., iron, copper, zinc, and manganese are considered essential for humans but lead to toxic effects when ingested in excess, while lead and cadmium are considered toxic, environmental contaminants found in food [2]. Food and water are the main sources of metals for humans [3,4].Almost all food products contain iron in varying portions, but food of animal origin constitutes the major iron source to human [5,6]. The key functions of iron encompass oxygen transport in blood and muscle tissue (through haemoglobin and myoglobin), taking part-along with copper-in redox processes, and an important role in the biosynthesis of certain proteins like collagen and elastin.
The germ part of wholegrain seeds, nuts, pulses, liver, crustaceans, and molluscs are rich in copper [7]. Copper acts as a cofactor in various redox enzymes, in mitochondrial respiration, iron absorption, and elastin synthesis. It is also required for the catalytic activities of many metalloenzymes like cytochrome C oxidase, superoxide dismutase, dopamine β-hydroxylase, lysyl oxidase, and tyrosinase [7,8]. Recently, copper has been identified as a factor in prion disease prevention [9].
Zinc is widely distributed in foods and beverages, but similar to other chemical elements, contents are extremely variable and generally low. Food products of marine origin, mainly shellfish (oysters and crustaceans), are rich in zinc, followed by red meat, dairy (including eggs), and wholegrain cereals [10-12]. Zinc is involved in biochemical processes, such as cell respiration and the use of oxygen by the cell, both DNA and RNA synthesis, the preservation of the cell membrane integrity, and the elimination of free radicals, a process executed through a cascade of enzymatic systems [13].
The metals that act in redox processes in the brain, as is the case with iron, zinc, and copper, play an important role in cellular function. The maintenance of their adequate levels has been related to the prevention of diseases like Alzheimer and multiple sclerosis [14].
Manganese is widely distributed in plants and animal tissues, though at relatively low concentrations. Wholegrain cereals, dry pulses, nuts, and tea are rich in manganese. Foods of animal origin are considered poor sources of this element [6,7,15,16]. Manganese is an essential nutrient to humans as it acts as an activator and a constituent of various enzymes. It is involved in carbohydrate and fatty acid metabolism and the synthesis of arginase and coenzyme A and represents a component of metalloenzymes like superoxide dismutase A [6,17].
A high intake of iron as well as a high storage in the body has been associated with a wide variety of chronic diseases [18]. Although the problems by an overload of iron in the body’s tissues are less common than the deficiency, these may cause complications such as cirrhosis, liver cancer, diabetes, cardiomyopathy, hypogonadism and arthritis among others. But perhaps the best known and most studied syndrome caused by an excess of iron deposits in the body is the hereditary hemochromatosis (an autosomal recessive disease that causes an increase in the absorption of iron) [19,20].
Copper is an essential trace element that can be extremely toxic in excess due to the pro-oxidant activity of its ions [21]. It is an element toxic to prokaryotic and eukaryotic cells, due to it can bind to proteins and nucleic acids and cause the oxidation of lipids and proteins [22]. Intoxication occurs by intakes voluntary or accidental contamination of drinks. The regulation of the intracellular activity of copper, as well as the mechanisms that maintain homeostasis of this element, are considered crucial to maintain cell viability and prevent phenomena of toxicity [22].In general, zinc may be considered a non-toxic mineral; in fact it is the least toxic trace element of all. However, zinc supplementation in excess, can produce toxicity which would be characterized by the decrease of immune function and gastrointestinal problems (discomfort, vomiting, etc.). In patients with kidney failure who are undergoing haemodialysis, the problem is more accused, characterizing this toxic syndrome by anaemia, fever and disorders of the central nervous system [23,24].
The toxic action of manganese is exerted on the pulmonary epithelium and cerebral cortex, resulting in degenerative lesions. There are also modifications of the voice, the word and writing, as well as neurovegetative disorders and psychiatric symptoms, irritability, violent behaviour and hallucinations [7,25,26].
Considering the nutritional value of the trace metals and the fact that they can accumulate in food and lead to toxic effects in the densely populated Canary Islands with their millions of tourists every year, determination of metal contents in the different food types consumed in the Islands is of major importance.
