Journal of Surgery
Download PDF
Review Article
Role of Wall Shear Stress in an Intracranial Aneurysm Formation: A Systematic Review
Neupane D1*, Lageju N1, Dahal A2, Jaiswal LS3, Manandhar S1, Chhetri S1, Acharya R1, Pokhrel N1 and Panthi S1
1Department of Surgery, B. P. Koirala Institute of Health Sciences,
Dharan, Nepal.
2Division of Neurosurgery, B. P. Koirala Institute of Health Sciences,
Dharan, Nepal.
3Division of CTVS, B. P. Koirala Institute of Health Sciences,
Dharan, Nepal
*Address for Correspondence
Neupane D, Department of Surgery, B. P. Koirala Institute of Health
Sciences, Dharan, Nepal; E-mail: neupanedurga26@gmail.com
Submission: 06 June, 2022
Accepted: 25 July, 2022
Published: 29 July, 2022
Copyright: © 2022 Neupane D, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Background: Recent evidence suggests a link between
hemodynamic factors and intracranial aneurysm inception. However,
the role of wall shear stress is not clearly understood.
Aim: To elucidate the role of wall shear stress in intracranial
aneurysm formation.
Methods: We performed areview of literature by recruiting
articles from 2000 through 2019. 2134 unique articles were identified,
of which 32 were retrieved for more detailed evaluation. 17 articles
met the inclusion criteria and were involved in the qualitative analysis.
Standard guidelines were followed.
Results: Wall shear stress showed to have a significant role in
intracranial aneurysm inception. Other hemodynamic factors also
played role in the inception of cerebral aneurysms. The geometry
and optimality principle along with social factors like smoking is also
responsible for the formation of cerebral aneurysms.
Conclusion: Wall shear stress plays a major role in intracranial
aneurysm formation. Gradient oscillatory number is the emerging
hemodynamic factor for the inception of cerebral aneurysms.
However, limited experiments in humans have resulted in inconclusive
results.
Keywords
Intracranial; Aneurysm; Cerebral; Hemodynamics; Wall
Shear Stress; Computational Fluid Dynamics
Abbreviations
CA-Cerebral Aneurysm; IA-Intracranial Aneurysm; WSS-Wall
Shear Stress; AComA-Anterior Communicating Artery; PComAPosterior
Communicating Artery; ACA- Anterior Cerebral Artery;
ICA- Internal Carotid Artery; MCA- Middle Cerebral Artery;
OA- Ophthalmic Artery; AChA- Anterior Choroidal Artery GONGradient
Oscillatory Number; CFD-Computational Fluid Dynamics;
WSSG-Wall Shear Stress Gradient; SWSS-Spatial Wall Shear Stress;
SW- Side wall; BF- Bifurcation; IEL- Internal Elastic Lamina
Background
Aneurysm formation is a continuous remodelling process that
involves the breakdown of the extracellular matrix and is described
by the loss of the internal elastic lamina, media layer thinning, and
bulge formation [1,2]. Cerebral aneurysms (CAs) are distinguished
by a pathological wall structure characterized by rupture of the
internal elastic lamina and media, resulting in focally weakened
arterial wall pouches [3,4]. The incidence of unruptured CAs in
the general population has been approximated to be 2% to 5% [5].
Hemodynamics has been found to play a significant role in the
genesis, progression, and rupture of intracranial aneurysms (IA) [6-8]. Specific elements, such as site, blood pressure, boundary condition,
and vascular shape, do, nevertheless, influence hemodynamic factors
[9-11]. Recent fluid dynamics research has highlighted the close
connections between hemodynamics and IAs [12]. Among numerous
hemodynamic characteristics, high wall shear stress placed on artery bifurcation points, where IAs originates, is related to IA formation
and growth [12]. As a result, high wall shear stress (WSS) can be
identified as a cause of IA development. WSS is flow-induced stress
that acts on the endothelium surface and is analogous to the frictional
force of viscous blood [13,14]. WSS is regarded as a crucial factor
of artery diameter and is involved in vascular remodeling [15]. An
growing number of research in recent years have shown that WSS
is intimately associated with determining aneurysm development,
growth, and rupture [16-20].
