Journal of Syndromes
Download PDF
Research Article
Are there Racial Differences in β-cell Compensation among Pregnant Women with Gestational Diabetes?
Naito M, Furukawa S*, Ohno T And Kawase R
Department of Obstetrics and Gynecology, Kawakita General
Hospital, Asagaya-kita, Suginami City, Tokyo, Japan
*Address for Correspondence: Seishi Furukawa, Department of Obstetrics and Gynecology,
Kawakita General Hospital, Asagaya-kita, Suginami City, Tokyo, Japan. E-mail Id: shiiba46seishi@gmail.com
Submission: September 13, 2025
Accepted: October 07, 2025
Published: October 09, 2025
Accepted: October 07, 2025
Published: October 09, 2025
Copyright: © 2025 Naito M, et al. This is an open access article
distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction
in any medium, provided the original work is properly cited.
Keywords:Pregnancy/β cell compensation /GDM / interracial differences/obese
Abstract
Introduction: To compare β cell compensation between foreign
and Japanese women with gestational diabetes mellitus (GDM).
Methods: We retrospectively evaluated pregnant women diagnosed with GDM who underwent measurements of HOMA-IR and HOMA-β. Subjects were divided into subgroups based on the cutoff value of HOMA-IR (≥1.4) indicating GDM positivity and nationality. Based on this classification, we compared HOMA-β and HOMA-IR during pregnancy and the postpartum period, as well as changes in body weight from pre-pregnancy to postpartum. Data are expressed as a number or median.
Results: The study included the following groups: foreign women with HOMA-IR ≥1.4 (G-F, n=18), Japanese women with HOMA-IR ≥1.4 (G-JH, n=31), and Japanese women with HOMA-IR <1.4 (G-JL, n=27). During pregnancy, there was no significant difference in HOMA-β between G-F and G-JH (134% vs.127%, p=0.75), whereas G-JL showed the lowest value (72%). Postpartum HOMA-β was significantly higher in G-F compared to G-JH (98% vs. 63%, p<0.01), with G-JL showing the lowest value (40%). In G-F, there were no significant differences in HOMA-β or HOMA-IR between the pregnancy and postpartum periods. In contrast, both G-JH and G-JL showed significant decreases in postpartum HOMA-β and HOMA-IR. Postpartum body weight did not return to pre-pregnancy levels in G-F, while it was recovered in G-JH.
Conclusions: In comparison with the postpartum period, an augmentation of insulin secretion was observed in Japanese GDM women, whereas it was not prominent in foreign women with HOMAIR ≥1.4. Elevated insulin resistance at the postpartum period, along with a lack of weight reduction, suggested that altered metabolic adaptation may be involved
Methods: We retrospectively evaluated pregnant women diagnosed with GDM who underwent measurements of HOMA-IR and HOMA-β. Subjects were divided into subgroups based on the cutoff value of HOMA-IR (≥1.4) indicating GDM positivity and nationality. Based on this classification, we compared HOMA-β and HOMA-IR during pregnancy and the postpartum period, as well as changes in body weight from pre-pregnancy to postpartum. Data are expressed as a number or median.
Results: The study included the following groups: foreign women with HOMA-IR ≥1.4 (G-F, n=18), Japanese women with HOMA-IR ≥1.4 (G-JH, n=31), and Japanese women with HOMA-IR <1.4 (G-JL, n=27). During pregnancy, there was no significant difference in HOMA-β between G-F and G-JH (134% vs.127%, p=0.75), whereas G-JL showed the lowest value (72%). Postpartum HOMA-β was significantly higher in G-F compared to G-JH (98% vs. 63%, p<0.01), with G-JL showing the lowest value (40%). In G-F, there were no significant differences in HOMA-β or HOMA-IR between the pregnancy and postpartum periods. In contrast, both G-JH and G-JL showed significant decreases in postpartum HOMA-β and HOMA-IR. Postpartum body weight did not return to pre-pregnancy levels in G-F, while it was recovered in G-JH.
