Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: Sani Ali Aji, Urology Unit, Department of Surgery, Bayero University, Aminu Kano Teaching Hospital, Kano, Nigeria, E-mail: saniaji2004@yahoo.com
Citation: Abdullahi M, Aji SA. Phytotherapy for Benign Prostatic Hyperplasia: The Clinical Efficay and Safety of Use of Prostacare in Patients with Mild to Moderate Lower Urinary Tract Symptoms. J Surgery. 2016;4(2): 4.
Copyright © 2016 Abdullahi M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 4, Issue: 2
Submission: 28 September, 2016 | Accepted: 19 November, 2016 | Published: 25 November, 2016
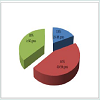
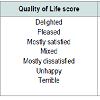
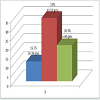
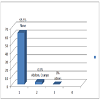
The estimated prostate size (by abdominal ultrasound) of the patients at presentation is shown in Figure 2: Majority of the patients had Qmax of less than 10 mls/s at presentation as shown in Figure 3: Table 1 shows the QOL scores the patients had if they were to have their LUTS for the rest of their life. After six weeks on prostacare capsules, majority of the patients recorded a significant improvement in the LUTS as shown in Figure 4.
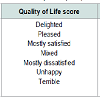
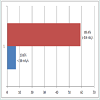
However, Majority of the patients had a significant improvement in their urinary flow as Qmax rises in most of the patients (Figure 5). No significant change in prostate size recorded after six weeks of prostacare (Figure 6). Similarly, most of the patients reported a better QOL based on the LUTS as shown in Table 2. The side effects profile of prostacare in our patients were very low (Figure 7).
Phytotherapy for Benign Prostatic Hyperplasia: The Clinical Efficay and Safety of Use of Prostacare in Patients with Mild to Moderate Lower Urinary Tract Symptoms
Muzzammil Abdullahi and Sani Ali Aji*
- Urology Unit, Department of Surgery, Bayero University, Aminu Kano Teaching Hospital, Kano, Nigeria
*Address for Correspondence: Sani Ali Aji, Urology Unit, Department of Surgery, Bayero University, Aminu Kano Teaching Hospital, Kano, Nigeria, E-mail: saniaji2004@yahoo.com
Citation: Abdullahi M, Aji SA. Phytotherapy for Benign Prostatic Hyperplasia: The Clinical Efficay and Safety of Use of Prostacare in Patients with Mild to Moderate Lower Urinary Tract Symptoms. J Surgery. 2016;4(2): 4.
Copyright © 2016 Abdullahi M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 4, Issue: 2
Submission: 28 September, 2016 | Accepted: 19 November, 2016 | Published: 25 November, 2016
Abstract
Background: Benign prostatic Hyperplasia (BPH) is the commonest benign tumour affecting men worldwide often causing structural or functional obstruction of the bladder neck with manifestation of lower urinary tract symptoms (LUTS). This may have significant effect on the quality of life of the individual.Aim and objective: The study was conducted to find the clinical efficacy and safety of use of prostacare in patients with mild and moderate LUTS secondary to BPH.
Patients and methods: It was a prospective interventional study on 72 patients, 40 years and above who presented with LUTS (International Prostate Symptoms Score; IPSS ≤ 19) to urology outpatient clinic ofAminu Kano Teaching Hospital (AKTH), Kano-Nigeria and who were diagnosed to have BPH. On presentation patients were evaluated for symptoms and signs of bladder outlet obstruction secondary to BPH.All patients recruited into the study had uroflowmetry (for peak urinary flow rate), laboratory investigations and abdominal ultrasound at presentation, and then they were given prostacare capsules for three months, one capsule (320 mg) daily. They were seen after six weeks of commencement of the drug and re-assessed.The generated data was analyzed using statistical package for social sciences (SPSS) version 20 software.
Results: Seventy two (72) patients were recruited over the period of the study; however, 6 patients were lost to follow up during subsequent review in the clinic. The ages of the patients ranged from 40 to 77 years with a mean age of 58±18.4 SD.