Materials and Methods
SamplesDifferent types of meat, made up of 140 samples, were analysed and assigned to the following groups: cold meat, poultry-rabbit, viscera, and red meat. Selection criteria were based on the percentage of consumption of these products in the Canary Islands. The latter data is published in the Canary Nutrition Survey 2000 [27].
The following samples were analysed in this study (number of samples given in parenthesis): cooked ham (8), Serrano ham (6), spicy sausage (4), hard cured sausage (3), salami (3), mortadella (4), chop (6), chicken liver (10 ), calf’s liver (12), pork kidney (4), calf’s kidney (6), chicken drumsticks (12), chicken breast (12), turkey breast (8), rabbit (8), pork loin (6) pork chop (4), pork stew (3), pork tenderloin (3), ground pork (2), calf steak (6), veal stew (4), ground veal (2), bull veal (2), fillet of unweaned veal (2).
The meat samples were purchased randomly at different points of sale throughout the island of Tenerife. Approximately 500-1000 g per sample, depending on the sample type, was acquired. Samples were chosen from different brands to enhance sampling quality of each type of sample material. The identified and classified samples were homogenised. A representative portion was separated and homogenized and a representative portion separated and stored in clean polyethylene containers at -18° C until analysis, which was done in triplicate.
The plastic and glass laboratory material used for sample treatment and storage was maintained in 5% nitric acid for 24 hours and subsequently washed twice with Milli-Q (Millipore, Milford, MA) water to clean from and remove traces of metal.
Assessment of Iron, Copper, Zinc, and Manganese
A total of 20 g of homogenised sample was weighed into porcelain crucibles. In order to prevent potential metal contamination, disposable plastic was used for handling. Samples were then ovendried for at least 12 hours at a temperature of 60-80° C.
The crucibles with the samples were placed in a temperaturecontrolled muffle furnace, the temperature gradually raised (approximately 25° C/hour) to 450 ± 25° C, which was held for 18-24 hours. The obtained white ash was dissolved in 1.5% nitric acid and completed to a volume of 50 ml.
Metals were analysed by Inductively Coupled Plasma Optical Emission Spectrophotometry (ICP-OES) [28,29] using the iCAP 6300 Duo spectrometer (Thermo Scientific). The following ICP-OES conditions were applied: wavelength 259.9 nm for iron, 327.3 nm for copper, 206.2 nm for zinc, and 257.6 nm for manganese; nebuliser gas flow 0.5 l/min; auxiliary gas flow 0.5 l/min; approximate radio frequency power 1150 W; sample injection pump flow 50 rpm.
The instrument detection and quantification limits for the analysed metals were: iron 0.003 and 0.009 mg/l, respectively, copper 0.004 and 0.012 mg/l, respectively, zinc 0.002 and 0.007 mg/l, respectively, manganese 0.002 and 0.008 mg/l, respectively. These were estimated based on the instrumental response of the equipment, analysing 15 blank samples under conditions of reproducibility. The standard curves of iron, copper, zinc and manganese were as follows: Iron: 0.5-1-5-10-20-50 mg/l, respectively; Copper: 0.01-0.05-0.2-5-20-100 mg/l, respectively; Zinc: 0.5-1-5-10-20-50 mg/l, respectively and Manganese: 0.01-0.05-0.2-1-2-5 mg/l, respectively.
Quality control of the analytical assay was performed using Standard Reference Material 1577b Bovine Liver. The obtained recovery was higher than 93% (Table 1). Throughout analysis, a blank and a reference sample were measured every 20 samples.
Data homogeneity was determined by Kolmogorov-Smirnov test, and Levene’s test was used for analysis of variance. As data were not distributed normally, the non-parametric tests Kruskal-Wallis and Mann Whitney U-test were performed.