Conclusions: In comparison with the postpartum period, an augmentation of insulin secretion was observed in Japanese GDM women, whereas it was not prominent in foreign women with HOMAIR ≥1.4. Elevated insulin resistance at the postpartum period, along with a lack of weight reduction, suggested that altered metabolic adaptation may be involved
Introduction
Studies on type 1 diabetes have shown that insulin requirements
during pregnancy increase by ca. 1.5 to 2 times compared to the
non-pregnant state [1,2]. To meet this increased insulin demand,
pancreatic β-cells undergo hypertrophy and neogenesis, thereby
enhancing insulin production [3,4]. This adaptive process is referred
to as β-cell compensation during pregnancy. Gestational diabetes
mellitus (GDM) is thought to occur when this compensatory response
is insufficient relative to the heightened insulin resistance associated
with pregnancy [5,6].
It is well established that Japanese individuals are more susceptible to developing diabetes than Western populations, even in the absence of obesity. This predisposition is primarily attributed to an inherent defect in insulin secretion, which plays a central role in the pathogenesis of diabetes in the Japanese population [7].
It is well established that Japanese individuals are more susceptible to developing diabetes than Western populations, even in the absence of obesity. This predisposition is primarily attributed to an inherent defect in insulin secretion, which plays a central role in the pathogenesis of diabetes in the Japanese population [7].
This characteristic is also observed among Japanese women with
GDM, ca. 40% of whom present a lean phenotype with both low
insulin resistance and reduced insulin secretion capacity [8]. If
this inherently low basal insulin secretory ability contributes to the
development of GDM, it is reasonable to hypothesize that impaired
β-cell compensation during pregnancy may also be involved. In an
effort to investigate this possibility, cross-ethnic comparative studies
including both GDM and non-GDM individuals are necessary.
However, no such studies have been conducted in Japan to date.
Our hospital provides obstetric care to a diverse population,
including foreign nationals such as women from Nepal. Although
our current study is limited to individuals with GDM, this clinical
setting allows for a comparison of insulin secretion capacity in
relation to insulin resistance across different ethnic groups. In this
study, we aimed to examine differences in insulin secretory function
between Japanese and non-Japanese women with GDM.
Part of this study was presented as a poster presentation at the 77th Annual Scientific Meeting of the Japan Society of Obstetrics and Gynecology in Okayama, Japan, 2025.
Part of this study was presented as a poster presentation at the 77th Annual Scientific Meeting of the Japan Society of Obstetrics and Gynecology in Okayama, Japan, 2025.
Materials and Methods
This observational study was approved by the Ethics Committee
of our institution (Kawakita General Hospital Approval Number:
2024-15). Opt-out consent was implemented through our hospital’s
website in accordance with the Helsinki Declaration. The publicly
available study outline allowed potential participants to decline
research participation.
We undertook a retrospective study of pregnant women who
were diagnosed with GDM who delivered at our hospital between
January 2021 and March 2024. GDM was diagnosed based on the
criteria of the Japan Diabetes Society [9]. Fasting insulin levels were
also measured with the oral glucose tolerance test (OGTT). Cases
without fasting insulin measurements were excluded. From the
medical charts of the group under investigation, we extracted fasting
plasma glucose and fasting insulin data from the patients’ medical
records. Using these data, we calculated the Homeostatic Model
Assessment of beta-cell function (HOMA-β) and Homeostasis Model
Assessment of Insulin Resistance (HOMA-IR) as indicators of insulin
secretion and insulin resistance, respectively, during pregnancy and
postpartum. HOMA-β was calculated using the formula: fasting
insulin × 360/ (fasting glucose [mg/dL] – 63), and HOMA-IR with:
fasting insulin × fasting glucose/405. The postpartum OGTT was
performed at 6–8 weeks after delivery.
Background characteristics also extracted from medical records
included ethnicity (Japanese or foreign national), parity (primiparous
or multiparous), pre-pregnancy and postpartum body weight
(measured at the time of postpartum OGTT), pre-pregnancy body
mass index (BMI), and gestational age at GDM diagnosis.
We initially classified subjects based on HOMA-IR cutoff values
(≥1.4 or <1.4), which indicate GDM positivity, and their nationality.