All the patients enrolled into the study had moderate LUTS (IPSS 9-19) and after 6 weeks on prostacare 86.4% of them had improvement in the LUTS. Similarly both quality of life (QOL) due to LUTS and peak urinary flow rate (Qmax) of majority of the patients recorded significant improvements after 6 weeks on the prostacare. From the results up to 54 patients felt happy or delighted about their LUTS after 6 weeks on prostacare when compared to 54 patients and 6 patients who felt unhappy and terrible respectively before given the drug. Also up to 89.4% of the patients had a Qmax of > 10 mls/s a significant improvement over the 16.7% with similar Qmax before prostacare. However no significant change in prostate size was recorded after 6 weeks on prostacare. Prostacare also was clinically safe in our patients as only 0.5% of our patients reported abdominal cramps.
Conclusion: Prostacare a monotherapy containing saw palmetto berries significantly relieves LUTS and improves urinary flow and thereby improving quality of life of patients with mild and moderate LUTS secondary to BPH. However, prostacare does not significantly affect the size of the prostate in short term use. The drug is generally safe to use as it does not have significant side effect on our patients.
Keywords
Phytotherapy; Prostacare; Efficacy; SafetyIntroduction
Benign prostatic hyperplasia (BPH) is a natural ageing process which involves the proliferation of both prostatic stroma and epithelium [1]. The increase in the size of the prostate may cause structural or functional obstruction of the bladder neck with manifestation of lower urinary tract symptoms (LUTS) which comprise of voiding (obstructive) symptoms, such as reduced stream, hesitancy, and straining as well as storage (irritative) symptoms, including frequency, nocturia, urgency and urge incontinence [1,2].Prostate gland usually begins to enlarge at the age of 40 years and by 60 years up to 50% of men will have a histologically enlarged prostate and this may increase to 90% in men of more than 80 years [1,2]. However, only about half of these men will show LUTS at corresponding age group [1].
Medical treatment of BPH is increasingly becoming popular owing to less morbidity among other reasons [3]. The use of plants and plant extracts (phytotherapy) is one of the medical therapies for BPH that have been in use for centuries [4]. Phytotherapy for BPH has been popular in many European countries and its use is increasingly been explored in the united state of America (USA) [4,5]. Several plant extracts have been used in the management of BPH; however, Saw palmetto extract (Serenoa repens) is the most extensively studied and scientifically proven [4,5].
Prostacare contains lipophylic extract of Saw palmetto 320 mg per capsule for treatment of LUTS secondary to BPH and improving sexual function for decades [6]. The use of phytotherapy as a modality for the treatment of BPH is limited in Africa owing to paucity of data on its efficacy in our patients. Phytotherapeutic agents are also comparatively less costly than the more commonly used α- adrenergic blockers and 5 α- reductase inhibitors [4].
The need for alternative drugs that have less side effects and with less cost is imperative in order to improve compliance of our patients hence the drive to conduct this study to determine the efficacy and safety of the use of PROSTACARE in the management of mild and moderate LUTS secondary to BPH in our environment.
Patients and Method
It was a prospective interventional study of 72 patients, 40 years and above who presented with LUTS (International Prostate Symptoms Score; IPSS ≤ 19) to urology outpatient clinic, Aminu Kano Teaching Hospital (AKTH), Kano-Nigeria and who were diagnosed to have BPH. The patients who were not on other forms of treatment for BPH were recruited from September 2015 to February 2016 and each patient followed up for three months. On presentation history was taken with details on the LUTS and symptoms of associated complications such as post void residual urine, uraemia, UTI and inguinal swellings among others. Patients were then examined to establish presence of BPH and to find signs of associated complications. All findings were documented in the questionnaire. Patients with causes of bladder outlet obstruction such as urethra stricture, stone diseases and bladder cancer were excluded. Those suspected to have stricture based on age and other aspect of the history had urethrocystoscopy. Those found with stricture were also excluded.Patients that consented to participate in the study were asked to fill a prepared International Prostate Symptoms Score (IPSS) and the Quality of life (QOL) score due to LUTS by themselves or through an interpreter for those who could not read. Patients found to have mild and moderate LUTS (IPSS ≤ 19) were then enlisted into the study. Others were treated by other modalities of treatment for BPH.
