Journal of Surgery
Download PDF
Case Report
Open Repair of Pediatric Aortoenteric Fistula from A Remote Gastric Transposition in Congenital Esophageal Atresia: A Multidisciplinary Approach
Snyder KB1,2*, Farnell C3, Buonpane C1,2, Gierman JL2,3, Hunter CJ1,2 and Landmann A1,2
1Division of Pediatric Surgery, Oklahoma Children’s Hospital, 1200
Everett Drive, ET NP 2320 Oklahoma City, USA.
2The University of Oklahoma Health Sciences Center, Department of Surgery, 800 Research Parkway, Suite 449, Oklahoma City, OK USA.
3The University of Oklahoma Health Sciences Center, Department of Vascular Surgery, 800 Research Parkway, Suite 449, Oklahoma City, OK USA
2The University of Oklahoma Health Sciences Center, Department of Surgery, 800 Research Parkway, Suite 449, Oklahoma City, OK USA.
3The University of Oklahoma Health Sciences Center, Department of Vascular Surgery, 800 Research Parkway, Suite 449, Oklahoma City, OK USA
*Address for Correspondence: Katherine B. Snyder, Department of Pediatric Surgery Oklahoma Children’s Hospital, Oklahoma City, USA. Email Id: Katherine-snyder@ouhsc.edu
Submission:11 January 2024
Accepted:07 February 2024
Published:12 February, 2024
Copyright: © 2024 Snyder KB, et al. Powell BS, et al. This is an open
access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Keywords
Esophageal Atresia; Aortoenteric Fistula; Ulcer; Pediatric Surgery; Vascular Surgery
Abstract
A 12-year-old male with history of long gap esophageal atresia
with a gastric transposition at one year of age presented with multiple
episodes of hematemesis. He recently had been prescribed high
dose NSAIDs for pericarditis. He underwent multiple endoscopic
cauterizations of a large gastric ulcer and despite this required MTP.
CTA was obtained showing hypoattenuation of the gastric conduit
along the aorta near the area that was cauterized. The patient
underwent a left-thoracotomy and gastrotomy. Once hematoma was
evacuated, a large pulsatile bleed was encountered. Pressure was
held and control of the aorta was obtained. The gastric conduit was
dissected off the aorta, revealing a large defect. The gastric conduit
was repaired, the aorta was repaired with bovine pericardium and
pleural flap was placed. On POD 8 a swallow study demonstrated no
leak and the patient was discharged on POD 15. Outpatient follow-up
CTA demonstrated an intact repair.
Introduction
Aortoenteric fistulas are a fairly rare cause of gastrointestinal
bleeding and if seen, it is typically seen in adults [1,2]. They
are historically separated into primary aortoenteric fistula and
secondary aortoenteric fistulas [1,2]. Primary AEF is defined as a
spontaneous communication between native aorta and any portion
of the gastrointestinal tract resulting from compression against
an abdominal aortic aneurysm [1,2]. Secondary AEF is defined as
typically resulting from a complication following vascular surgery
and rarely GI surgery [1-3]. When noted to be a sequala of GI
surgery, AEF characteristically presents 2-3 weeks following the
operation, typically an esophagectomy or esophago-gastrectomy at
the anastomosis site [4-6]. Patients typically present with massive
hematemesis and regardless of type of AEF, the mortality rate
for AEF is high, with it reaching as high as almost 60% with an in
hospital mortality rate almost 30% [7]. There is little to no literature
demonstrating AEF in the pediatric population. We present a case
of a 12-year-old male presenting with a thoracic gastro aortoenteric
fistula following a gastric transposition for long gap esophageal
atresia when he was 1-year-old.
Case report
The patient is a 12-year-old male with a history of long-gap
esophageal atresia with a gastric transposition at one year of age
presented with multiple episodes of hematemesis. Interval history
involved primary care physician diagnosis of pericarditis-associated
with COVID vaccination, requiring high dose ibuprofen one month
prior to his presentation for hematemesis. Four weeks following the
diagnosis of pericarditis, he experienced hematemesis for which three
attempts at endoscopic cauterization of a large gastric ulcer were
attempted and unsuccessful. Computed tomography angiography
(CTA) was obtained that showed hypoattenuation of the gastric
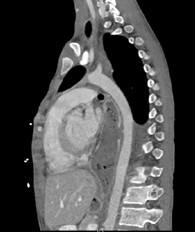
conduit along the aorta near the area that was cauterized [Figure 1].
Due to failure of non-operative management and concern for
AEF, operative intervention with pediatric surgery was indicated.