Previously, reviews on the involvement of hemodynamics in
the development of IAs were examined, with the role of WSS being
recognized as the most essential element. The purpose of this review
is to shed light on the involvement of WSS in the formation state of
intracranial aneurysms.
Methods
Literature Search:
PubMed and Google Scholar were used as sources for searching
published studies from 2000 through December 2019. Searches
were conducted using the keywords “intracranial aneurysm” in
combination with “computational fluid dynamics”, “hemodynamics”
and “wall shear stress”. Titles, abstracts, and full text were screened
for study and report characteristics that matched eligibility criteria.
References within studies were perused and incorporated if they
met our inclusion criteria. Two independent reviewers screened and
retrieved reports, and a third reviewer settled any confusion if any
between the two. Others were involved in manuscript preparation.
The final version of the paper was approved by all the authors.Eligibility Criteria:
The following criteria were designed for the selection of eligible
studies.Study design: Observational studies measuring the influence
of WSS on intracranial aneurysm inception using methods of
computational fluid dynamics were eligible.
Language of study: Only English language studies were reviewed.
Objective outcomes: Included studies were to objectively assess
the role of WSS in intracranial aneurysm formation.
Data abstraction: Data were manually extracted by investigators
from eligible studies. First author, type of study design, year of
publication, number of subjects, number of aneurysms, location
of aneurysms, type of aneurysms, sex of participants, mean age of
participants were extracted.
Exclusion Criteria:
Articles published in any other languages except English, animal
studies, case reports, review articles, viewpoints, study without
computational fluid dynamics (CFD) analysis were excluded.Results
Literature search and data extraction:
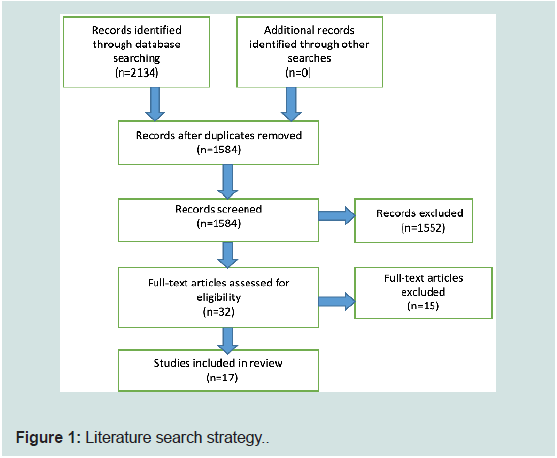
2134 unique articles were identified, of which 32 were retrieved
for more detailed evaluation. 17 articles met the inclusion criteria and
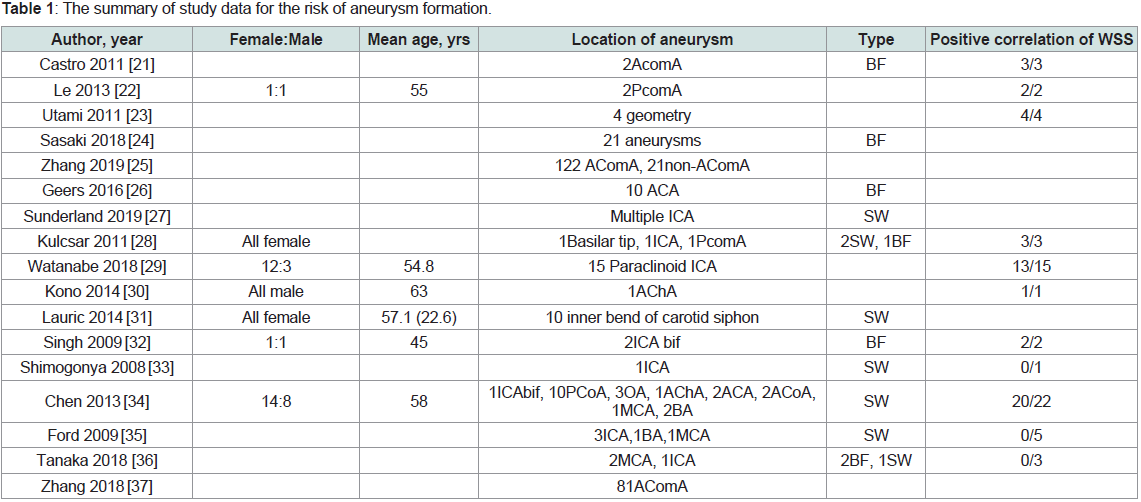
were involved in the qualitative analysis. Figure 1 shows the results
of our literature search and selection. Table 1 shows the summary of
study data for the risk of aneurysm formation.Study design:
All eligible studies were observational studies measuring the
influence of WSS in intracranial inception.Risk of formation:
In a study conducted by Castro et al, three individuals were chosen
for Model A (without aneurysms) and Model B (with aneurysms). A
comparison of the two models for Patient 1 reveals that the aneurysm
began to develop in the high WSS region. The aneurysm impacted
two unconnected high WSS areas in patient 2. In the case of patient 3,
the aneurysm developed across an area with both high and moderate
WSS values. In a study of Anterior Communicating Artery (AComA)
aneurysms, Castro et al discovered a probable link between areas of
high WSS prior to aneurysm development and aneurysm location
[21].Le et al performed a retrospective hemodynamic analysis before
