Journal of Surgery
Download PDF
Special Issue: Surgical Oncology
Case Report
*Address for Correspondence: Joshua T. Tieman, Department of General Surgery, University of Arizona, USA, Tel: 602-714-2518; Fax: 520-626-2247; E-mail:Tieman@email.arizona.edu
Citation: Tieman JT, Loebl H, Ilyas MIM, Nguyen T, Krouse R. Role of Gastrectomy in the Management of Gastric Pre-Malignancies - A Review of Current Literature and Illustrative Case Report. 2016; S(2): 5.
Copyright © 2016 Tieman JT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Special Issue: 2
Submission: 15 Febraury 2016 | Accepted: 15 March 2016 | Published: 21 March 2016
Editors:Dr. Marlon A Guerrero, Assistant Professor of Surgery, University of Arizona, USA
Dr. James A Warneke, Associate Professor of Surgery, University of Arizona, USA
Gastric cancer has generally been subdivided histologically into intestinal and diffuse, with the intestinal type representing the majority of cases. The geographic variability in disease distribution is closely related to the regional prevalence of H. pylori. Despite its position as the third leading cause of cancer-related deaths, there has been a global decrease in the overall incidence of gastric cancer in recent decades [1]. However, this pattern is not reflected uniformly across gastric cancer subtypes. Review of epidemiologic data in lowincidence areas including the United States shows a relative increase in the proportion of diffuse gastric cancers, particularly in Signet Ring Cell Carcinoma (SRCC) [2]. These epidemiologic trends support an accumulating body of evidence that suggests that intestinal GC and diffuse GC are two distinct diseases with different clinical behavior. These characteristics have significant clinical implications that impact both diagnosis and management and therefore warrant increased attention in current medical literature.
Pathogenesis
It is currently believed that the pathogenesis of intestinal type GC is related to environmental risk factors such as H. pylori infection, food, and smoking status. In comparison, it appears that diffuse type GC has a much larger genetic component related to its development. A proposed model of carcinogenesis has been developed based on studies of hereditary diffuse cancers, which only represent 1-3% of total GCs [3]. Hereditary Diffuse Gastric Cancer (HDGC) is an autosomal dominant germline mutation of the CDH1 gene, which encodes E-cadherin, a cellular adhesion protein. Mutation carriers have a 70% lifetime risk for developing diffuse GC [3-5]. Gastric specimens from patients with HDGC are characterized by the presence of SRCC, suggesting a role for E-cadherin in the sequence of carcinogenesis. Further investigations have supported the suggestion of E-cadherin as a specific factor in SRCC. In cases of sporadic gastric cancer somatic loss of function mutations of the CDH-1 gene have been identified in patients with diffuse GC but not those with intestinal GC [3]. Immunohistologic studies have provided compelling evidence supporting the relationship between E-cadherin and SRCC and its utility as a diagnostic marker by demonstrating that in cases of GC with mixed histologic features changes in E-cadherin expression are only detected in the diffuse components [3].Additional investigations of the molecular pathogenesis of SRCC have supported a genetic model of development. Malignant cells in SRCC have been found to show increased expression of TAZ protein, a known oncogene involved in the development of many cancers including non-small cell lung cancer, papillary thyroid cancer, colon cancer, and breast cancer [6]. High intracellular expression of TAZ is known to confer loss of differentiation and stem-cell characteristics in the development of poorly differentiated breast cancers, which supports the possibility that signet ring histology is indicative of neoplastic change [7].
Detection methods
As previously described, early changes of diffuse GC are located in the gastric glandular neck which is located in the deeper tissues of the mucosa, below an intact surface epithelium. Macroscopic changes are often absent or subtle and missed on routine endoscopy [11]. Furthermore, the early histologic changes are easily mistaken for benign reactive processes such as inflammation or regeneration [12]. Investigations of improved diagnostic techniques have identified special endoscopic screening protocols and immunohistochemical stains with potential for improving earlier detection. This is an area in need of further research and investigation [6,13,14].
