Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: Stefano Merigliano, Department of Surgery, University of Padua School of Medicine, Italy, Tel: 049.8215387; Fax: 049.8216514; E-mail: stefano.merigliano@unipd.it
Citation: Sarzo G, Finco C, Mungo B, Gruppo M, Cadrobbi R, et al. Anal Fistula Repair with Acellular Dermal Matrix Plug: Description of a Novel Technique and Early Results. J Surgery. 2013;1(1): 6.
Copyright © 2013 Sarzo G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 26 July 2013 | Accepted: 07 August 2013 | Published: 12 August 2013
Surgical technique
A second anchoring resorbable 2/0 suture was then positioned through the secondary orifice, in order to allow the end of the plug to protrude externally for a length of 3-4 mm. Finally the external orifice was enlarged with a scalpel blade to promote the drainage, and a trans-anal gauze was positioned and left in place for 12 hours after surgery, in order to prevent contamination of the wound, and to stabilize the flap.
Among healed patients, 5 (41.7%) had cryptoglandular AF and 4 (58.3%) had AF secondary to chronic fissure. Six of the healed patients were treated for the first time while other 3 had had previous surgeries (average 3, range 2-4). The fistula was posterior in 6 cases (66.7%), and anterior in the other 3 cases (33.3%); seven (77.8%) fistulas were linear and two (22.2%) fistulas had multiple tracts. The average time of seton persistence was 7.4 months (range 3-12).
Analyzing the results in terms of recurrence, we observed that in 75% of treated cases there was complete clinical healing, with an average follow-up of 9.33 months. This closure rate is slightly higher than that reported by other authors, with different devices [14,19-23].
We could hypothesize that this difference may be explained by the biomechanical principles described above: in a linear fistula, the positioning of a non collapsible plug, perfectly and fully fitting to the fistula tissues, allows, together with its self-locking shape, the primary stability of the implant, which is thought to be the necessary condition for the correct biomaterial incorporation and the subsequent healing of the fistula [15-17]. On the contrary, the presence of branched fistulas may facilitate over-infection and therefore the risk of recurrent abscess, due to the incomplete occupation of the fistulous tract and the persistence of residual recesses [25]. This finding suggests that a possible and logical treatment of branched fistulas could consist of several progressive stages, with the positioning of multiple plugs, approaching those pathological entities as if they were an ideal set of linear anal fistulas.
Anal Fistula Repair with Acellular Dermal Matrix Plug: Description of a Novel Technique and Early Results
G. Sarzo1, C. Finco2, B. Mungo1, M. Gruppo1, R. Cadrobbi1, L. Polese1, R. Rizzato1, and S. Merigliano1*
- 1Department of Surgery, University of Padua School of Medicine, Italy
- 2Department of General Surgery, Arzignano Hospital, Vicenza, Italy
*Address for Correspondence: Stefano Merigliano, Department of Surgery, University of Padua School of Medicine, Italy, Tel: 049.8215387; Fax: 049.8216514; E-mail: stefano.merigliano@unipd.it
Citation: Sarzo G, Finco C, Mungo B, Gruppo M, Cadrobbi R, et al. Anal Fistula Repair with Acellular Dermal Matrix Plug: Description of a Novel Technique and Early Results. J Surgery. 2013;1(1): 6.
Copyright © 2013 Sarzo G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 1
Submission: 26 July 2013 | Accepted: 07 August 2013 | Published: 12 August 2013
Abstract
Background: The interest of colorectal surgeons has shifted over the years towards the development of “sphincter-saving” surgical techniques. The aim of the present study was to evaluate the efficacy of the new device in terms of healing, biocompatibility and postoperative complications.Method: A total of 12 patients with high transphincteric anal fistulas were treated with a new acellular dermal matrix (ADM) plug. All patients were preoperatively examined by means of rectoscopy and transanal ultrasound and underwent placement of a silk or silicone seton for a minimum period of two months in order to consolidate and drain the fistular track. The plug was surgically positioned through a “press-fit” technique. Patient’s demographics, fistulas’ etiology and success rates were recorded. During the follow-up, healing time of the fistulae, continence and postoperative pain were evaluated.