and after aneurysm development in a ruptured aneurysm of the
posterior communicating artery and an unruptured aneurysm of
the posterior communicating artery using a vessel surface repair
approach [22]. The first patient was a 62-year-old male who had a
ruptured aneurysm, and the second was a 48-year-old man who had
an unruptured aneurysm. Their early findings revealed that the artery
wall was exposed to higher WSS, WSSG, and blood-flow velocity
before the formation of an aneurysm [22]. Utami et al. achieved
consistent findings [23].
Sasaki and colleagues investigated twenty-one bifurcation models
with varied in-branch angles and branch sizes. The highest WSS
value was studied in all models using a steady-flow simulation using
computational fluid dynamics. The amplitude of WSS was shown
to be substantially linked with bifurcation geometry and aneurysm
development [24].
The role of the optimality principle was discussed by Zhang et
al [25]. They demonstrated that normal anterior cerebral artery
bifurcations followed the optimality principle, but AComA
bifurcations did not, Disobeying the optimality principle results in
dramatically increased hemodynamic stress, which may damage the
bifurcation wall and lead to aneurysm formation. IA developed in
locations other than the bifurcation apex [25]. In support of this, Geers
et al investigated the involvement of WSS in aneurysm formation at
locations other than the bifurcation apex and the exterior wall of the
arterial bed [26].
Sunderland et al created 3D computational vasculatures using
angiographic pictures of 18 patients who had several closely spaced
IAs in the internal carotid artery. Two models were created: one with
all IAs computationally eliminated and one with one IA retained.
They discovered that a combination of hemodynamic parameters was
more important in the onset of an aneurysm than individual indices
[27]. Kulcsar et al and Watanabe et al obtained similar findings
[28,29].
Kono et al described a patient who had a freshly developed
aneurysm with proximal stenosis, which was validated by serial
imaging. They created two pre-aneurysm models, one with stenosis
and one without, and ran computational fluid dynamics simulations
on both. Because of the stenosis-induced jet flow, the maximal WSS
and WSSG at the aneurysm start site were roughly doubled and
tripled, respectively. As a result, they underlined the importance of
proximal stenosis in aneurysm formation [30]. Lauric et al, on the
other hand, investigated the curvature effects on hemodynamic
circumstances [31]. Many additional elements had an important
part in the development of IAs. Singh et al investigated the effects of
smoking and hypertension on the establishment of aneurysms [33].
Shimogonya et al, on the other hand, discovered no
significant correlation between WSS and aneurysm inception
[33]. They also developed a novel hemodynamic measure termed
Gradient Oscillatory Number (GON), which may impact aneurysm
development. Chen et colleagues found that regionally higher WSS
and GON were strongly linked with locations vulnerable to sidewall
IA development in the hypothesized pre-diseased geometries of 22
clinical sidewalls IAs [34]. Ford et al discovered similar findings [35].