Results and Discussion
The number of samples, their means, standard deviations, and minimum and maximum are presented in Table 2. All food groups studied had detectable concentrations of iron, copper, zinc, and manganese. The group poultry-rabbit showed the lowest concentrations in iron, copper, and zinc, (6.09 ±1.78 mg/Kg, 0.53 ±0.20 mg/Kg, and 13.23 ±5.77 mg/Kg, respectively). The lowest concentration of manganese was assessed in red meat (0.11 ±0.07 mg/Kg). The food group that had the highest concentrations of the herein presented elements was that of viscera, with concentrations ranging from 2.12 ±1.13 mg/Kg of manganese to 49.81 ±55.19 mg/ Kg of copper.The following food groups are listed in decreasing order of iron contents: viscera, red meat, cold meats, and poultry-rabbit. Decreasing concentrations of copper were found from viscera to cold meat, red meat, and meat from the poultry-rabbit group. Zinc concentrations from high to low were detected in viscera, red meat, cold meat, and poultry-rabbit. Finally, manganese contents decreased from viscera to cold meat, poultry-rabbit, and red meat.
Consequently, the highest levels among all the studied metals were those of copper in viscera, and the lowest detected metal concentrations corresponded to manganese in red meat (Table 2).
Comparison of the concentrations of iron, copper, zinc, and manganese in the different studied meat groups led to the conclusion that iron and copper concentrations were significantly higher in viscera than in cold meat and red meat (p<0.05), but in meat from poultry and rabbits significantly lower than in the two aforementioned groups (p<0.05). Hence, the groups of cold meat and red meat turned out to be statistically similar. As to zinc, the groups poultry-rabbit and cold meat were characterised by significantly lower concentrations than viscera and red meat (p<0.05). Finally, all food groups differed significantly from each other (p<0.05) with respect to manganese concentrations.
Of note, there was high data variability in some of the analysed meat groups, particularly in viscera. However, this variability in biological samples is considered standard since the content of metals in foods, both from plant and animal origin, accumulate from several sources, ranging from ambient conditions to processing and production methods [6].
Table 3 gives a comparison between the data obtained in this work and studies published between 1980 and 2011.
The iron concentration observed in the food group cold meat (12.53 mg/Kg) was slightly higher than that described by Nuurtamo et al., 1980 for Finland (7 mg/Kg in cooked ham) [30], similar to that reported by Brito et al. for samples of loin and ham (10.08 and 8.33 mg/Kg, respectively) [31] and above the one found by Moreiras et al. in 1992 in spicy sausage samples (2.4 mg/Kg) [32]. The concentration of copper (1,13 mg/Kg) resembled that detected in mortadella (1.05 mg/Kg) by Ybanez et al. in loin (1.59 mg/Kg) [33], and (1.77 mg/ Kg) by Brito et al. in ham [31], although it was higher than in the samples of cooked ham (0.60 mg/Kg and 0.078 mg/Kg) described by Nuurtamo et al. and Ybanez et al., respectively [30,33] and that in hard cured sausage (0.52 mg/Kg) reported by Hernandez et al. [34]. The concentrations of zinc detected in this work (20.54 mg/Kg) were comparable to those described by Brito et al. and Moreiras et al. for loin and hard cured sausage with concentrations of 18.05 and 17 mg/ Kg, respectively [31,32], although it was higher than that from the other works consulted for comparison. Manganese concentrations from this study (0.56 mg/Kg) were lower than those reported by Nuurtamo et al. for spicy sausage samples (3 mg/Kg), but it was similar to that described by Brito et al. for samples of loin and ham with 0.38 and 0.39 mg/Kg, respectively [30,31].
In our samples from the poultry-rabbit group, iron (6.09 mg/Kg), copper (0.53 mg/Kg), zinc (13.23 mg/Kg), and manganese (0.23 mg/Kg) levels were characterised by lower concentration ranges than those proposed by Falandysz in 1991 for samples of duck, goose, chicken, hen, and rabbit [35]. Furthermore, the iron concentrations from this study were quite similar to those described by Gerber et al. for chicken breast (6 mg/Kg) [36] and Valenzuela et al. in 2011 for rabbit samples (8.3 mg/Kg) [37]. With regard to copper, our detected concentrations were lower than those found by Ysart et al. in poultry samples (0.85 mg/Kg) [38], although higher than those by Gerber et al., in 2009 in chicken breast samples (0.048 mg/Kg) [36]. Mean zinc concentrations in our samples were similar to those observed by Ysart et al. in poultry (15 mg/Kg) [38], but higher than those detected by Gerber et al. and Valenzuela et al. in samples of chicken breast (7 mg/ Kg) and of rabbit (9.5 mg/kg), respectively [36,37]. Manganese levels were, alike zinc concentrations, above those published by Gerber et al. for chicken breast (0.043 mg/Kg) [36], but lower than those by Valenzuela et al. in rabbit samples (0.8 mg/Kg) [37].
