Journal of Surgery
Download PDF
2. Pectus Excavatum - PE
3. Pectus Carinatum - PC
4. Pectus Arcuatum - PA
5. Poland Syndrome - PS
6. Connective Tissue Disorder - CTD
7. Echocardiogram - ECHO
8. Left ventricle ejection fraction - LVEF
9. Mitral valve prolapse - MVP
10. Patent foramen ovale - PFO
11. Aortic root dilation - ARD
12. Marfan’s Syndrome - MFS
13. Ehlers-Danlos Syndrome - EDS
14. Loeys-Dietz Syndrome - LDS
15. Mixed Connective Tissue Disease - MCTD
16. Anterior-to-Posterior - AP
17. Right ventricle - RV
18. Ventricular premature complexes - VPC
Most patients were not seen by a Cardiologist based on their ECHO findings (n=425, 86.0%; [Table 3]. For those seen by Cardiology, common plans included repeat follows up at various intervals (1, 2 or 3 years usually), repeating an ECHO with follow up, outpatient monitors for arrhythmias, and genetics referrals for CTD workup. Of the few seen by Cardiology, even less required cardiologic interventions (n=5, 1%). Interventions included heart catheterization, starting medications (atenolol and losartan), two radiofrequency ablations, and one dual chamber transvenous pacemaker placement.
Research Article
The Utility of Screening Echocardiograms in Congenital Chest Wall Deformities
Gershner GH, He D, Pardue K, Stewart K, Cave A and Hunter CJ
1The University of Oklahoma Health Sciences Center, Department of Surgery, 800 Research Parkway, Suite 449, Oklahoma City,
2Division of Pediatric Surgery, Oklahoma Children’s Hospital, 1200 Everett Drive, ET NP 2320 Oklahoma City
3Division of Pediatric Cardiology, Oklahoma Children’s Hospital, 1200 Everett Drive, ET NP 2320 Oklahoma City
2Division of Pediatric Surgery, Oklahoma Children’s Hospital, 1200 Everett Drive, ET NP 2320 Oklahoma City
3Division of Pediatric Cardiology, Oklahoma Children’s Hospital, 1200 Everett Drive, ET NP 2320 Oklahoma City
*Address for Correspondence: Catherine J. Hunter, Department of Pediatric Surgery, Oklahoma Children’s Hospital, Oklahoma City. E-mail Id: catherine-hunter@ouhsc.edu
Submission: 30 September, 2025
Accepted: 24 October, 2025
Published: 28 October, 2025
Copyright: © 2025 Gershner GH, et al. This is an open access article
distributed under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original work is properly cited.
Keywords: Congenital chest wall deformities; connective tissue
disease; echocardiograms; screening tests; pectus excavatum;
pectus carinatum
Abstract
Objective: Congenital chest wall deformities (CCWD) are bony
or cartilaginous defects of the chest. These deformities result in both
an abnormal appearance and, in many cases, cardiopulmonary
compromise. Evidence is growing regarding the cardiovascular
implications of CCWD. Screening echocardiograms (ECHOs) are
increasingly utilized for patients with chest wall deformities to assess
cardiac function. We sought to describe the impact of screening
ECHOs on patients with CCWD.
Methods: This is a retrospective study. Using ICD10 diagnosis codes, a cohort was generated. Manual chart review performed for demographic data, CCWD diagnosis, and ECHO reports. Additional chart review was performed to see if patients were seen by Pediatric Cardiology, and what the documented plan was including potential intervention. Results: We found 494 patients that fit inclusion criteria. Patients with CCWD most often had pectus excavatum or pectus carinatum. The most common ECHO findings included mitral valve prolapse, patent foramen ovale, and aortic root dilation. Most patients did not require cardiology follow up (86%), with even less requiring cardiologic interventions (1%). We found 10 patients with connective tissue disease.
Conclusions: Patients with CCWD benefit from screening ECHOs in order to find cardiac anomalies that could impact pre, intra, and post-operative management. Findings could also initiate the workup for CTD and Cardiology involvement, further improving patient care.