Case report
67 year old male was admitted to the hospital with complaints of melena, early satiety, abdominal pain and a 50 pounds weight loss over the course of one year. Patient has social history that includes 40 years of smoking as well as occasional alcohol intake. On EGD an antral mass was found and biopsy results revealed that most of the specimen consisted of benign gastric mucosa with ulceration and reactive changes. Multiple step sections showed small foci of adenocarcinoma with poorly differentiated signet ring cytoarchitectural features. MAK-6 immunostain highlighted the abnormal epithelial architecture and did not demonstrate any definite invasion of tumor into the submucosa. No evidence of H. Pylori was found. The surgical service performed a preoperative work up and found no evidence of metastasis and decision was made to proceed to the operating room.Laparotomy was performed using a Chevron incision and a suspicious lesion was noted in segment 5 of the liver. Wedge biopsy of the lesion was performed using scalpel and hemostasis at the site of liver biopsy obtained using electrocautery. Frozen section of the wedge biopsy of liver lesion returned as granuloma. Celiac lymph nodes were sampled and sent to pathology. Greater curvature of the stomach was mobilized and short gastric vessels were taken with energy device. Using sharp dissection, lesser sac was accessed along the superior margin of transverse colon. Kocherization of duodenum was performed to enable distal transection at the Pyloro-Duodenal junction. To identify the site of preoperative biopsy and guide the proximal resection margin, an intraoperative gastroscopy was performed. Proximal transection was performed using gastrointestinal anastomosis (GIA) stapler 5 cm proximal to the superior margin of the gastric ulcer. Frozen section of the gastric margins was negative.Final pathology demonstrated that the area of the previous biopsy showed marked submucosal edema and focal ulceration. Occasional scattered glands showed neutrophils and signet ring cells within the lumen. These glands also showed signet ring cells replacing the glandular mucosa. (See Figures 1-3) These glands were similar to those done at time of biopsy on initial EGD. Staining with Her2 was indeterminate. This is considered a neoplastic precursor lesion of diffuse types of gastric adenocarcinoma. All lymph nodes biopsied were benign with no evidence of malignancy. Postoperative surveillance included gastroscopy and computerized tomography (CT) scan which were negative for any local / regional recurrence.
In cases of intestinal GCs, pre-malignant lesions (neoplastic, non-invasive) that are identified as high-risk according to cytologic changes are treated with endoscopic resection. Early gastric cancer (EGC) is defined as an invasive malignancy that has not expanded beyond the submucosa, regardless of LN involvement [4]. In general, EGC has a significantly improved prognosis in comparison to advanced GC with 5YS reported as >90%. Studies of intervention strategies in EGC have suggested that patients meeting the following criteria are potential candidates for endoscopic resection: (1) tumor limited to the mucosa, (2) size < 1-2 cm and (3) no histologic evidence of ulceration or lymphovascular involvement [24]. Studies on the risks for LN metastasis in early SRCand others comparing behaviors of different SRCCs have demonstrated that these criteria are unreliable in patients with SRC histology [25,26]. The strongest evidence comes from multiple studies identifying SRC histology as a risk factor for lymphatic involvement [21-23]. Overwhelmingly, recommendations for EGC and findings of signet ring cell histology are primary surgical treatment with gastrectomy and LN resection [21-24]. There is a general paucity of evidence regarding the use of perioperative chemotherapy. Large European clinical trials have proven the benefit of neoadjuvant chemotherapy in EGC, establishing it as the standard of care [27]. However, subsequent studies specifically investigating neoadjuvant therapy in early SRCC indicate that there is no survival benefit, and delaying surgery may in fact allow for disease progression [28]. Adjuvant chemotherapy following primary surgical resection is currently under investigation in a phase III RCT [29].
Future risk stratification
A final consideration in patient with SRC histology is evaluation of the need for referral to a genetic expert. Recently updated guidelines on HDGC have outlined criteria for initiating testing or considering familial testing patients with disease features suggestive of a hereditary syndrome (Table 2) [5]. In addition to gastric cancer, female HDGC mutation carriers have a 40% lifetime risk for developing lobular breast cancer [3-5]. Thus, genetic information may be important in future healthcare considerations and in determining appropriate screening practices for at-risk populations.