Cutoff values were obtained from a previous study comparing
pregnant women diagnosed with GDM and those with normal
glucose tolerance following OGTT [8]. We then compared HOMA-β
and HOMA-IR during pregnancy and postpartum between these
groups, in addition to pre-pregnancy and postpartum body weight.
For the statistical analyses, categorical variables among groups were compared using the chi-square test, and continuous variables using the Kruskal–Wallis test followed by Steel–Dwass post hoc analyses. For comparisons between two groups, Welch’s t-test and paired t-test (two-tailed) were employed. A p-value <0.05 was considered statistically significant. Data are presented as the number of cases and median (IQR).
For the statistical analyses, categorical variables among groups were compared using the chi-square test, and continuous variables using the Kruskal–Wallis test followed by Steel–Dwass post hoc analyses. For comparisons between two groups, Welch’s t-test and paired t-test (two-tailed) were employed. A p-value <0.05 was considered statistically significant. Data are presented as the number of cases and median (IQR).
Results
A total of 80 pregnant women with GDM were included in the
study. Of these, 58 were Japanese and 22 were of foreign nationality,
including 19 Nepalese women. Based on HOMA-IR cutoff value,
participants were classified into four groups: 18 foreign nationals with
HOMA-IR ≥1.4 (G-F), 31 Japanese with HOMA-IR ≥1.4 (G-JH), and
27 Japanese with HOMA-IR <1.4 (G-JL). Due to the small number
of foreign participants with HOMA-IR <1.4 (n=4), this group was
excluded from the intergroup comparison.
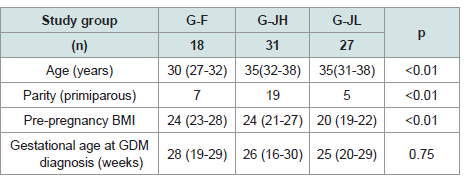
In the comparison of background characteristics among the three
groups, G-F was the youngest (p<0.01), while there was no significant
difference in age between G-JH and G-JL (p=0.96). The proportion
of multiparous women was highest in G-JH (p<0.01). Pre-pregnancy
BMI was lowest in G-JL (p<0.01), with no significant difference
between G-F and G-JH (p=0.46). Gestational age at GDM diagnosis
did not differ significantly among the three groups (p=0.75) [Table 1].
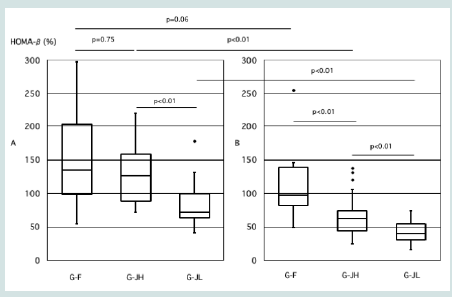
During pregnancy, there was no significant difference in HOMA-β
between G-F and G-JH (134% vs.127%, p=0.75), while G-JL showed
the lowest value (72%, p<0.01) [Figure 1]. In the postpartum period,
HOMA-β was significantly higher in G-F compared to G-JH (98%
vs.63%, p<0.01), with G-JL again showing the lowest value (40%,
p<0.01) (Figure 1).
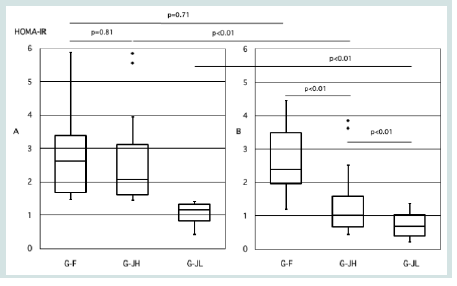
Regarding HOMA-IR during pregnancy, no significant difference was observed between G-F and G-JH (2.6 vs.2.1, p=0.81), while G-JL had the lowest value (1.2, p<0.01) [Figure 2A]. In the postpartum period, HOMA-IR remained significantly higher in G-F compared to G-JH (2.4 vs.1.0, p<0.01), with G-JL again having the lowest value (0.7, p<0.01) [Figure 2].