In viscera, the iron concentrations from this study (43.61mg/Kg) were virtually identical to those described by Falandysz in 1993 for calf’s liver samples (44 mg/Kg) [39] although lower than those found by the same author in calf’s kidney (72 mg/Kg). Comparing our data for copper contents (49.81 mg/Kg) with other studies, we found them lower than those detected by Lopez Alonso et al. in samples of calf’s liver (64.6 mg/Kg) and beef liver (60.3 mg/Kg) [40], higher than those by Falandysz in calf’s liver (29 mg/Kg) and calf’s kidney (5.6 mg/Kg) samples [39] and the ones by Lopez Alonso et al. in calf’s and beef kidney samples (4.91 and 3.67 mg/Kg, respectively) [40] and very close to those described by Ysart et al. for viscera (50 mg/Kg) [29] and by Lopez Alonso et al. for calf’s liver (53.3 mg/Kg) [41]. The zinc concentrations detected in this study (36.35 mg/Kg) were lower than the ones described by Falandysz in calf’s liver (43 mg/Kg) [39], by Lopez Alonso et al. in calf’s and beef liver samples (47.7 and 59.8 mg/ Kg, respectively) [40], by Ysart et al. in samples of viscera (52 mg/ Kg) [29], and by Lopez Alonso et al. in calf’s liver (45.4 mg/Kg) [41]. The manganese concentrations detected in this study (2.12 mg/Kg) resembled those in calf’s liver (1.8 mg/Kg), though lower than those in calf’s kidneys (0.93 mg/Kg), both described by Falandysz [39].
As to red meat, we observed similar iron concentrations (12.93mg/Kg) to the overall described ones, although they were slightly lower than those found by Falandysz in 1993 in veal samples (23 mg/Kg) [39] and by Gerber et al. in samples of lamb chop and veal sirloin (20 mg/Kg in each) [36]. Copper contents in our samples (0.87 mg/kg) resembled closely those described by most of the consulted authors: 1.2 mg/Kg in calf muscle [35], concentrations ranging between 0.666 and 1.26 mg/Kg in beef and veal samples [40,41], 0.8 mg/Kg and 1.1 mg/Kg in lamb samples [36,42]. However, the herein assessed copper concentrations were lower than those found by Gerber et al. in veal sirloin (0.408 mg/Kg) and pork loin (0.405 mg Kg) [36]. Zinc concentrations in our samples (33.15 mg/Kg) were higher compared to those described by Gerber et al. for samples of pork loin and lamb chop (15 and 23 mg/Kg, respectively) [36], similar to those found by Falandysz and again Gerber et al. in calf muscle (34 mg/Kg) [36] and lamb (34.2 mg/Kg) [35], but lower than in the rest of the studies consulted for comparison. Finally, manganese concentrations assessed in this study (0.11 mg/Kg) were identical to those described by Falandysz for calf muscle (0.11 mg/Kg) [39] and higher than those set by Bellof et al. and Gerber et al. for Germany and Switzerland [36,42] except for the value described by Gerber et al. for lamb chop samples (0.167 mg/Kg) [36].
The total intakes of iron, copper, zinc, and manganese from the consumption of cold meats, poultry-rabbit, viscera, and red meats, as well as from the individual food groups in the Canary Islands and each of the seven islands of the archipelago are shown in Table 4.
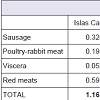
Iron intake in the Canary Islands stemming from the consumption of cold meats, poultry-rabbit, viscera, and red meat was set at 1.1658 mg/day, with the highest intake in the island of Tenerife (1.3078 mg/ day) and the lowest in La Gomera (1.0105 mg/day). Among the four food groups studied, red meat provided the most (0.5935 mg/day) and viscera the least (0.0523 mg/day) iron intake to the overall population of the Canary Islands. These food groups provide a total copper intake of 0.1460 mg/day. The greatest intake of copper was detected on the island of Fuerteventura (0.2465 mg/day) and the lowest in Lanzarote (0.0867 mg day). In the Canary Islands, the group that contributes most to the intake of this element is that of viscera, with an intake of 0.0598 mg/day; the smallest contribution stems from poultry-rabbit meat with 0.0170 mg/day.