Methods: This is a retrospective study. Using ICD10 diagnosis codes, a cohort was generated. Manual chart review performed for demographic data, CCWD diagnosis, and ECHO reports. Additional chart review was performed to see if patients were seen by Pediatric Cardiology, and what the documented plan was including potential intervention. Results: We found 494 patients that fit inclusion criteria. Patients with CCWD most often had pectus excavatum or pectus carinatum. The most common ECHO findings included mitral valve prolapse, patent foramen ovale, and aortic root dilation. Most patients did not require cardiology follow up (86%), with even less requiring cardiologic interventions (1%). We found 10 patients with connective tissue disease.
Conclusions: Patients with CCWD benefit from screening ECHOs in order to find cardiac anomalies that could impact pre, intra, and post-operative management. Findings could also initiate the workup for CTD and Cardiology involvement, further improving patient care.
Glossary of Abbreviations:
1. Congenital chest wall deformities - CCWD2. Pectus Excavatum - PE
3. Pectus Carinatum - PC
4. Pectus Arcuatum - PA
5. Poland Syndrome - PS
6. Connective Tissue Disorder - CTD
7. Echocardiogram - ECHO
8. Left ventricle ejection fraction - LVEF
9. Mitral valve prolapse - MVP
10. Patent foramen ovale - PFO
11. Aortic root dilation - ARD
12. Marfan’s Syndrome - MFS
13. Ehlers-Danlos Syndrome - EDS
14. Loeys-Dietz Syndrome - LDS
15. Mixed Connective Tissue Disease - MCTD
16. Anterior-to-Posterior - AP
17. Right ventricle - RV
18. Ventricular premature complexes - VPC
Introduction
Congenital chest wall deformities (CCWD) are bony or
cartilaginous defects of the chest. This includes pectus excavatum
(PE), pectus carinatum (PC), pectus arcuatum (PA), and Poland
syndrome (PS). These occur in 1 in 300 to 1000 live births [1]. PE
and PC are the most common, and present with a depression and
protrusion of the chest wall, respectively. These deformities result in
both an abnormal appearance and, in many cases, cardiopulmonary
compromise. Multicenter studies have shown that patients with
these deformities have negative body image that improves following
surgical correction [2]. There is growing and substantial evidence
regarding the cardiovascular implications of CCWD. The severity of
cardiovascular symptoms ranges from mild, including palpitations or
chest pain, to severe, such as life-threatening arrhythmias [2]. Most
commonly patients report exercise intolerance and dyspnea.
While CCWD are an isolated entity, they can be indicative of
underlying connective tissue disorders (CTD). The most associated
CTD with CCWD are Noonan syndrome and Marfan syndrome.
Both syndromes are inherited in an autosomal dominant fashion and
can have various cardiac defects including valvular abnormalities,
cardiomyopathies, and aortic dilation or dissection [3]. While
certainly not all CCWD have a direct link to CTD, this close association
heightens suspicion for potential underlying cardiac defects.
Screening echocardiograms (ECHOs) are increasingly utilized for patients with chest wall deformities to assess cardiac compression, function, and screen for underlying cardiac anomalies. Several studies have evaluated the implications of pectus deformities on the heart such as mitral valve prolapse, reduced right ventricle filling, and reduced cardiac output [4]. These are most significant in PE patients where the heart can be displaced or depressed secondary to the indentation of the chest wall. These anomalies could contribute to the most reported symptoms of chest pain, palpitation, and exercise intolerance by pectus patients. Given the association of CCWD and CTD, screening ECHOs also help identify cardiac or aortic anomalies that need to be addressed before pectus correction.
Screening echocardiograms (ECHOs) are increasingly utilized for patients with chest wall deformities to assess cardiac compression, function, and screen for underlying cardiac anomalies. Several studies have evaluated the implications of pectus deformities on the heart such as mitral valve prolapse, reduced right ventricle filling, and reduced cardiac output [4]. These are most significant in PE patients where the heart can be displaced or depressed secondary to the indentation of the chest wall. These anomalies could contribute to the most reported symptoms of chest pain, palpitation, and exercise intolerance by pectus patients. Given the association of CCWD and CTD, screening ECHOs also help identify cardiac or aortic anomalies that need to be addressed before pectus correction.
