Journal of Surgery
Download PDF
Review Article
*Address for Correspondence: Vishnumurthy Shushrutha Hedna, MD, Assistant Professor, Department of Neurology, Room L3-100, McKnight Brain Institute, 1149 Newell Drive, Gainesville, FL 32611, USA, Tel: (352)273-5550; Fax: (352)273-5575; E-mail: vhedna@mail.ufl.edu
Citation: Cai PY, Derequito R, Mishra M, Tenkabail S, Bodhit A, et al. A Non-Surgeon’s Guide to Surgical Management of Atrial Fibrillation. J Surgery. 2013;1(2): 5.
Copyright © 2013 Cai PY, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 26 August 2013 | Accepted: 09 October 2013 | Published: 14 October 2013
Reviewed & Approved by: Dr. Hong-Shuo Sun, Department of Surgery, University of Toronto, Canada
A Non-Surgeon’s Guide to Surgical Management of Atrial Fibrillation
Peter Y Cai1,2, Roselle Derequito1, Monica Mishra1, Spandana Tenkabail1, Aakash Bodhit1, Saeed Ansari2, Sarah Ganji1, Pradeepan Saravanapavan1, Sheema Khan1, Fawzi Abukhalil1, Michael F Waters 1, Thomas M Beaver 3 and Vishnumurthy Shushrutha Hedna1*
- 1Department of Neurology, University of Florida College of Medicine, Gainesville, FL, USA
- 2Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL, USA
- 3Department of Cardiothoracic Surgery, University of Florida College of Medicine, Gainesville, FL, USA
*Address for Correspondence: Vishnumurthy Shushrutha Hedna, MD, Assistant Professor, Department of Neurology, Room L3-100, McKnight Brain Institute, 1149 Newell Drive, Gainesville, FL 32611, USA, Tel: (352)273-5550; Fax: (352)273-5575; E-mail: vhedna@mail.ufl.edu
Citation: Cai PY, Derequito R, Mishra M, Tenkabail S, Bodhit A, et al. A Non-Surgeon’s Guide to Surgical Management of Atrial Fibrillation. J Surgery. 2013;1(2): 5.
Copyright © 2013 Cai PY, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 26 August 2013 | Accepted: 09 October 2013 | Published: 14 October 2013
Reviewed & Approved by: Dr. Hong-Shuo Sun, Department of Surgery, University of Toronto, Canada
Abstract
Atrial Fibrillation (AF) is the most common cardiac arrhythmia associated with substantial increases in death, heart failure, and stroke. It is important for healthcare providers in all fields to also gain an understanding of the novel techniques used in surgical treatment of AF. Clinicians must now decide between many different options. There are modified Maze procedures, catheter-based or minimally invasive surgical approaches to isolate the triggers and foci in the left atrium responsible for AF. A recently proposed radial approach can also be employed in substitution of the traditional geographical maze surgery. Finally, different energy sources, such as cryoablation, radiofrequency, microwave, and laser, can be used to create lesions in the atrium. Especially in the fields of neurology, psychiatry, and psychology, an understanding of these treatments is important for the management of AF patients with neurological pathology.Background
With an estimated prevalence of 2.66 million Americans, Atrial Fibrillation (AF) is the most common cardiac arrhythmia [1]. Substantial increases in death, heart failure, and stroke are all related to the occurrence of AF [2-5]. Within the affected population, incidence increases with age with the median age for men at 66.8 years and for women at 74.6 years [1,6]. Lifetime risk of AF appears is comparable in two large population-based studies in North America (Framingham Heart Study) and Europe (Rotterdam study) [7,8]. There is a diverse range of risk factors for AF, such as advancing age and male sex, or a variety of diseases (diabetes mellitus, hypertension, valvular disease, myocardial infarction, heart failure, obesity) and pathologies (elevated inflammatory marker concentrations, PRinterval prolongation) [9,10].AF is currently classified as either lone or recurrent AF. Patients with lone AF tend to be under 60 years of age who do not have evidence of cardiopulmonary disease. Recurrent AF is further divided into paroxysmal AF (within seven days), persistent AF (longer than seven days), or permanent AF (longer than one year) [11]. In general, AF is thought to arise from interactions between initiating triggers and abnormal tissue in the atria that can maintain arrhythmia. For example, in younger patients with relatively normal hearts, short intervals of paroxysmal AF are proposed to be triggered by the pulmonary veins. Focal triggers can be in the muscular sleeves arising from the proximal parts of the pulmonary veins down to the left atrium, the proximal superior vena cava, ligament of Marshall, or other parts of the right and left atria. Ultimately, AF can itself cause electrophysiological remodeling and further facilitate pathological arrhythmia [12]. Recent advances in the molecular pathophysiology of atrial fibrillation have suggested that acetylcholine-dependent current (IK,ACh), Ca2+-dependent K+ current (IKCa), and ryanodine receptor (RyR2) Ca2+ release are all potential therapeutic targets in the future, especially through the emerging role of miRNAs [13].