Case Report
Role of Gastrectomy in the Management of Gastric Pre- Malignancies - A Review of Current Literature and Illustrative Case Report
JT Tieman1*, H Loebl2, M I M Ilyas1,T Nguyen3 and R Krouse4
- 1Department of General Surgery, University of Arizona, USA
- 2University of Arizona College of Medicine, USA
- 3Southern Arizona VA Health Care System, University of Arizona, USA
- 4Southern Arizona VA Health Care System, University of Arizona, USA
*Address for Correspondence: Joshua T. Tieman, Department of General Surgery, University of Arizona, USA, Tel: 602-714-2518; Fax: 520-626-2247; E-mail:Tieman@email.arizona.edu
Citation: Tieman JT, Loebl H, Ilyas MIM, Nguyen T, Krouse R. Role of Gastrectomy in the Management of Gastric Pre-Malignancies - A Review of Current Literature and Illustrative Case Report. 2016; S(2): 5.
Copyright © 2016 Tieman JT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Special Issue: 2
Submission: 15 Febraury 2016 | Accepted: 15 March 2016 | Published: 21 March 2016
Editors:Dr. Marlon A Guerrero, Assistant Professor of Surgery, University of Arizona, USA
Dr. James A Warneke, Associate Professor of Surgery, University of Arizona, USA
Abbreviations
GC: Gastric Cancer; EGC: Early Gastric Cancer; SRCC: Signet Ring Cell Carcinoma; NSRC: Non-Signet Ring Cell; LN: Lymph NodeBackground
EpidemiologyGastric cancer has generally been subdivided histologically into intestinal and diffuse, with the intestinal type representing the majority of cases. The geographic variability in disease distribution is closely related to the regional prevalence of H. pylori. Despite its position as the third leading cause of cancer-related deaths, there has been a global decrease in the overall incidence of gastric cancer in recent decades [1]. However, this pattern is not reflected uniformly across gastric cancer subtypes. Review of epidemiologic data in lowincidence areas including the United States shows a relative increase in the proportion of diffuse gastric cancers, particularly in Signet Ring Cell Carcinoma (SRCC) [2]. These epidemiologic trends support an accumulating body of evidence that suggests that intestinal GC and diffuse GC are two distinct diseases with different clinical behavior. These characteristics have significant clinical implications that impact both diagnosis and management and therefore warrant increased attention in current medical literature.
Pathogenesis
It is currently believed that the pathogenesis of intestinal type GC is related to environmental risk factors such as H. pylori infection, food, and smoking status. In comparison, it appears that diffuse type GC has a much larger genetic component related to its development. A proposed model of carcinogenesis has been developed based on studies of hereditary diffuse cancers, which only represent 1-3% of total GCs [3]. Hereditary Diffuse Gastric Cancer (HDGC) is an autosomal dominant germline mutation of the CDH1 gene, which encodes E-cadherin, a cellular adhesion protein. Mutation carriers have a 70% lifetime risk for developing diffuse GC [3-5]. Gastric specimens from patients with HDGC are characterized by the presence of SRCC, suggesting a role for E-cadherin in the sequence of carcinogenesis. Further investigations have supported the suggestion of E-cadherin as a specific factor in SRCC. In cases of sporadic gastric cancer somatic loss of function mutations of the CDH-1 gene have been identified in patients with diffuse GC but not those with intestinal GC [3]. Immunohistologic studies have provided compelling evidence supporting the relationship between E-cadherin and SRCC and its utility as a diagnostic marker by demonstrating that in cases of GC with mixed histologic features changes in E-cadherin expression are only detected in the diffuse components [3].Additional investigations of the molecular pathogenesis of SRCC have supported a genetic model of development. Malignant cells in SRCC have been found to show increased expression of TAZ protein, a known oncogene involved in the development of many cancers including non-small cell lung cancer, papillary thyroid cancer, colon cancer, and breast cancer [6]. High intracellular expression of TAZ is known to confer loss of differentiation and stem-cell characteristics in the development of poorly differentiated breast cancers, which supports the possibility that signet ring histology is indicative of neoplastic change [7].