Regarding HOMA-IR during pregnancy, no significant difference was observed between G-F and G-JH (2.6 vs.2.1, p=0.81), while G-JL had the lowest value (1.2, p<0.01) [Figure 2A]. In the postpartum period, HOMA-IR remained significantly higher in G-F compared to G-JH (2.4 vs.1.0, p<0.01), with G-JL again having the lowest value (0.7, p<0.01) [Figure 2].
Table 1:Clinical Characteristics of the three GDM groups. Results are
expressed as number or median. G-F: foreign nationals with HOMA-IR ≥1.4,
G-JH: Japanese with HOMA-IR ≥1.4, G-JL: Japanese with HOMA-IR <1.4.
Three groups were compared using the chi-square test and the Kruskal–Wallis
test followed by Steel–Dwass post hoc analyses. A p-value <0.05 was considered
statistically significant. GDM: gestational diabetes mellitus.
Figure 1:Comparison of HOMA-β during pregnancy and postpartum among
the three groups. A: During Pregnancy, B: Postpartum, G-F: foreign nationals
with HOMA-IR ≥1.4, G-JH: Japanese with HOMA-IR ≥1.4, G-JL: Japanese
with HOMA-IR <1.4. Three groups were compared using the Kruskal–Wallis
test followed by Steel–Dwass post hoc analyses. Welch’s t-test was used
for comparisons between two groups. A p-value <0.05 was considered
statistically significant. GDM: gestational diabetes mellitus.
Figure 2: Comparison of HOMA-IR during pregnancy and postpartum among
the three groups. A: During Pregnancy, B: Postpartum, G-F: foreign nationals
with HOMA-IR ≥1.4, G-JH: Japanese with HOMA-IR ≥1.4, G-JL: Japanese
with HOMA-IR <1.4. Three groups were compared using the Kruskal–Wallis
test followed by Steel–Dwass post hoc analyses. Welch’s t-test was used
for comparisons between two groups. A p-value <0.05 was considered
statistically significant. GDM: gestational diabetes mellitus.
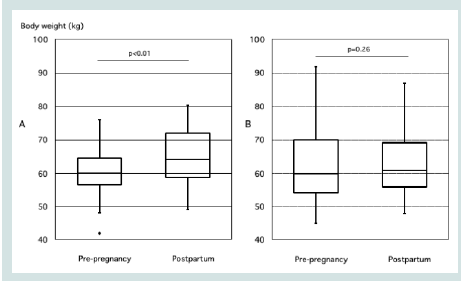
Figure 3: Comparison of pre-pregnancy and postpartum body weight in
GDM cases with HOMA-IR ≥ 1.4 during Pregnancy. A: Foreign nationals with
HOMA-IR ≥1.4, B: Japanese with HOMA-IR ≥1.4. A paired t-test was used
for comparisons. A p-value <0.05 was considered statistically significant.
GDM: gestational diabetes mellitus.
Intra-group comparisons revealed no significant difference
between pregnancy and postpartum HOMA-β or HOMA-IR in
G-F (0.06 and 0.71, respectively). However, in both G-JH and G-JL,
HOMA-βand HOMA-IR during pregnancy was significantly higher,
being ca. twice that observed postpartum (p<0.01 and p<0.01,
respectively).
Among four foreign participants with HOMA-IR <1.4 who were excluded from the intergroup comparison, the median HOMA-β during pregnancy was 121% (89-189), the median HOMA-β in the postpartum period was 62% (54-74), and the median HOMA-IR in the postpartum period was 0.8 (0.64-0.95).
When comparing body weight before pregnancy and postpartum, G-F had not returned to pre-pregnancy weight by the time of postpartum testing (p=0.26), unlike the case with G-JH (p<0.01) [Figure 3]. Among four foreign participants with HOMA-IR <1.4 who were excluded from the intergroup comparison, three out of the four cases had returned to their pre-pregnancy weight.
Among four foreign participants with HOMA-IR <1.4 who were excluded from the intergroup comparison, the median HOMA-β during pregnancy was 121% (89-189), the median HOMA-β in the postpartum period was 62% (54-74), and the median HOMA-IR in the postpartum period was 0.8 (0.64-0.95).