This study aims to further our understanding of pectus deformities
impact on the cardiovascular system by analyzing echocardiographic
data. We additionally sought to elucidate the impact of screening
ECHOs in those with positive findings. This was achieved by
examining ECHO reports for positive findings, and if these findings
warranted cardiology visits, additional follow up. Findings from
this study could shape cardiovascular screening, cardiology referral
practices, and monitoring protocols in this patient population.
Methods
Cohort Generation:
This was a retrospective study. All patient gathering was
performed after IRB approval (IRB# 17618). Informed consent was
waived given the retrospective nature of the study and all patient
information being de-identified after collection. Inclusions criteria
included all patients seen in the Oklahoma Children’s Hospital
Congenital Chest Wall Clinic between July 1st, 2020 through July 1st,
2024 between the ages of 0 to 17. All patients were seen and managed
by a single provider during this time. Patients were selected based on
ICD 10 code diagnosis. The codes used were: Q67.6, Q67.7, Q67.8,
Q76.6, Q76.7, Q76.8, Q76.9, and M95.4. Exclusion criteria included
those who had seen the provider for reasons other than our inclusion
criteria, those older than 17, and those without ECHO reports.
This list of patients was then uploaded to REDCap. We performed
manual chart review. Demographic data gathered included age at
initial clinic visit and gender. CCWD diagnosis was confirmed, and
ECHO reports reviewed for structural abnormalities and ejection
fraction. Further chart review was done for if the patient was seen
by Pediatric Cardiology, and what the documented plan was at
their initial visit. Additionally, it was noted if the patient has had
any Cardiology based intervention such as started medications,
catheterizations, or pacemaker placements. Finally, patient charts
were examined to see if the patient had a CTD diagnosis.Statistical Analysis:
Descriptive statistics were used to summarize the data. Bivariate
testing of associations was performed using chi-square tests. T-tests
were used to evaluate differences in continuous variables. All data
management and analyses were performed using SAS v9.4 (SAS
Institute, Cary, NC). Significance was set at a value of p=0.05.Results
Cohort:
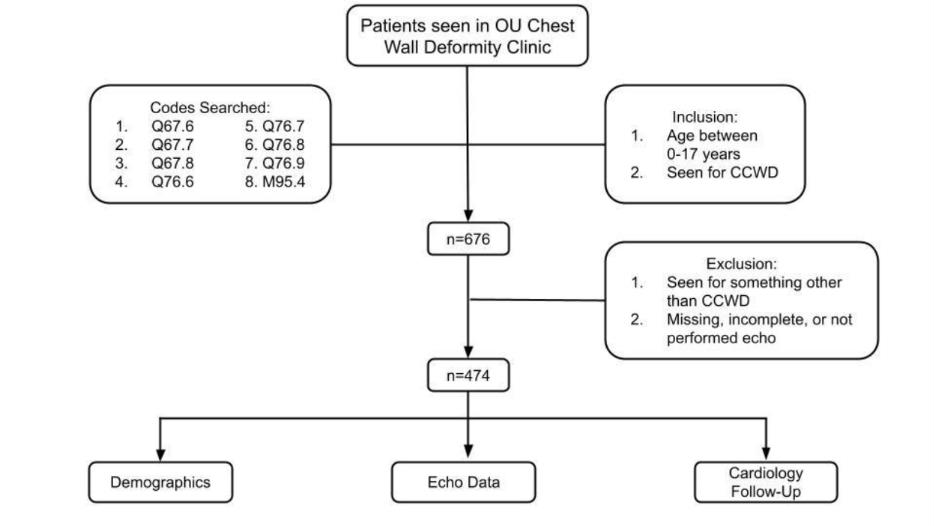
A total of 676 patients were found [Figure 1]. Of these, 182 were
excluded for incomplete ECHO data, or ECHOs being reported as
shortened interpretations in clinic notes instead of formal reports
by faculty when full reports were unavailable (n= 494), [Table 1].