AF can cause significant pathology in the heart, with rapid atrial pacing altering atrial architecture due to uneven stretch of myocytes, activation of stretch receptors and channels, and apoptosis leading to irreversible damage [14]. Additionally, AF results in blood adhering to static atrial walls due to loss of normal mechanical activity of the atria, resulting in a 5 times increased risk of ischemic stroke due to thromboembolism. AF is thought to contribute to stasis, endothelial dysfunction, and a hypercoagulable state (Virchow’s triad) in promoting the formation of thrombus [15]. About 5 to 14% of patients will develop left atrial thrombi, ranging from a few millimeters to about 4 cm, if AF lasts more than 2 days [16]. Ultimately, 15% of all stroke etiology can be traced back to AF [1,4,17]. In addition, two prospective trials have shown conflicting results on the significance of atrial fibrillation on the severity of leukoaraiosis in stroke patients [18,19]. The role of AF in cognitive impairment and dementia, independent of stroke, is uncertain. However, the idea that a pathologic heart can cause cognitive deterioration through vascular dementia was discovered in the 1970s and was termed “cardiogenic dementia” [20]. While some suggest that AF can directly cause cognitive and functional decline even in the absence of stroke, epidemiologic studies have failed to consistently demonstrate this relationship [21-23].
AF treatments include surgery and/or medications that target heart rate or rhythm, inhibit clot formation to prevent strokes, or target risk factors for AF (eg. high blood pressure, obesity, diabetes). Because of novel surgical techniques and both difficulties in managing medication, such as coumadin levels in minimizing risk for major bleeding, as well as misconceptions that lead to limited utilization in clinical practice, surgical treatments are increasingly important in the management of AF [24]. The use of anti-arrhythmic drugs (AADs) to treat AF has declined in clinical practice due to proarrhythmic side effects, lack of adequate control, and the need for lifelong drug use [25].
Patients with paroxysmal AF are typically provided pharmacological interventions to control the arrhythmia. However, in patients with persistent AF, there is no consensus on whether to provide rhythm control, restoring sinus rhythm, or to provide rate control, controlling ventricular rate. Nevertheless, patients should always be considered for anticoagulation regardless of arrhythmia pattern [12]. Patients with AF have been shown to develop more severe strokes with worse outcomes than patients with sinus rhythm, further supporting the importance of treating the AF in order to reduce the risk of developing stroke [26]. The use of ECG may also be an important diagnostic tool for creating patient-tailored treatment strategies. Certain P-wave morphologies may have important clinical implications for invasive AF therapies by allowing for the identification of interatrial conduction routes in order to guide the placement of atrial leads during atrial resynchronization [27]. Interestingly, the risk of thromboembolism in patients with AF has been suggested to depend on the presence of well recognized risk factors and not the clinical type of AF [28]. Specific guidelines can be found in the recently amended ACCF/AHA Practice Guideline [29].