When comparing body weight before pregnancy and postpartum, G-F had not returned to pre-pregnancy weight by the time of postpartum testing (p=0.26), unlike the case with G-JH (p<0.01) [Figure 3]. Among four foreign participants with HOMA-IR <1.4 who were excluded from the intergroup comparison, three out of the four cases had returned to their pre-pregnancy weight.
Discussion
During normal pregnancy, insulin requirements typically
increase by 50–100% [1,2]. To meet this demand, insulin secretion
must increase by ca. 1.5- to 2-fold. In the present study, although
Japanese women with GDM exhibited lower insulin secretory capacity
in the postpartum period compared to foreign GDM women, their
insulin secretion during pregnancy was significantly higher, being
ca. twice that observed postpartum. This relative increase suggested
that β-cell compensation during pregnancy is active in Japanese
women with GDM. In contrast, foreign GDM women (primarily
of Nepalese origin), who exhibited higher insulin resistance during
pregnancy, showed no significant difference in insulin secretion or
insulin resistance between the pregnancy and postpartum periods.
This finding indicated a limited amplification of β-cell compensation
during pregnancy in this group (Figure 1) (Figure 2). On the
otherhood, in foreign participants with HOMA-IR <1.4 who were
excluded from the intergroup comparison, insulin secretory capacity
was reduced by half in the postpartum period, accompanied by a
decrease in insulin resistance.
In the present study, foreign GDM women, who exhibited
higher insulin resistance during pregnancy, did not return to their
pre-pregnancy weight by the postpartum period. Notably, their prepregnancy
BMI did not differ from that of Japanese GDM women
who had high insulin resistance during pregnancy. Furthermore,
with increasing age or multiparity, insulin resistance due to obesity
tends to increase [10,11]. Japanese GDM women with high insulin
resistance were generally older and more likely to be multiparous
than their foreign national counterparts, suggesting that foreign
GDM women may be at higher risk of developing insulin resistance
due to obesity. Nevertheless, these Japanese GDM women returned
to their pre-pregnancy weight by the postpartum period, and their
insulin resistance significantly decreased. These sequential changes
observed in Japanese GDM women with high insulin resistance
appear to parallel the pattern of fat mass gain during pregnancy and
its subsequent reduction in the postpartum period [12]. In contrast,
among foreign GDM women, insulin resistance remained elevated
even at 6 to 8 weeks postpartum, a period when the physiological effects
of pregnancy typically subside. This persistent insulin resistance may
be explained by minimal postpartum changes in fat mass. Most of
the foreign GDM women in this study were of Nepalese origin, whose
dietary habits differ substantially from those of Japanese women.
Traditionally, the Nepalese diet has been high in carbohydrates
and low in protein and fat. However, the recent introduction of
Westernized dietary patterns has led to excessive intake of both
carbohydrates and fats, raising concerns about increased risk of noncommunicable
diseases [13]. It is therefore plausible that foreign
GDM women with high insulin resistance had already experienced
qualitative and/or quantitative alterations in fat metabolism prior to
pregnancy, in contrast to their Japanese counterparts.
During pregnancy, insulin production increases to meet rising
insulin demands, primarily through pancreatic β-cell hypertrophy
and neogenesis [3,4]. This β-cell expansion is stimulated by
pregnancy-related hormones such as human placental lactogen
(hPL) and prolactin (PRL) [4]. Independently, obesity also induces
β-cell compensation. As insulin resistance increases with obesity, the
demand for insulin rises, leading to β-cell proliferation and an overall
increase in β-cell mass [14,15]. If both pregnancy-related hormonal
stimulation and obesity-induced insulin resistance act independently,
it would be expected that insulin secretion capacity during pregnancy
would increase even further due to their cumulative effects. However,
in the present study, foreign GDM women with high insulin
resistance exhibited no significant changes in insulin secretion
capacity or insulin resistance between the pregnancy and postpartum
periods. This suggested that synergistic enhancement of β-cell
compensation did not occur. One possible explanation is that β-cell
compensation due to obesity was already active prior to pregnancy,
and the capacity for further β-cell hypertrophy or neogenesis had
reached its physiological limit. As a result, in foreign GDM women
with high insulin resistance, additional β-cell compensation during
pregnancy may have been limited or mitigated. In contrast, among
the four foreign GDM women with low insulin resistance, who were
not included in the intergroup comparison, both insulin secretion
capacity and insulin resistance declined in the postpartum period as
observed in Japanese women with GDM. This suggests that, rather
than reflecting ethnic differences, variations in fat metabolism may
account for the presence of two types of women with GDM: those
who exhibit elevated insulin resistance during pregnancy followed
by a decline in the postpartum period, and those in whom insulin
resistance remains unchanged.