Results: From January 2012 to May 2013, 7 men and 5 women underwent a plug insertion in our institution. The average age was 52.3 years (range 31-70). The fistulas were either of cryptoglandular origin (5; 41.7%) or secondary to chronic fissure (7; 58.3%). Six patients (50%) had history of previous failed surgery, while the remaining 6 patients (50%) were surgically treated for the first time. 9 of the 12 patients (75%) achieved clinical healing with average follow-up of 9.33 months.
Conclusion: The anal fistula ADM plug was associated with a good success rate and less postoperative morbidity, compared to similar series in the literature. The product demonstrated high biocompatibility. The new developed surgical technique allowed plug implantation maintaining both intimate contact with the fistular track and primary stability, which are known to be crucial requirements for the device’s incorporation. Further studies will be needed to evaluate the impact of patient selection on overall therapeutic success.
Keywords
Anal fistula plug; Porcine dermis; Cryptoglandular fistulas; Incontinence; Fissure; Press-fit procedure; ADMIntroduction
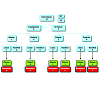
Anal fistulas (AF) represent one of the most frequent pathologies of the anal canal. According to Park’s classification, anal fistulae may be divided into four groups: Type I-Intersphinteric, Type II-Transphinteric, Type III-Suprasphincteric, Type IV-Extrasphincteric [1]. It is useful for clinical purposes to distinguish between simple and complex AF. The latter include: type II fistulas with internal orifice located above the first third of the anal canal; Type III-IV AF; horseshoe AF; rectovaginal fistulas; perianal fistulas in Crohn’s disease; anterior AF in women; post radiotherapy anal fistulae (Figure 1) [2].The management of complex AF is challenging and it is burdened by a high recurrence rate and a significant rate of fecal incontinence. The surgical treatment of AF aims to eradicate the sepsis and to promote healing through closure of the internal anal orifice and removal of the fistulous tract [3]. Fistulectomy and fistulotomy represent the gold standard for the treatment of submucosal fistulas, intersphinteric AF and low type II AF (within the lower third of the anal canal) [4]. These two procedures lead to good results and low recurrence rates (0-9%) and incidence of iatrogenic fecal incontinence (0-33%) [5]. However, if used for the treatment of complex anal fistulas (CAF), fistulectomy may cause permanent damage to the sphincters, which can easily cause mild to severe fecal incontinence [5,6]. The traditional surgical options for CAF include advancement flaps, loose seton technique, and confectioning of a stoma. Unfortunately, these techniques are associated with a high recurrence rate and postoperative FI [6]. In order to overcome these limits, the interest of colorectal surgeons has shifted, over the years, towards the development of “sphincter saving” surgical techniques, such as Ligation Intersphincteric Fistula Technique (LIFT) [7], Video Assited Anal Fistula Treatment (VAAFT) [8], OTCS Proctology [9] and the use of plugs (Gore Bio-A, Surgisis COOK) [10,11], fibrin glues, liquid collagen and collagen paste [12]. The development and availability of biocompatible materials, within the last few years, have led to the possibility of treating this disease with the application of a biologic prosthetic plug through the fistulous tract. The use of biocompatible materials, moreover, respects the anatomy and physiology of the anal canal, thus not precluding the possibility of recourse to other solutions in case of failure [13,14,18]. The aim of the present study is to prospectively evaluate the efficacy of a new biological prosthetic plug made of acellular dermal matrix (ADM) in the treatment of the CAF.
Materials and Methods
Patient selectionConsecutive patients with complex transphincteric anal fistulas were evaluated for eligibility. After approval of the study protocol by the ethics committee, all patients released their informed consent for the application of a biological device.
Patients with inflammatory bowel disease (Crohn’s disease, ulcerative colitis) or colorectal cancer were excluded.
Data collected included age, patient’s demographics, fistula etiology, fistula type, presence of a previously placed seton, duration of seton permanence and previous anorectal surgeries.
All patients included in the study underwent a preoperative proctological examination (including rectoscopy) and instrumental evaluation of the sphincter apparatus through anorectal manometry and two-dimensional ERUS with hydrogen peroxide. A pelvic MRI was performed for those patients in whom relations of the fistular tract with the sphincter apparatus could not be assessed in a satisfactory way with the above procedures.
Each fistula was first treated for a minimum period of two months using seton, until complete regression of the residual abscess cavities visualized in the preoperative study with ERUS and/or MRI. Each patient underwent preoperative rectum cleaning with osmotic laxative and was treated with antibiotics (Amoxicillin/Clavulanicacid) for the first six postoperative days.