Histopathology and Early Diagnosis
Early histologic studies of gastric cancer suggest that intestinal and diffuse cancers originate from different anatomic regions of the gastric mucosa with the former developing from the surface epithelium and the latter from the tubular neck of gastric glands [8,9]. The tubular neck is situated at the junction between the gastric pit and the gastric gland and is an area of high proliferation [9]. Subtle architectural abnormalities of the tubule neck have been described in the literature as “tubule neck dysplasia” (TND). These changes have also been reported in association with atypical signet ring cells, and many authors have speculated that TND may be representative of early cellular changes ultimately leading to SRCC. Pagetoid spread has been considered a unique feature of HDGC [5], however sporadic cases of GC have been described in which foci of atypical tubule neck cells demonstrate upward and lateral spreading in clusters [9], suggesting that pagetoid spread may not be unique to HDGC, but rather a feature of SRCC in general. Signet ring cells have also been identified in varying number in cases of poorly-differentiated gastric cancer and have been observed to later transform into poorly differentiated cancers [10]. It is therefore reasonable to consider the possibility that these various histologic changes may represent a spectrum of changes that occur in the progression of the same pathologic process.Detection methods
As previously described, early changes of diffuse GC are located in the gastric glandular neck which is located in the deeper tissues of the mucosa, below an intact surface epithelium. Macroscopic changes are often absent or subtle and missed on routine endoscopy [11]. Furthermore, the early histologic changes are easily mistaken for benign reactive processes such as inflammation or regeneration [12]. Investigations of improved diagnostic techniques have identified special endoscopic screening protocols and immunohistochemical stains with potential for improving earlier detection. This is an area in need of further research and investigation [6,13,14].
Case report
67 year old male was admitted to the hospital with complaints of melena, early satiety, abdominal pain and a 50 pounds weight loss over the course of one year. Patient has social history that includes 40 years of smoking as well as occasional alcohol intake. On EGD an antral mass was found and biopsy results revealed that most of the specimen consisted of benign gastric mucosa with ulceration and reactive changes. Multiple step sections showed small foci of adenocarcinoma with poorly differentiated signet ring cytoarchitectural features. MAK-6 immunostain highlighted the abnormal epithelial architecture and did not demonstrate any definite invasion of tumor into the submucosa. No evidence of H. Pylori was found. The surgical service performed a preoperative work up and found no evidence of metastasis and decision was made to proceed to the operating room.Laparotomy was performed using a Chevron incision and a suspicious lesion was noted in segment 5 of the liver. Wedge biopsy of the lesion was performed using scalpel and hemostasis at the site of liver biopsy obtained using electrocautery. Frozen section of the wedge biopsy of liver lesion returned as granuloma. Celiac lymph nodes were sampled and sent to pathology. Greater curvature of the stomach was mobilized and short gastric vessels were taken with energy device. Using sharp dissection, lesser sac was accessed along the superior margin of transverse colon. Kocherization of duodenum was performed to enable distal transection at the Pyloro-Duodenal junction. To identify the site of preoperative biopsy and guide the proximal resection margin, an intraoperative gastroscopy was performed. Proximal transection was performed using gastrointestinal anastomosis (GIA) stapler 5 cm proximal to the superior margin of the gastric ulcer. Frozen section of the gastric margins was negative.Final pathology demonstrated that the area of the previous biopsy showed marked submucosal edema and focal ulceration. Occasional scattered glands showed neutrophils and signet ring cells within the lumen. These glands also showed signet ring cells replacing the glandular mucosa. (See Figures 1-3) These glands were similar to those done at time of biopsy on initial EGD. Staining with Her2 was indeterminate. This is considered a neoplastic precursor lesion of diffuse types of gastric adenocarcinoma. All lymph nodes biopsied were benign with no evidence of malignancy. Postoperative surveillance included gastroscopy and computerized tomography (CT) scan which were negative for any local / regional recurrence.