Clinical Indication for Surgical Therapy
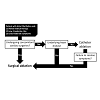
As with all clinical decisions, treatment is ultimately dependent on the discussion between a clinician and the patient about patientimportant outcomes. Different treatments can have different affects on not only outcome, but also costs, lifestyle, and risks of mortality and morbidity that should be addressed individually. With these important considerations in mind, appropriate guidelines can still serve as useful references for clinicians. Guidelines from the European Society of Cardiology (ESC) suggest considering atrioventricular node ablation and cardiac resynchronization therapy (CRT) when medical therapy has failed [30]. Surgical ablation is often considered for patients at the lower spectrum of risk and symptom presentation who are already planning to undergo other cardiac surgeries. Catheter ablation can be considered for patients with paroxysmal symptomatic AF and no underlying heart disease. However, failed catheter ablation subsequently requires consideration of minimally invasive surgical techniques. A simple representation of the considerations involved with these decisions is illustrated in Figure 1. Despite this simplification, in practice, each treatment approach can involve different procedures and each procedure can involve a range of different techniques. In addition, patients with persistent AF may require extensive and repeated ablation procedures [31].Treatment and Brief History
Left atrial isolation was first described in 1980 and involved a leftsided arteriotomy to limit arrhythmia to the left atrium [32]. Another approach, the Corridor technique, was later proposed which involved isolating a portion of the atrial septum that contains the sinoatrial and atrioventricular nodes to allow for appropriate contractions [33]. The disadvantages of these procedures were failure to achieve sinus rhythm and persistent risk of thromboembolism.The Maze procedure was first presented in 1991 as a technique for interrupting potential atrial circuits in a Maze-like manner and directing sinus impulse from the sinoatrial to atrioventricular node [34]. If correctly performed, this procedure preserve atrial transport function in most patients by allowing majority of the atrial myocardium to remain activated [35]. Unfortunately, this initial version of the Maze procedure could lead to significant chronotropic incompetence due to incisions near the sinus node and atrioventricular dyssynchrony due to prolonged interatrial conduction time. Modifications to incision technique were made to address these disadvantages in the Maze II procedure [36].
Next, the Maze III procedure was developed by including isolation of the pulmonary veins and posterior left atrium or left atrial appendage (LAA) [37]. Similar to earlier versions, this procedure involves making multiple incisions to interrupt reentrant circuits and guide electrical impulses to the atrioventricular node. The Maze III procedure is the gold standard for surgical treatment of AF, has been shown to effectively address atrial fibrillation while also having relatively low peri-operative morbidity and late morbidity rates [38-40]. In addition, the high success rate of eliminating AF and amputating the LAA was correlated with a significant reduction in the rate of cerebrovascular accidents [41]. However, failure of the Maze III procedure is often associated with increased duration of pre-operative AF, increased size of the left atrium, and advanced age [42,43]. Because this procedure is complex, requires prolonged aortic cross-clamp and cardiopulmonary bypass times, and can have morbidities associated with failed surgeries, its use for treatment of AF has been limited despite evidence for its efficacy.
New advances to the traditional Maze procedures involve using hypothermic sources, such as cryoablation, and hyperthermic sources, such as laser, ultrasound, microwave and radiofrequency, to interrupt electrical pathways [44]. By allowing for an epicardial approach to making transmural ablations that effectively eliminate AF, these energy sources can help reduce invasiveness and improve patient recovery if their efficacy can be established. For example, The Maze IV operation replaces the traditional cut-and-sew incisions with linear lines of ablation using newer technologies, such as radiofrequency [45]. This version of the Maze procedure can be performed in the traditional, thoracoscopic, or catheter-based approaches and has been shown to have high success rate that is equivalent to the classical method, in addition to the simplified and shortened procedure [46-48]. A further simplified version, the mini Maze procedure, involves making three essential lesions and has been shown to be nearly as effective as the Maze III version [49,50]. In these modified procedures, successful left atrial isthmus lesion is strongly associated with effective operation.
The traditional surgical approach centers on the idea that AF is mainly due to reentry that can be inhibited by incisions at critical locations to create a geographical maze [38]. A radial approach was developed to create a more physiological atrial contraction sequence by creating incisions that radiate from the sinus node towards the atrioventricular annular margins [51]. Because these incisions are parallel to the coronary arteries, this technique avoids creating incisions that cut across the atrial coronary arteries. However, adoption of this technique has been limited.
Catheter-based ablation is considered to be the least invasive approach to treating AF when compared to Maze procedures involving sternotomy or thoracoscopy. The different energy sources to achieve ablation previously mentioned for Maze procedures can also be employed in the catheter-based approach. However, early radiofrequency catheter ablation techniques demonstrated 40 to 50% success rate with high complication rates [52]. Another study of 200 patients suggested that success rate was only 28%, although the success rate was 69% in the subgroup of patients with paroxysmal AF, the optimal candidates for this procedure [53]. However, there is evidence that in select patients, radiofrequency catheter ablation can decrease symptoms and improve quality of life scores [54,55].