Tanaka et al discovered no obvious connections between
hemodynamic parameters and aneurysm initiation, contradicting
all of the preceding studies [36]. The magnitude of WSS in ruptured
and unruptured aneurysms has been determined in certain studies.
Nonetheless, the degree of WSS observed varies, and the threshold of
high or low WSS has been documented very infrequently. WSS values
ranging from 7.8 to 12.3 dyne/cm2 were shown to independently
describe aneurysm development by Zhang et al. There was a one-fold
increase in the likelihood of AComA aneurysm development with
each additional unit of WSS [37].
Discussion
The analysis of cerebral arteries from autopsies and IA animal
models has been critical in understanding the early alterations in the
emerging aneurysm. The aneurysm wall frequently has a disturbed
internal elastic lamina (IEL), media thinning, and bulge development.
For the etiology of IAs, several theories have been proposed.
The first theory claimed a congenital etiology, but the second theory
proposed that IAs are acquired lesions that develop over time, with
hemodynamic conditions having a role in their onset. Indeed, the
preferred position of the aneurysm at artery bifurcations with a
certain flow pattern suggested a role for hemodynamics in aneurysm
development. Meng et al. investigated how “a hemodynamic insult”
may result in maladaptive remodelling of the vessel wall. They
identified three regions at arterial bifurcations with different blood
patterns using CFD, the first one being the impingement zone,
where blood from the parent artery hits the apex of the bifurcation
and creates a stagnation point before accelerating into the branches
(WSS ≤ 20 dynes/cm2; velocity < 0.05 m/s; positive Wall Shear Stress
gradient(WSSG);the second one being the acceleration region, where
blood flow continues to accelerate until the maximum velocity (WSS >
20 dynes/cm2, high positive WSSG); and the third one being recovery
region where the velocity of blood reaches the maximum and starts to
decelerate until the physiological level of WSS ~20 dynes/cm2(negative
WSSG). They discovered early alterations mimicking IA initiation
in places subjected to SWSS and positive WSSG by mapping CFD with histological analysis of the arterial bifurcation. Further research
found that aneurysmal remodeling occurs only when hemodynamic
forces exceed a particular threshold (WSS > 1.22 x 103 dynes/cm2 and
WSSG > 530 Pa/mm) in their rabbit model.
Although these findings assist to clarify the function of
hemodynamic pressures in the onset of IA, Meng et al investigations
were conducted on an artificial extracranial bifurcation in a small
number of animals and require additional validation in other,
preferably intracranial, animal models. Furthermore, the stated
hemodynamic threshold values are only for rabbits and may change
in humans where vessel diameter is varied and several confounding
variables, such as genetic risk factors and habits such as smoking,
might impact IA development.
The idea of the WSS threshold, on the other hand, explains why
IAs occur more frequently at particular bifurcations and individuals
than others. Each arterial bifurcation has a unique flow pattern
based on its location, bifurcation angle, and parent and daughter
artery sizes, making some more likely to reach the threshold.
Singh et al33 investigated the effects of smoking and hypertension
on aneurysm development. As a result, a weaker wall caused by
age, smoking, hypertension, and genetic disorders would be more
susceptible to hemodynamic disturbances. A meta-analysis of
patients’ hemodynamic forces and the development of IAs supports
the notion that IAs are caused by spatial WSS (SWSS) and positive
WSSG [38,39].
There are some limitations to this study. Firstly, only CFDrelated
studies are examined. The current IA CFD models produce
poor results due to improper simplifications, reliance on physically
meaningless parameters, and a sloppy computational representation
of physiologic blood flow. As a result, new generation IA CFD models
should be explored in order to understand the function of various
hemodynamic variables in the onset of IA. Secondly, only items
published in English were considered.
Conclusion
Intracranial aneurysm formation is thought to have a complex origin, with hemodynamics playing a crucial part in the process. A rise in local WSS, as well as other hemodynamic indicators such as
GON, comes into play. The geometry and curvature effects, as well as
hemodynamic and therapeutically important variables like smoking
and hypertension, all influence the development of IA.