Figure 1: The gastric mucosa shows multifocal intramucosal signet-ringcarcinoma cells confined within the gastric glands. In some glands, tumor cells about the non-neoplastic foveolar counterparts. No tumor cells are detected in the lamina propia or beneath the epithelial lining. Tumor cells are shed into the lumen, some underwent necrosis. Few eosinophils are present in the vicinity, the importance of which is unclear. Helicobacter pyloriorganisms are absent from this case (H&E, 20X).
Clinicopathology and Impact on Treatment
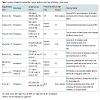
Retrospective studies comparing the clinic-pathologic features of SRC vs. NSRC cancer have yielded variable results (Table 1) [14-21]. The applicability of these studies has been questioned by Western authors as most were conducted in high prevalence geographic locations, which differ in population characteristics as well as screening practices and intervention strategies [22]. However, there is agreement between Eastern and Western authors that SRCC demonstrates unique behavior that often deviates from that of other GC subtypes. A 2009 case-control series identified SRC histology as a statistically significant negative prognostic indicator in patients with GC in addition to pTNM stage and tumor location. Through further analysis, the study concluded that SRC histology conferred a poor prognosis due to its invasive potential, increased rate of peritoneal carcinomatosis, and affinity for lymphatic tissue resulting in both higher frequency and greater extent of lymph node involvement [22]. Many of these behaviors were supported by findings from previous studies [15,19,21], as well as subsequent literature identifying SRC or diffuse type histology as a positive predictive factor for lymph node involvement in EGC [21-23]. Endoscopic resection vs. primary surgery
In cases of intestinal GCs, pre-malignant lesions (neoplastic, non-invasive) that are identified as high-risk according to cytologic changes are treated with endoscopic resection. Early gastric cancer (EGC) is defined as an invasive malignancy that has not expanded beyond the submucosa, regardless of LN involvement [4]. In general, EGC has a significantly improved prognosis in comparison to advanced GC with 5YS reported as >90%. Studies of intervention strategies in EGC have suggested that patients meeting the following criteria are potential candidates for endoscopic resection: (1) tumor limited to the mucosa, (2) size < 1-2 cm and (3) no histologic evidence of ulceration or lymphovascular involvement [24]. Studies on the risks for LN metastasis in early SRCand others comparing behaviors of different SRCCs have demonstrated that these criteria are unreliable in patients with SRC histology [25,26]. The strongest evidence comes from multiple studies identifying SRC histology as a risk factor for lymphatic involvement [21-23]. Overwhelmingly, recommendations for EGC and findings of signet ring cell histology are primary surgical treatment with gastrectomy and LN resection [21-24]. There is a general paucity of evidence regarding the use of perioperative chemotherapy. Large European clinical trials have proven the benefit of neoadjuvant chemotherapy in EGC, establishing it as the standard of care [27]. However, subsequent studies specifically investigating neoadjuvant therapy in early SRCC indicate that there is no survival benefit, and delaying surgery may in fact allow for disease progression [28]. Adjuvant chemotherapy following primary surgical resection is currently under investigation in a phase III RCT [29].
Future risk stratification
A final consideration in patient with SRC histology is evaluation of the need for referral to a genetic expert. Recently updated guidelines on HDGC have outlined criteria for initiating testing or considering familial testing patients with disease features suggestive of a hereditary syndrome (Table 2) [5]. In addition to gastric cancer, female HDGC mutation carriers have a 40% lifetime risk for developing lobular breast cancer [3-5]. Thus, genetic information may be important in future healthcare considerations and in determining appropriate screening practices for at-risk populations.