The anal fistula ADM plug
The new acellular dermal matrix (ADM) plug is innovative in both material and design. The collagen matrix, not cross-linked, is obtained through a deantigenation process which preserves the integrity of the native proteins and acts as a natural scaffold for the development and the regeneration of new tissue, promoting remodeling without slowing down the timescale of physiological turnover.
The design of the plug (wedge-shaped with sharp edges; patent pending) neutralizes the forces of axial displacement and rotation. The solid body, not collapsible, allows intimate contact with the fistulous tract. The intimate contact with the vascularized tissue and primary stability obtained through the “press-fit” positioning aim to ensure the incorporation of the biomaterial (Figure 2) [15-17].
Surgical technique
The surgical procedure was performed by two accredited colorectal surgeons with no less than 10 years of experience in colorectal surgery and with no less than 500 colo-proctological operations per year performed at our colo-proctological surgical unit. All selected patients underwent spinal anesthesia and the procedure was carried out in lithotomy position. Before plug placement, a mechanical courettage of the fistulous tract was performed, until a complete evacuation of fibrin and corpuscular slags deposited in the fistulous tunnel was obtained. The blood oozing from the tissues promotes healing of the fistula and regeneration of the tissues after self-locking plug positioning.
After soaking the plug in saline solution for about 15 minutes, the device was pulled into the fistular track, from the internal opening to the external one, through interference fit, using a non-absorbable suture previously passed through the distal end of the plug as a handhold. The plug design neutralized rotation forces as a result of its sharp edges, axial extrusion by its wedged shape, and collapse risk due to its solid body (Press-fit principle). After further accurate toilette of the fistulous tract with saline solution, a small mucous periorificial flap was created. The plug was then secured to the internal sphincter with 2 or 3 loose interrupted re-absorbable sutures (2/0 polyglactin), knotted after plug placement in order to close the primitive orifice with a mucous plastic (Figure 3).
A second anchoring resorbable 2/0 suture was then positioned through the secondary orifice, in order to allow the end of the plug to protrude externally for a length of 3-4 mm. Finally the external orifice was enlarged with a scalpel blade to promote the drainage, and a trans-anal gauze was positioned and left in place for 12 hours after surgery, in order to prevent contamination of the wound, and to stabilize the flap.
Postoperative protocol
Anamnestic data of all patients were collected, as well as perioperative variables, including operative time, any intra-operative complication, the position of the internal and external fistulous orifices, the track of the fistula and its etiology (cryptoglandular or secondary to the presence of an anal fissure). The evaluation of the outcome was made through proctological examination 7 days after surgery and thereafter at 1-3-6-12 months. During the examinations, the site was cleaned with saline solution and sodium hypochlorite based disinfectant solution; hydrogen peroxide was not used. Clinical parameters were evaluated, such as the closure of the fistulous orifices, postoperative pain, presence and type of secretions, presence/absence of signs of local flogosis (redness, swelling, perianal skin reactions). We also collected functional information, such as presence of urgency, anal incontinence and the degree of functional limitation. Healing was defined as the closure of the internal and external fistulous orifices, assessed with anoscopy and clinical evaluation. Moreover, healing was defined as the absence of purulent secretions and swelling related to persistent active fistulous tracts or abscess formation. In doubtful cases ERUS was performed.
Results
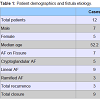
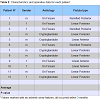
From January 2012 to May 2013, 12 patients (5M/7F) with an average age of 52.3 years (range 31-70), underwent ADM plug insertion. Patients treated for AF secondary to chronic fissure were 7 (58.3%), while those treated for cryptoglandular fistula were 5 (41.7%).50% of the patients (n = 6) suffered from a recurrent fistula and had previously undergone multiple fistula surgical repairs (average 3.3, range 2-4); the remaining 50% were treated for the first time. The overall success was 75% (n = 9), with an average follow-up of 9.33 months (range 2-14). Table 1 shows patients characteristics. Table 2 shows detailed information for each patient.
Among healed patients, 5 (41.7%) had cryptoglandular AF and 4 (58.3%) had AF secondary to chronic fissure. Six of the healed patients were treated for the first time while other 3 had had previous surgeries (average 3, range 2-4). The fistula was posterior in 6 cases (66.7%), and anterior in the other 3 cases (33.3%); seven (77.8%) fistulas were linear and two (22.2%) fistulas had multiple tracts. The average time of seton persistence was 7.4 months (range 3-12).