All patients recruited into the study had uroflowmetry at presentation and peak urine flow rate (Qmax) was determined for each patient and recorded. Some investigations (urinalysis, urine microscopy culture and sensitivity, abdominal ultrasound, serum urea, electrolytes and creatinine, and serum prostatic specific antigen) were done on all the patients, others such as prostatic biopsy, urethrocystoscopy were done where indicated. Results of all the investigations were recorded.
Each of the patients was given prostacare capsules for three months to take 1 capsule (320 mg) daily after food and was seen after six weeks on the drug. Lower urinary tract symptoms were re-assessed by re-filling a fresh IPSS and QOL score questionnaire by patients in a similar way as done 6 weeks previously. Patients were also clinically re-examined with emphasis on bladder and per rectal examination. Peak urine flow rate and abdominal ultrasound were repeated. Side effects of prostacare use were also asked for. The results in all of these were recorded in the appropriate sections of the questionnaire.
Patients who had significant improvement in LUTS (IPSS < 8) were asked to continue on the drug, while those with poor response to drug (IPSS ≥ 8) were either given additional medical treatment (α-adrenergic blocker mostly tamsulosin) or had prostatectomy (if patient chose). Some of the patients had urethrocystoscopy for reasons of worsening LUTS, haematuria and recurrent UTI.
The generated data was analyzed using statistical package for social sciences (SPSS) version 20 software.
Results
Seventy two (72) patients were recruited over the period of the study; however, 6 patients were lost to follow up during subsequent review in the clinic. The ages of the patients ranged from 40 to 77 years with a mean age of 58 ±18.4 SD. The distribution is shown in Figure 1: All the 72 patients had moderate LUTS (IPSS 9-19) at presentation and only 7 (9.7%) patients had UTI at presentation, none had any of the other associated complications looked for.The estimated prostate size (by abdominal ultrasound) of the patients at presentation is shown in Figure 2: Majority of the patients had Qmax of less than 10 mls/s at presentation as shown in Figure 3: Table 1 shows the QOL scores the patients had if they were to have their LUTS for the rest of their life. After six weeks on prostacare capsules, majority of the patients recorded a significant improvement in the LUTS as shown in Figure 4.
However, Majority of the patients had a significant improvement in their urinary flow as Qmax rises in most of the patients (Figure 5). No significant change in prostate size recorded after six weeks of prostacare (Figure 6). Similarly, most of the patients reported a better QOL based on the LUTS as shown in Table 2. The side effects profile of prostacare in our patients were very low (Figure 7).
Discussion
Prostacare is a monotherapy containing lipophylic extract of Saw palmetto 320 mg per capsule for treatment of LUTS secondary to BPH and improving sexual function [6,7].The Saw palmetto berry which is the main ingredient contains over 100 known compounds. The active ingredients in saw palmetto are contained in the purified lipid soluble extract of the saw palmetto berry. This has been found to contain 85 to 95 percent fatty acids (predominantly lauric, caprylic, and caproic), long chain alcohols, and sterols (including beta-sitosterol, stigmasterol, cycloartenol, lupeol, lupenone, and methylcycloartenol) [5,8].
Proposed mechanisms of actions include anti-androgenic effects, inhibition of type 1 and type 2 isoenzymes of 5-alpha-reductase, inhibition of growth factors such as the insulin-like growth factor-I, relaxation of lower urinary tract smooth muscle by non-competitively inhibiting both α 1a and 1d adrenergic receptors, anti-inflammatory effects through inhibition of lipooxygenase, cyclooxygenase, and leukotrienes, alteration of cholesterol metabolism; anti-estrogenic effects; and a decrease in available sex hormone-binding globulin [4,5,8].