Conclusions
The recent changes in epidemiologic trends of diffuse gastric cancer make it a relevant topic warranting increased focus and attention. Based on the available literature, SRCC may be a potentially aggressive disease process and the finding of SRC histology thereforerequires special consideration. If uncertain of the presence of change immunohistochemical evaluation may help establish a diagnosis. Patients with SRCC should be considered for immediate surgical intervention.Potential Areas of Future Research
Molecular studies: biomarkers/targeted drug therapy.Development of an SRC Specific treatment strategy.Feasibility of less invasive surgical options.References
- Mcmanus LM, Mitchell R (2014) Pathobiology of human disease: A dynamic encyclopedia of disease mechanisms, 1st Edn, Academic Press, USA.
- Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J (2004) Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med 128: 765-770.
- Lynch HT, Grady W, Suriano G, Huntsman D (2005) Gastric cancer: new genetic developments. J Surg Oncol 90: 114-133.
- Alfaro EE, Lauwers GY (2011) Early gastric neoplasia: diagnosis and implications. Adv Anat Pathol 18: 268-280.
- van der post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, et al. (2015) Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 52: 361-374.
- Yue G, Sun X, Gimenez-Capitan A, Shen J, Yu L, et al. (2014) TAZ is highly expressed in gastric signet ring cell carcinoma. Biomed Res Int 2014: 393064.
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, et al. (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147: 759-772.
- Kumarasinghe MP, Lim TK, Ooi CJ, Luman W, Tan SY, et al. (2006) Tubule neck dysplasia: precursor lesion of signet ring cell carcinoma and the immunohistochemical profile. Pathology 38: 468-471.
- Grundmann E (1975) Histologic types and possible initial stages in early gastric carcinoma. Beitr Pathol 154: 256-280.
- Humar B, Fukuzawa R, Blair V, Dunbier A, More H, et al. (2007) Destabilized adhesion in the gastric proliferative zone and c-Src kinase activation mark the development of early diffuse gastric cancer. Cancer Res 67: 2480-2489.
- Phalanusitthepha C, Grimes KL, Ikeda H, Sato H, Sato C, et al. (2015) Endoscopic features of early-stage signet-ring-cell carcinoma of the stomach. World J Gastrointest Endosc 7: 741-746.
- Mayinger B, Horner P, Jordan M, Gerlach C, Horbach T, et al. (2000) Light-induced autofluorescence spectroscopy for tissue diagnosis of GI lesions. Gastrointest Endosc 52: 395-400.
- Nagami Y, Tominaga K, Machida H, Nakatani M, Kameda N, et al. (2014) Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol 109: 845-854.
- Kim JP, Kim SC, Yang HK (1994) Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 3: 221-227.
- Otsuji E, Yamaguchi T, Sawai K, Takahashi T (1998) Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol 67: 216-220.
- Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, et al. (1998) Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med 186: 121-130.
- Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, et al. (2002) Early gastric carcinoma with signet ring cell histology. Cancer 94: 78-83.
- Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, et al. (2004) Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 74: 1060-1064.
- Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, et al. (2004) Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg 91: 1319-1324.
- Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M, et al. (2011) Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a chinese mono-institutional study. J Surg Oncol 103: 700-703.
- Li C, Kim S, Lai JF, Hyung WJ, Choi WH, et al. (2007) Advanced gastric carcinoma with signet ring cell histology. Oncology 72: 64-68.
- Piessen G, Messager M, Leteurtre E, Jean-pierre T, Mariette C (2009) Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 250: 878-887.
- Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, et al. (2001) Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 31: 495-499.
- Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, et al. (2010) Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg 97: 732-736.
- Wang Z, Zhang X, Hu J, Zeng W, Liang J, et al. (2014) Predictive factors for lymph node metastasis in early gastric cancer with signet ring cell histology and their impact on the surgical strategy: analysis of single institutional experience. J Surg Res 191: 130-133.
- Kim BS, Oh ST, Yook JH, Kim BS (2014) Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery 155: 1030-1035.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, et al. (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355: 11-20.
- Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, et al. (2011) The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 254: 684-693.
- Piessen G, Messager M, Le Malicot K, Robb WB, Di Fiore F, et al. (2013) Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC Cancer 13: 281.