A brief summary of the advantages and disadvantages associated with some of these procedures are presented in Table 1.
Energy Sources
Cryoablation and radiofrequency ablation techniques are two possible ablation techniques [44]. Cryoablation, a commonly used technique, induces targeted scarring by cooling tissue to low temperatures (-50 to -75° C). Advantages include a time-tested safety record, minimal vascular and collagen damage in adjacent structures with a low risk for bleeding or perforation, reduced endocardial thrombus formation, ease of use, and a short operating room time. Unfortunately, this technique is not practical for an epicardial or endocardial approach due to warming blood flow. Radiofrequency can also be used to cause coagulation and destruction of cell structures and collagen. Previously, this process often involved estimation because maximal tissue temperature occurs in tissue planes below the contact surface, resulting in a risk of excessive heating. The addition of saline irrigation to unipolar radiofrequency ablation has allowed for ablation that is faster, deeper, more efficient, and with improved transmurality. The use of bipolar radiofrequency helps increase speed and precision of ablation but with low accuracy of algorithms to predict adequate tissue damage. Advantages of radiofrequency include improved safety, speed, minimization of bleeding, and realtime assessment of lesion thickness. However, this technique also has the highest risk for thrombus formation and increased risk of hyperthermic stress to adjacent tissue. Esophageal or mediastinal injury has been reported following radiofrequency ablation [56]. Microwave and laser ablation techniques are no longer used due to poor efficacy.Current Trends
The current trend in surgical management of AF is shifting towards a minimally invasive approach via mini thoracotomy for all procedures to be done in one sitting (eg. isolating the pulmonary vein, exclusion of the LAA, and ablation) [57]. In addition, there is a great deal of interest in developing novel therapeutic strategies, such as LAA exclusion as a means of reducing embolic complications in AF patients. In a trial of 1181 patients, 98% of arterial thrombi were found in the LAA [58]. Currently, LAA occlusion is conducted to reduce the risk of stroke in AF. This added procedure can be performed without increasing surgical complications and duration [59]. The American College of Cardiology (ACC) and American Heart Association (AHA) recommend that LAA ligation be performed during the course of valvular surgery [60].A percutaneous endovascular technique with transesophageal and/or intracardiac echocardiographic guidance for LAA occlusion can be performed with three devices: the Percutaneous LAA Transcatheter Occlusion (PLAATO, EV3, Plymouth, MN, USA), the Amplatzer Cardiac Plug (AGA Medical, Plymouth, MN, USA), and the WATCHMAN LAA system (Atritech Inc., Plymouth, MN, USA). The endocardial approach reduces recovery time and bleeding risk as well as eliminates the need for long-term anticoagulation [61]. However, this technique is associated with a steep learning curve that may initially increase complications such as pericardial effusion, cardiac tamponade or air embolization, increases risk for endovascular infection, requires post-procedural antithrombotic therapy and can result in incomplete LAA occlusion [62-64].
More recently, a percutaneous epicardial approach has also been developed using the LARIAT snare device (SentreHEART Inc., Palo Alto, CA, USA). By combining endocardial and percutaneous subxiphoid epicardial magnet-tipped wire-guided approaches, this technique allows for ligation of the LAA at its ostium. An initial report showed ten out of eleven patients had successful acute LAA ligation and four out of six patients demonstrating successful and complete LAA closure at 60 days [65].
Another device that can be used is the Atriclip, which was approved by the FDA to be applied epicardially via a median sternotomy approach that allows for effective deployment during other cardiac procedures. It should be noted that surgeons have also used the clip via an off label thoracoscopic approach. The Atriclip is available in a number of sizes to account for LAA size variability and has been shown to safely and effectively apply pressure to ensure consistent, secure occlusion of the LAA [66]. A 6 month-follow up showed that 98% of patients (60 out of 61) were confirmed to have clip stability, successful LAA closure and absence of LAA thrombus [66].