Cold meats, poultry-rabbit, viscera, and red meat provide a total intake of 2.5219 mg/day of zinc in the Canary Islands, the highest intake being observed in the island of Tenerife and the lowest in La Gomera with 2.8166 and 2.2305 mg/day, respectively. Of note, red meat (1.5216 mg/day) and viscera (0.0436 mg/day) represent the food groups studied that provide the highest and lowest intake of zinc, respectively. As to manganese, the herein presented food groups contribute to a total of 0.0295 mg of manganese intake per day in the Canary Islands. The islands of Fuerteventura and Tenerife have the highest manganese intakes (0.334 and 0.333 mg/day) and La Gomera the lowest (0.0245 mg/day). Cold meat and viscera (0.0145 mg/day and 0.0025 mg/day, respectively) are the groups that contribute most and least manganese to the diet.
Considering an average consumption of 25.9 g of cold meat per person and day, 32.1 g of poultry-rabbit, 1.2 g of viscera, and 45.9 g of red meat, as set by ENCA, 2000 for the population in the Canary Islands, the contribution of the daily intake of these foods to the Dietary Reference Intakes (DRI) should be taken into account. Even though these DRIs are dietary recommendations both Canadian and US, we opted for them to evaluate the metal intakes via the herein studied food groups because they represent the most recent, established dietary recommendations.
The DRIs for adults (men and women) for the elements analysed in this study are as follows: 8-18 mg of Fe/day, 700-900 μg of Cu/ day, 8-11 mg of Zn/day, and 1.6-2.3 mg of Mn/day [43]. Therefore, a consumption of 25.9 g of cold meat per person and day provides 0.3245 mg Fe/day, resulting in 1.803-4.056% of the DRI; 0.0293 mg Cu/day (29.3 μg Cu/day), corresponds to 3.255-4.186% of its DRI, 0.5320 mg Zn/day, corresponds to 4.836-6.650 % of its DRI, and 0.0145 mg de Mn/day represents 0.630-0.906 % of its DRI. On the other hand, the consumption of 32.1 g of poultry-rabbit meat provides 0.1955 mg Fe/day, i.e., 1.086-2.444% of the DRI, 0.0170 mg Cu/day (17.0 μg Cu/day), which corresponds to 1.889-2.429% of the DRI, 0.4247 mg Zn/day, i.e., 3.861-5.309% of the DRI, and 0.0074 mg Mn/ day, corresponding to 0.322-0.463% the DRI. The consumption of 1.2 g per person per day of viscera provides 0.0523 mg Fe/day, resulting in 0.291-0.654% of its DRI, 0.0598 mg Cu/day (59.8 μg Cu/day), i.e., 6.644-8.543% of the DRI, 0.0436 mg Zn/day, corresponding to 0.396-0.545% of the DRI, and 0.0025 mg Mn/day, which corresponds to 0.109-0.156% of the DRI. Finally, the consumption of 45.9 g of red meat provides 0.5935 mg Fe/day, resulting in 3.297-7.419% of the DRI, 0.0399 mg Cu/day (39.9 Cu μg/day), i.e., 4.433-5.7% of the DRI, 1.5216 mg Zn/day, corresponding to 13.833-19.02% of the DRI, and 0.0050 mg Mn/day, i.e., 0.217-0.313 of its DRI.
The daily meat consumption in the population of Canary Island accounts for 8% of all types of food consumed. Therefore, other food sources that can provide iron, copper, zinc, and manganese have to be taken into account.
Conclusion
Whereas meat consumption in the Canary Islands accounts for only 8% of intake of the herein analysed metals, other sources that can contribute to their intake should be considered. The data resulting from this study demonstrate the need to assess metal concentrations in food to establish safe, overall intakes for the population.References
- Gutierrez AJ, Gonzalez-Weller D, Gonzalez T, Burgos A, Lozano G, et al. (2007) Content of toxic heavy metals (Hg, Pb, Cd) in Canned Variegated Scallops (Chlamys varia). J Food Prot 70: 2911-2915.