The latter usually had pertinent findings but usually did not include
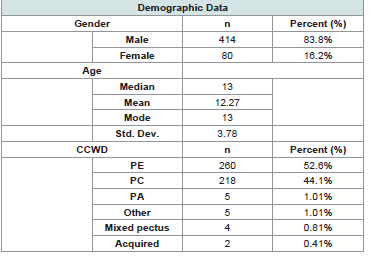
LVEF. The cohort was vastly male (83.8%) and the median age was
13 (mean 12.3, mode 13, std. dev 3.78). Over half of the patients were
seen for PE, followed closely by PC.Echocardiogram Findings and Cardiology Evaluation:
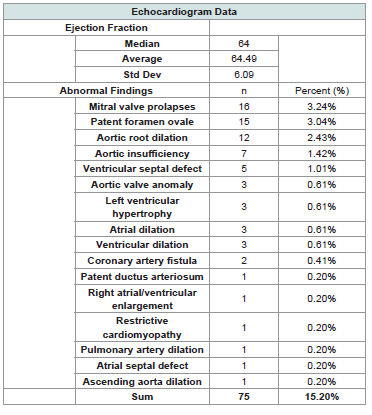
The median LVEF was 64% (mean 64.49%, std dev 6.09; [Table 2]. There were 53 positive screening ECHOs (10.7%), with 75 total findings (as some patients had multiple findings). In PE and
PC, the most common anomalies included: Mitral valve prolapse
(MVP), Patent foramen ovale (PFO), aortic root dilation (ARD).
Abnormalities were most often seen in PE (PE 13%, PC 7%; p=0.032).
All cases were newly diagnosed and were not a pre-existing condition
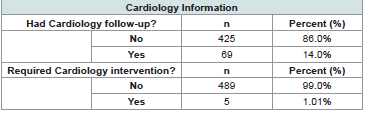
for these patients.Most patients were not seen by a Cardiologist based on their ECHO findings (n=425, 86.0%; [Table 3]. For those seen by Cardiology, common plans included repeat follows up at various intervals (1, 2 or 3 years usually), repeating an ECHO with follow up, outpatient monitors for arrhythmias, and genetics referrals for CTD workup. Of the few seen by Cardiology, even less required cardiologic interventions (n=5, 1%). Interventions included heart catheterization, starting medications (atenolol and losartan), two radiofrequency ablations, and one dual chamber transvenous pacemaker placement.
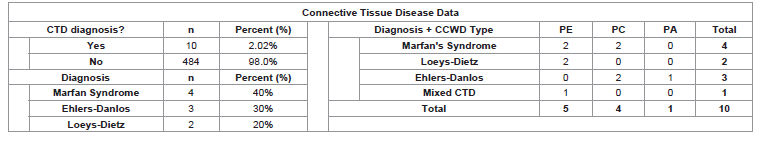
Connective Tissue Disease Prevalence:
Ten patients were found to have CTD (2.02%) [Table 4]. We
found four different CTDs in our patient population. These include
Marfan Syndrome (MFS; n=4), Ehlers-Danlos Syndrome (EDS; n=3),
Loeys-Dietz Syndrome (LDS; n=2), and Mixed CTD (MCTD; n=1).
Patients with CTD had either PE (n=5; 50%), PC (n=4; 40%) or PA
(n=1; 10%).Discussion
CCWD are a diverse family of pathologies. This group of diseases
are often associated with underlying cardiac abnormalities, and have
a predisposition to CTD such as MFS or EDS. Through our study,
we better describe the relationship between CCWD and associated
findings on screening ECHOs, further validating the need for
preoperative screening.
A recent study by Pitt et. al. examined 1510 patients with CCWD.
They found that 80% of their patients were male, which is similar to
our findings. PE was the most common CCWD between our two
studies at ~50%, followed closely by PC at ~45% and then all others
[5]. Previous research by our group focused on PC. In that study there
were 155 patients with PC, and 10% were found to have a cardiac
anomaly. These were most usually MVP and aortic root dilation
[6]. Of the 228 patients with PC, 16 had a positive ECHO finding.
The most common were PFO, ARD, and MVP. Other than a slightly
lower incidence of positive findings, this is in line with the previously
PE was significantly more often associated with positive ECHO
findings compared to PC. There were not enough instances of all
CCWD to make any meaningful conclusions. A recent systematic
review by Sonaglioni et. al. found that children with PE had a threefold
higher chance of having MVP compared to adults. This is thought
to be due to the smaller thoracic volume of children, resulting in more
anterior-to-posterior (AP) compression of the right ventricle (RV)
and great distortion of the mitral valve annulus [7].