Extensive operative planning was performed including resuscitative
line placement, preparation for possible cardiopulmonary bypass,
and vascular and cardiac surgery on standby.The patient underwent
a left thoracotomy and gastrotomy. Once hematoma was evacuated
from the conduit, a large pulsatile bleed was encountered. Pressure
was held and proximal and distal control of the aorta was obtained.
Vascular surgery was consulted emergently intraoperatively and the
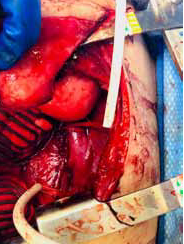
gastric conduit was dissected off the aorta, revealing a large defect
Figure 1: CTA demonstrating hypoattenuation of the gastric conduit along the
aorta near the area that was cauterized with concern for aortoenteric fistula.
and an aortoenteric fistula [Figure 2]. Proximal and distal control of
the aorta was obtained using umbilical tape. A 20F chest tube was
then used as a shunt and the aorta was clamped. Pediatric surgery
worked to separate the gastric conduit from the aorta, while vascular
surgery repaired the aorta with a rifampin soaked bovine patch sewed
in using 4-0 prolene suture. Upon removal of the gastric conduit from
the aorta, a large ulcer in the posterior aspect of the stomach was
discovered and repaired with several figure of eight 3-0 PDS and the
gastrotomy was closed with a blue load 60mm stapler. Cardiothoracic
surgery was called intraoperatively to perform a thoracic pleural flap
placed between the gastric and aortic repair as a buttress.
Post operatively, he was resuscitated in the pediatric intensive
care unit for several days. Post-operative medications included IV
antibiotics for a 6-week course, twice daily proton-pump inhibitor,
and Plavix which was started on post-operative day (POD) three. On
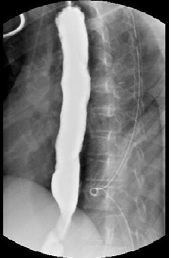
POD 8 we performed a swallow study demonstrating no leak and
diet was advanced [Figure 3]. He was discharged on POD 15 with an
outpatient follow-up with pediatric surgery and vascular surgery and
progressed well.
Discussion
Aortoenteric fistulas are rare, however if found they are
typically in the adult population predominantly associated with
aortic aneurysms or following vascular operations [1,3,7,8]. Nonaneurismal
aortoenteric fistulas a are extremely rare, but have been
reported in adults to be associated with carcinomas, tuberculosis,
abscess, radiation, or duodenal ulcers [8]. The presentation of these is
usually with gastrointestinal bleeding, and if from an operative cause,
typically within one month postoperatively [4]. Management in the
adult population often consists of endovascular stent placement and
resection and reconstruction with intestinal reconstruction [6,7].
Our case is unique in many ways, our patient is a 12-year-old male
without history of vascular disease or surgery who presented with
gastrointestinal bleeding in the form of hematemesis 11 years post
operatively. He was prescribed high dose ibuprofen one month prior
to his episodes of hematemesis which almost certainly led to the
development of the gastric ulcer found intraoperatively eroding in the
aorta. His unique anatomy following the gastric pull through likely
contributed to the ease of development of the fistula from the gastric
ulcer. Our patient was trial managed non-operatively multiple times
with failure leading to hemorrhagic shock. We would recommend
early involvement of a pediatric surgery team when dealing with
unique anatomy and continued hematemesis following endoscopic
intervention.
To our knowledge, this is the first case of this nature in pediatrics
and it presented several challenging components. Our institution
is fortunate to have all needed available specialties for adequate
operative repair. Obtaining proximal and distal control in this
instance was crucial, we were able to use a chest tube as a shunt to
avoid prolonged ischemic time. Ideally, the aorta would have been
repaired primarily, however the ulcer site tissue was extremely friable
and thus sutures did not hold. Rifampin soaked bovine patches have
shown good results in adult literature for vascular repair, and we
would recommend that for treatment should primary repair not be
attainable. Removal of the ulcerated gastric tissue and repair of the
area is crucial in this operation as well, and we were able to primarily
repair this without evidence of leak. In a hostile field, the worry
would be that this would recur for this patient, which prompted us
to perform the thoracic pleural flap to act as a barrier between the
stomach and the newly repaired aorta, which is crucial in this case. He
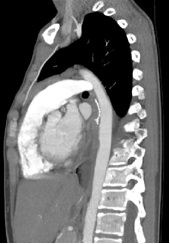
recovered well postoperatively and has close follow up surveillance
with outpatient CTA demonstrating an intact repair [Figure 4]. We
would recommend follow up imaging with CTA’s at 3 months, and
one year due to the serious nature of this aortoenteric fistula and his
unique anatomy.