- Frias I, Rubio C, Gonzalez-Iglesias T, Gutierrez AJ, Gonzalez-Weller D, et al. (2008) Metals in Fresh Honeys from Tenerife Island, Spain. Bull Environ Contam Toxicol 80: 30-33.
- Rubio C, Gonzalez-Iglesias T, Revert C, Reguera JI, Gutierrez AJ, et al. (2005) Lead Dietary Intake in a Spanish Population (Canary Islands). J Agric Food Chem 53: 6543-6549.
- Gonzalez-Weller D, Karlsson L, Caballero A, Hernandez F, Gutierrez AJ, et al. (2006) Lead and Cadmium in meat and meat products consumed by a Spanish population (Tenerife Island. Spain). Food Addit Contam 23: 757-763.
- National Research Council (1991) Raciones Dieteticas Recomendadas. Subcommitee on the Tenth Edition of RDAs. 1st Spanish edition of the 10th original edition of: Recommended dietary allowances. Ediciones Consulta S.A., Barcelona.
- Reilly C (2002) Metal contamination of food. Its significance for food quality and human health. (Third Edition), Blackwell Science Ltd, United Kingdom.
- Aranda P, Llopis J (1993) Minerales. In: Aranceta J, Aranda P, Barrionuevo MM et al. (dirs). Nutricion y dietetica. Aspectos sanitarios. Consejo General de Colegios Oficiales de Farmaceuticos, Girona.
- Tapiero H, Townsend DM, Tew KD (2003) Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 57: 386-398.
- Harris ED (2003) Basic and clinical aspects of copper. Crit Rev Anal Chem 40: 547-586.
- Sandstead H (1995) Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am J Clin Nutr 61: 621S-624S.
- Honda R, Tsuritani I, Ishizaki M (1997) Zinc and copper levels in ribs of cadmium-exposed persons with special reference to osteomalacia. Environ Res 75: 41-48.
- Camara F, Amaro MA (2003) Nutritional aspect of zinc availability. Int J Food Sci Nutr 54: 143-151.
- Rubio Armedariz C, Gonzalez Weller D, Alonso S, Revert Girones C, Hardisson de la Torre A (2004) Zn, Mn, Cu, Se, Cr: Nutricion y suplementacion. Alimentaria 353: 37-44.
- Todorich BM, Connor JR (2004) Redox metals in Alzheimer's disease. Ann NY Acad Sci 1012: 171-178.
- Lozano A, Barbera R, Farre R (1987) Manganeso: Funciones en el organismo e importancia en alimentacion. Alimentaria 186: 55-59.
- Keen CL, Zidenberg-Cherr S (1991) Manganeso. In: Brown ML, Filer LJ, Guthire HA et al. (dirs). Conocimientos actuales sobre nutricion. OPS, Washington.
- Nielsen FH (1994) Ultratrace elements. In: Shils ME, Olsen JA, Shike M (dirs). Modern Nutrition in Health and Disease. Lea & Febiger, Philadelphia.
- Swanson CA (2003) Iron intake and regulation: implications for iron deficiency and iron overload. Alcohol 30: 99-102.
- Burke W, Imperatore G, Reyes M (2001) Iron deficiency and iron overload: effects of diet and genes. Proc Nutr Soc 60: 73-80.
- Burke W, Reyes M, Imperatore G (2002) Hereditary haemochromatosis: i disease in the population. Best Pract Res Clin Haematol 15: 315-328.
- Llanos RM, Mercer JF (2002) The molecular basis of copper homeostasis copper-related disorders. DNA Cell i 21: 259-270.
- Florianczyk B (2003) Copper in the organism-transport and storage in the cells. Ann Univ Mariae Curie Sklodowska 58: 85-88.
- Saltzman B, Gross S, Yeager D, Meiners B, Gartside P (1990) Total Body burdens and tissue concentrations of lead, cadmium, cooper, zinc and ash in 55 human cadavers. Environ Res 52: 126-145.