While most of these findings may be mild, they have the potential
to significantly impact the perioperative period. The altered sternal
body of PE can cause RV compression and decreased stroke volumes
due to insufficient filling [8-10]. MVP is also associated with increased
incidences of arrhythmias such as ventricular premature complexes
(VPCs, [11]).
CCWDs’ association with CTDs puts patients at a higher risk for cardiac anomalies. PE has been associated with 20 unique genetic disorders, of which 5 of primary CTD [12]. In a study by Rhee et. al., patients with PE had increased rates of ARD. Further genetic evaluation of those with ARD found that 2 of 5 patients were newly diagnosed with MFD [13]. Depending on the degree of ARD, this can increase operative and anesthetic risk. A study by Elsäcker et. al., that ARD become more pronounced at 8 years old. Those who underwent intervention for ARD usually happened around 16 years of age [14]. Finding these patients early could allow for earlier medical or surgical intervention, or closer surveillance of aortic root diameter.
CCWDs’ association with CTDs puts patients at a higher risk for cardiac anomalies. PE has been associated with 20 unique genetic disorders, of which 5 of primary CTD [12]. In a study by Rhee et. al., patients with PE had increased rates of ARD. Further genetic evaluation of those with ARD found that 2 of 5 patients were newly diagnosed with MFD [13]. Depending on the degree of ARD, this can increase operative and anesthetic risk. A study by Elsäcker et. al., that ARD become more pronounced at 8 years old. Those who underwent intervention for ARD usually happened around 16 years of age [14]. Finding these patients early could allow for earlier medical or surgical intervention, or closer surveillance of aortic root diameter.
Our study has its limitations. Our patient cohort was generated
from a single center and only pediatric patients. This can limit
generalizability to other locations, as well as to adult patients. While
every patient in our study had an ECHO ordered, not all patients
had an ECHO on file. This could be due to a recent transition in
our electronic medical record (EMR) system. Other reasons for this
included that a screening ECHO was not indicated at the time due to
patient age, or that it was obtained at an outside facility with results
not uploaded to the EMR.
Future directions would be continued analysis of patients. This would increase the power of the study and help draw more meaningful conclusions. We could also further bolster our study by partnering with others for a multi-institutional study. Doing so would again increase power and would also make our results more generalizable. Finally, another common alteration of CCWD includes arrhythmias. During chart review, we did note that some patients who saw cardiology were noted to have positive EKG findings, but these were noted in an informal way. A formal evaluation of EKGs as an additional screening tool in this patient population is warranted.
Another potential area of interest would be cost-benefit analysis. ECHOs can cost anywhere from $204 to $2,588 depending on the patient’s insurance [15]. Given those with CCWD have a higher risk of CTD and CTD patients are at the highest risk during operative intervention, this population would be of particular interest.
Future directions would be continued analysis of patients. This would increase the power of the study and help draw more meaningful conclusions. We could also further bolster our study by partnering with others for a multi-institutional study. Doing so would again increase power and would also make our results more generalizable. Finally, another common alteration of CCWD includes arrhythmias. During chart review, we did note that some patients who saw cardiology were noted to have positive EKG findings, but these were noted in an informal way. A formal evaluation of EKGs as an additional screening tool in this patient population is warranted.
Another potential area of interest would be cost-benefit analysis. ECHOs can cost anywhere from $204 to $2,588 depending on the patient’s insurance [15]. Given those with CCWD have a higher risk of CTD and CTD patients are at the highest risk during operative intervention, this population would be of particular interest.
Conclusion
Based on this study, we will continue to pursue preoperative
screening ECHOs in all patients with CCWD. While the incidence of
positive findings is low, the impact of the findings can greatly impact
pre, intra, and post-operative management of this patient population.
Positive findings can prompt evaluation by cardiology, allowing for
preoperative risk stratification and long-term follow-up if indicated.
Screening ECHOs can also help start the process of genetic screening
for CTDs, further improving patient care.