Maternal nutritional status, including obesity, is thought to
influence β-cell compensation during pregnancy, although no
consensus has been reached regarding this issue [16]. For instance,
although insulin resistance is typically increased in obese women,
reports on insulin secretory responses during pregnancy are
conflicting, with some studies indicating an increase, while others
suggest a decrease compared to non-obese women [17,18]. In
contrast, animal studies have provided important insights into the
mechanisms underlying changes in β-cell compensation. In one
study, mice fed a high-calorie diet more than three months before
conception exhibited β-cell hypertrophy accompanied by increased
apoptosis and expression of inflammatory markers in pancreatic
islets [19]. Another study demonstrated that pregnant rats fed a high fat
diet exhibited enhanced glucose-stimulated insulin secretion in
vivo, while isolated islet perfusion experiments showed a reduced
insulin secretory response to glucose stimulation [20]. The former
study suggested that morphological adaptations of β-cells, such as
hypertrophy, may be accompanied by apoptosis, indicating functional
decline. The latter study suggested that while β-cell function may be
impaired, a compensatory response to increased insulin resistance
during pregnancy still occurred. These experimental findings help to
explain our clinical observation: in foreign GDM women with high
insulin resistance, the amplification of β-cell compensation during
pregnancy appeared limited, and elevated insulin resistance persisted
even into the postpartum period.
Whether the observed differences in β-cell compensation during
pregnancy among women with GDM truly originate from differences
in pre-pregnancy fat metabolism requires precise assessment not only
of insulin secretion capacity and insulin resistance prior to pregnancy,
but also of qualitative and quantitative changes in fat metabolism.
In our experience, abdominal ultrasonography performed in GDM
cases often revealed fatty liver predominantly in foreign women
with GDM, suggesting a metabolic state prone to hepatic fat
accumulation. Therefore, future prospective studies incorporating
evaluations of body fat volume and fat tissue distribution, including
imaging diagnostics, are warranted. Additionally, this study focused
exclusively on women with GDM and did not include comparisons
with non-GDM pregnant women. It is also necessary to investigate
whether the ethnic differences in β-cell compensation identified here
are similarly observed in non-GDM pregnant women. Generally,
sustained β-cell compensation leads to oxidative and endoplasmic
reticulum stress in β-cells, resulting in progressive functional decline
[21,22,23]. A study conducted in Japan similarly reported more
pronounced β-cell dysfunction in obese individuals [24]. Based on
these observations, women with a history of GDM who maintain
prolonged β-cell compensation may experience rapid β-cell
deterioration and an earlier transition to overt diabetes.
In conclusion, irrespective of basal insulin secretory capacity,
Japanese women with GDM exhibited an approximately twofold
increase in insulin secretory capacity during pregnancy compared
with the postpartum period, whereas it was not prominent in
foreign GDM women with high insulin resistance. These differences
are considered to arise not from ethnic disparities but rather from
variations in metabolic states that induce insulin resistance prior
to pregnancy. Metabolic alterations resulting in excessive insulin
resistance may induce functional changes in β-cells. Therefore,
longitudinal investigations are needed to evaluate the risk of
progression to impaired glucose tolerance or type 2 diabetes in this
population.
Conflicts of interest
There is no conflict of interest.
Approval code issued by the institutional review board (IRB) and the name of the institution(s) that granted the approval; Ethics Committee of Kawakita General Hospital (Approval Number: 2024- 15).
Approval code issued by the institutional review board (IRB) and the name of the institution(s) that granted the approval; Ethics Committee of Kawakita General Hospital (Approval Number: 2024- 15).