All the patients enrolled into the study had moderate LUTS (IPSS 9-19) and after 6 weeks on prostacare, 86.4% of them had improvement in the LUTS out of which 54.6% still had few LUTS which were not bothersome. Similarly both QOL due to LUTS and Qmax of majority of the patients recorded significant improvements after 6 weeks on the prostacare. From the results up to 54 patients felt happy or delighted about their LUTS after 6 weeks on prostacare when compared to 54 patients and 6 patients who felt unhappy and terrible respectively if they were to live with their LUTS without medication. Also up to 89.4% of the patients had a Qmax of ≥ 10 mls/s a significant improvement over the 16.7% with similar Qmax before prostacare. The possibilities for the failure in 10.6% might be as a result on unresponsiveness to the drug. This can occur if the symptoms are mainly caused by the mechanical component of the BPH and not the dynamic. The period of the study was also short during which time there was no significant change in the size of the prostate. There might also be coexisting pathology such as neurogenic bladder. However no significant change in prostate size was recorded after 6 weeks on prostacare. This might also be due to the short period of the study. Prostacare also was clinically safe in our patients as majority of them did not report any side effect such as erectile dysfunction, headache, or vomiting. Only 0.5% of our patients reported abdominal cramps.
The findings in this study were similar to the ones in about 18 randomized clinical trials on use of saw palmetto in the treatment of BPH which were summarized by Wilt and colleagues 9. The results of their analysis showed mild to moderate improvement in IPSS and Qmax without decreasing prostate size. The side effects were comparable with placebo and were reportedly mild 9. Similarly, a meta-analysis of 7 placebo controlled trials of permixon saw palmetto by Lowe and associates showed an improvement in symptoms (nocturia was the only symptom that occurred in all studies) and urine flow rate when compared to placebo 9. However, our study showed an improvement in the findings of the widely quoted study of permixon Saw palmetto in comparison to finasteride, the 5 α-reductase inhibitor, a 6-month, randomized, double-blind, placebo-controlled study. In the study, IPSS improved in both groups (37% with Permixon versus 39% with finasteride), Qmax improved to a similar extent in both groups (2.7 mL/sec with Permixon versus 3.2 mL/sec with finasteride). The differences were significant compared with baseline for both drugs. Prostate size decreased more in the finasteride group than in the Permixon group (by 18% versus 6%) [9-11].
Some of the limitations of this study include short period of follow up on the drugs. This might be the reason why there was no significant change in the size of the prostate. The sample size was also small compared to other similar studies done elsewhere.
We recommend the study to be repeated using large sample size and also over a long period (more than one year).
Conclusion
Prostacare a monotherapy containing saw palmetto berries significantly relieves LUTS and improves urinary flow and thereby improving quality of life of patients with mild and moderate LUTS secondary to BPH. However, prostacare does not significantly affect the size of the prostate in short term use. The drug is generally safe to use as it does not have significant side effect on our patients.Acknowledgements
We thank Mega We Care Life Science for proving the drugs free to the patients for the purpose of this study.References
- Presti JC, Kane CJ, Shinohara K, Carroll PR (2008) Neoplasms of the prostate gland. In: Tanagho EA, McAninch JW (Eds), Smith’s general urology. 17th Edition. The McGraw-Hill companies, New York, USA, pp. 348-374.
- McVary KT (2003) Clinical evaluation of benign prostatic hyperplasia. Rev Urol 5: S3-S11.
- Shrivastava A, Gupta VB (2012) Various treatment options for benign prostatic hyperplasia: A current update. J Midlife Health 3: 10-19.
- Gordon AE, Shaughnessy AF (2003) Saw palmetto for prostate disorders. Am Fam Physician 67: 1281-1283.
- Saper RB (2016) Clinical use of saw palmetto. UpToDate®.
- (2014) Mega Life Sciences launches Prostacare to curb prostate enlargement. Pharmanews.
- Prostacare 60 capsules. Pharmacy Direct.
- Suzuki M, Ito Y, Fujino T, Abe M, Umegaki K, et al. (2009) Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol Sin 30: 227-281.
- Fagelman E, Lowe FC (2001) Saw palmetto berry as a treatment for BPH. Rev Urol 3: 134-138.
- Boyle P, Robertson C, Lowe F, Roehrborn C (2004) Updated meta-analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. BJU Int 93: 751-756.
- Carraro JC, Raynaud JP, Koch G, Chisholm GD, Di Silverio F, et al. (1996) Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. Prostate 29: 231-240.