Conclusion
Because AF is associated with both stroke and dementia, it is important for healthcare providers in the fields of neurology, psychiatry, and psychology to also gain an understanding of the novel techniques used in surgical treatment of AF. Oral anticoagulation with warfarin remains an effective standard of care for AF but is limited by adverse effects like bleeding and difficulties achieving an adequate therapeutic level. Clinicians can now turn to newer options, such as modified Maze procedures, catheter-based or minimally invasive surgical approaches to isolate the triggers and foci in the left atrium responsible for AF.References
- Writing Group Members, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, et al. (2010) Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 121: e46-e215.
- Cohen M, Boiangiu C (2011) The management of patients with atrial fibrillation and dronedarone's place in therapy. Adv Ther 28: 1059-1077.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D (1998) Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 82: 2N-9N.
- Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988.
- Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, et al. (2012) Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 5: 85-93.
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e257-e354.
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, et al. (2004) Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 110: 1042-1046.
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, et al. (2006) Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 27: 949-953.
- Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, et al. (2012) Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options--a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 14: 8-27.
- Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, et al. (2011) Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 124: 1982-1993.
- Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, et al. (2001) ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation 104: 2118-2150.
- Markides V, Schilling RJ (2003) Atrial fibrillation: classification, pathophysiology, mechanisms and drug treatment. Heart 89: 939-943.
- Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S (2011) Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 121: 2955-2968.
- Aime-Sempe C, Folliguet T, Rucker-Martin C, Krajewska M, Krajewska S, et al. (1999) Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol 34: 1577-1586.
- Watson T, Shantsila E, Lip GY (2009) Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 373: 155-166.
- Thambidorai SK, Murray RD, Parakh K, Shah TK, Black IW, et al. (2005) Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study). Am J Cardiol 96: 935-941.
- Syed FF, Asirvatham SJ (2011) Left atrial appendage as a target for reducing strokes: justifiable rationale? Safe and effective approaches? Heart Rhythm 8: 194-198.
- Henon H, Godefroy O, Lucas C, Pruvo JP, Leys D (1996) Risk factors and leukoaraiosis in stroke patients. Acta Neurol Scand 94: 137-144.
- Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS (1995) Leukoaraiosis in stroke patients. The Copenhagen Stroke Study. Stroke 26: 588-592.
- (1977) Cardiogenic Dementia. Lancet 1: 27-28.
- Mead GE, Keir S (2001) Association between cognitive impairment and atrial fibrillation: a systematic review. J Stroke Cerebrovasc Dis 10: 35-43.
- Sabatini T, Frisoni GB, Barbisoni P, Bellelli G, Rozzini R, et al. (2000) Atrial fibrillation and cognitive disorders in older people. J Am Geriatr Soc 48: 387-390.
- Park H, Hildreth A, Thomson R, O'Connell J (2007) Non-valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing 36: 157-163.
- Hedna VS, Favilla CG, Guerrero WR, Patel A, Gottipati A, et al. (2012) Trends in the management of atrial fibrillation: A neurologist’s perspective. J Cardiovasc Dis Res 3: 255-264.
- Kuo CT, Luqman N, Lin KH, Lee YS (2003) Atrial fibrillation: new horizons. Chang Gung Med J 26: 712-721.
- Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS (1996) Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 27: 1765-1769.
- Platonov PG (2008) Atrial conduction and atrial fibrillation: what can we learn from surface ECG? Cardiol J 15: 402-407.
- Potpara T, Grujic M, Vujisic-Tesic B, Ostojic M, Polovina M, et al. (2009) [Relationship between the type of atrial fibrillation and thromboembolic events]. Vojnosanit Pregl 66: 887-891.
- American College of Cardiology Foundation, American Heart Association, European Society of Cardiology, Heart Rhythm Society, Wann LS, et al. (2013) Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 127: 1916-1926.
- European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369-2429.
- Oral H, Chugh A, Good E, Wimmer A, Dey S, et al. (2007) Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 115: 2606-2612.
- Williams JM, Ungerleider RM, Lofland GK, Cox JL (1980) Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg 80: 373-380.
- van Hemel NM, Defauw JJ, Kingma JH, Jaarsma W, Vermeulen FE, et al.(1994) Long-term results of the corridor operation for atrial fibrillation. Br Heart J 71: 170-176..
- Cox JL (1991) The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 101: 584-592.