- Czajka-Narins DM (1995) Minerales. In: Mahan LK and i MT (dirs). Nutricion y dietoterapia. Mc Graw-Hill i, Mexico.
- Villa Elizaga I, Navarro Blasco I, Martin Perez A (1999) Elementos traza. In: Hernandez M and Sastre A (dirs). Tratado de Nutricion. Ediciones Diaz de Santos, S.A., Madrid.
- Klaassen CD and Watkins JB (2001) Manual de toxicología. Mc i-Hill Interamericana, Mexico.
- Serra i L, Armas Navarro A, Ribas i L (2000) Food consumption and food sources of energy and nutrients in Canary Islands (1997-98). Arch Latinoam Nutr 50: 23-33.
- Chamberlain I, Adams K, Le S (2000) ICP-MS determination of trace elements in fish. At Spectrosc 21: 118-122.
- Millar-Ihli NJ, Baker SA (2001) Trace element composition of municipal waters in the United States: A comparision of ICP-AES and ICP-MS Methods. J Food Compos Anal 14: 619-629.
- Nuurtamo M, Varo P, Saari E, Koivistoinen P (1980) Mineral element composition of Finnish food. Acta Agr Scand 22: 57-87.
- Brito G, Diaz C, Galindo L, Hardisson A, Santiago D, et al. (1990) Levels of metals in canned meat products: intermetallic correlations. Bull Environ Contam Toxicol 44: 309-316.
- Moreiras O, Carvajal MB, Cabrera ML (1992) La composicion de los alimentos (contenido en energia, macronutrientes, minerales, vitaminas, acidos grasos y colesterol). Eudema, Madrid.
- i N, Montoso R, Catala R, Flores J (1982) Contenido de cadmio, plomo y cobre de productos carnicos. Rev i Tecnol 22: 419-425.
- Hernandez P, Hernandez L, Garcia C (1990) Separacion de cadmio, cobre, plomo y cinc en carnes con una resina sintetica de de negro eriocromo T. An i 86: 249-251.
- Falandysz J (1991) Manganese, copper, zinc, iron, cadmium, mercury and lead in muscle meat, liver and kidneys of poultry, rabbit and sheep slaughtered in the northern part of Poland, 1987. Food Addit Contam 8: 71-83.
- Gerber N, Brogioli R, Hattendorf B, i MR, Wenk C, et al. (2009) Variability of selected trace elements of different meat cuts determined by ICP-MS and DRC-ICPMS. Animal 3: 166-172.
- Valenzuela C, de Romana DL, Schmiede C, Morales MS, Olivares M, et al. (2011) Total iron, heme iron, zinc, and copper content in rabbit meat and viscera. Biol Trace Elem Res 143: 1489-1496.
- Ysart G1, Miller P, Croasdale M, Crews H, Robb P, et al. (2000) 1997 UK Total diet study-dietary exposures to aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Addit Contam 17: 775-786.
- Falandysz J (1993) Some toxic and essential trace metals in cattle from the northern part of Poland. Sci Total Environ 136: 177-191.
- Lopez Alonso M, Benedito JL, Miranda M, Castillo C, Hernandez J, et al. (2000) Toxic and trace elements in liver, kidney and meat from slaughtered in Galicia (NW Spain). Food Addit Contam 17: 447-457.
- Lopez Alonso M, Benedito JL, Miranda M, Castillo C, Hernandez J, et al. (2002) Contribution of cattle products to dietary intake of trace and toxic elements in Galicia, Spain. Food Addit Contam 19: 533-541.
- Bellof G, Most E, Pallauf J (2007) Concentration of copper, iron, manganese and zinc in muscle, fat and bone tissue of lambs of the breed German Merino Landsheep in the course of the growing period and different feeding intensities. J Anim Physiol Anim Nutr (i) 91: 100-108.
- Institute of Medicine, Food and Nutrition Board (2001) Dietary Reference Intakes for Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington, D.C.
- Valenzuela C, de Romana DL, Olivares M, Morales MS, Pizarro F (2009) Total iron and heme iron content and their distribution in beef meat and viscera. Biol Trace Elem Res 132: 103-111.