- Feinberg MS, Waggoner AD, Kater KM, Cox JL, Lindsay BD, et al. (1994) Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation 90: II285-292.
- Fragakis N, Pantos I, Younis J, Hadjipavlou M, Katritsis DG (2012) Surgical ablation for atrial fibrillation. Europace 14: 1545-1552.
- Cox JL, Jaquiss RD, Schuessler RB, Boineau JP (1995) Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 110: 485-495.
- Cox JL, Schuessler RB, Lappas DG, Boineau JP (1996) An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg 224: 267-273.
- McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D 3rd (2000) The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg 12: 25-29.
- Lee AM, Melby SJ, Damiano RJ Jr (2009) The surgical treatment of atrial fibrillation. Surg Clin North Am 89: 1001-1020.
- Ad N, Cox JL (2000) Stroke prevention as an indication for the Maze procedure in the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg 12: 56-62.
- Kim KC, Cho KR, Kim YJ, Sohn DW, Kim KB (2007) Long-term results of the Cox-Maze III procedure for persistent atrial fibrillation associated with rheumatic mitral valve disease: 10-year experience. Eur J Cardiothorac Surg 31: 261-266.
- Gillinov AM, Bhavani S, Blackstone EH, Rajeswaran J, Svensson LG, et al. (2006) Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Ann Thorac Surg 82: 502-513.
- Comas GM, Imren Y, Williams MR (2007) An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg 19: 16-24.
- Damiano RJ, Bailey M (2007) The Cox-Maze IV procedure for lone atrial fibrillation. Multimedia Manual of Cardio-Thoracic Surgery 2007.
- Damiano RJ Jr, Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, et al. (2011) The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 141: 113-121.
- Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, et al. (2004) A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 128: 535-542.
- Khargi K, Hutten BA, Lemke B, Deneke T (2005) Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg 27: 258-265.
- Cox JL (2004) Surgical treatment of atrial fibrillation: a review. Europace 5 Suppl 1: S20-S29.
- Cui YQ, Sun LB, Li Y, Xu CL, Han J, et al. (2008) Intraoperative modified Cox mini-maze procedure for long-standing persistent atrial fibrillation. Ann Thorac Surg 85: 1283-1289.
- Nitta T, Lee R, Watanabe H, Harris KM, Erikson JM, et al. (1999) Radial approach: a new concept in surgical treatment for atrial fibrillation. II. Electrophysiologic effects and atrial contribution to ventricular filling. Ann Thorac Surg 67: 36-50.
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, et al. (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339: 659-666.
- Cheema A, Vasamreddy CR, Dalal D, Marine JE, Dong J, et al. (2006) Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 15: 145-155.
- Kay GN, Ellenbogen KA, Giudici M, Redfield MM, Jenkins LS, et al. (1998) The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol 2: 121-135.
- Marshall HJ, Harris ZI, Griffith MJ, Gammage MD (1998) Atrioventricular nodal ablation and implantation of mode switching dual chamber pacemakers: effective treatment for drug refractory paroxysmal atrial fibrillation. Heart 79: 543-547.
- Ninet J, Roques X, Seitelberger R, Deville C, Pomar JL, et al. (2005) Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. J Thorac Cardiovasc Surg 130: 803-809.
- Han FT, Kasirajan V, Wood MA, Ellenbogen KA (2009) Minimally invasive surgical atrial fibrillation ablation: patient selection and results. Heart Rhythm 6: S71-S76.
- Blackshear JL, Odell JA (1996) Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 61: 755-759.
- Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, et al. (2005) Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 150: 288-293.
- Gillinov AM (2007) Advances in surgical treatment of atrial fibrillation. Stroke 38: 618-623.
- Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, et al. (2007) Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 49: 1490-1495.
- Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL (2008) Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 52: 924-929.
- Dawson AG, Asopa S, Dunning J (2010) Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion? Interact Cardiovasc Thorac Surg 10: 306-311.
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, et al. (2000) Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 36: 468-471.
- Bartus K, Bednarek J, Myc J, Kapelak B, Sadowski J, et al. (2011) Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 8: 188-193.
- Ailawadi G, Gerdisch MW, Harvey RL, Hooker RL, Damiano RJ, Jr., et al. (2011) Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 142: 1002-1009.