Recurrences were observed in 3 patients (25%). Recurrence occurred within three months, with continuous and persistent serous purulent secretions. All patients with recurrence had fistulous disease secondary to chronic fissure and had undergone previous surgeries (average 3.6; range 3-4). The fistula was anterior in 2 patients (66.7%) and linear in 1 patient (33.3%). Two of the patients with recurrence had maintained the seton in place for only two months.
All subjects were treated in Day Surgery and were discharged on the same day. There were no intra-operative complications and the average operative time was 30 minutes (range 20-45).
Discussion
The treatment of anal fistulae with a biocompatible material plug has been proposed in recent years as a “sphincter-saving” alternative to the classic treatment of AF with fistulectomy [3-5,14,18-20].The latest findings in biotechnological research have led to the development and commercialization of various biocompatible devices for the treatment of complex anal fistulas, such as Surgisis® Anal Fistula PlugTM (Cook Medical, Bloomington, IN) and Gore Bio-A® Fistula Plug (W. L. Gore Corporation, Newark, DE) [21,22]. Nevertheless, data from literature show low closure rate and remarkable incidence of postoperative complications (inflammation, sepsis, suppuration and pain) in patients treated with those devices [19,23]. We believe that the failed integration of the devices, could be due to their inability to maintain contact with the tissue of the fistula tract in the presence of stress such as muscle contraction [15,23]. In order to avoid these occurrences and inhibit the phenomenon of displacement of the plug, a new device, different in shape and material, was designed. The new ADM plug for anal fistula repair is a wedge-shaped plug made of deantigenated porcine dermis: its sharp edges prevent device rotation around its longitudinal axis while the wedge shape obstructs its dislocation (Figure 2). Secondly, the device has a solid body which doesn’t collapse even when subjected to external forces (e.g. contraction of muscle fibers), and fits into the fistulous tract “under pressure”, occupying the entire lumen (Pressfit principle). These features allow the ADM plug to meet the main biomechanical requirements necessary for the correct integration of the biomaterial: primary stability and intimate contact with vascularized tissue.
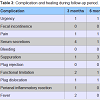
Our series, although limited, shows only one case of plug dislocation (Table 3), probably due to its incorrect positioning. Based on our experience, we recommend the positioning of the plug from the internal anal orifice toward the external orifice. In this way, the endorectal pressure, higher than that exerted at the cutaneous fistulous external orifice, contributes to the maintenance of the device in the correct position [3].
Analyzing the results in terms of recurrence, we observed that in 75% of treated cases there was complete clinical healing, with an average follow-up of 9.33 months. This closure rate is slightly higher than that reported by other authors, with different devices [14,19-23].
Table 3 shows the low rate of postoperative complications. A comparable symptomatic improvement has not been reported by other authors thus far [20-24]. In this regard, it is important to emphasize how every patient with cryptogenic linear anal fistula achieved full recovery; a similar result was not observed in those patients (42.9%) whose fistula originated from the bottom of a chronic fissure (Chart 1).
We could hypothesize that this difference may be explained by the biomechanical principles described above: in a linear fistula, the positioning of a non collapsible plug, perfectly and fully fitting to the fistula tissues, allows, together with its self-locking shape, the primary stability of the implant, which is thought to be the necessary condition for the correct biomaterial incorporation and the subsequent healing of the fistula [15-17]. On the contrary, the presence of branched fistulas may facilitate over-infection and therefore the risk of recurrent abscess, due to the incomplete occupation of the fistulous tract and the persistence of residual recesses [25]. This finding suggests that a possible and logical treatment of branched fistulas could consist of several progressive stages, with the positioning of multiple plugs, approaching those pathological entities as if they were an ideal set of linear anal fistulas.
Based on our experience, the placement of a drainage seton before surgery and its maintenance for at least 60 days, until the absence of detection of residual abscess with ERUS, represents a key step in the treatment of anal fistulas [26]. This hypothesis could be supported by the fact that many series in literature, characterized by a high rate of recurrence, do not describe the positioning of a seton before the placement of the prosthetic plug [20,23].
Chart 1 summarizes the patients’ complications: the plug showed an excellent tolerability; there were no cases of rejection and only one case of local skin inflammatory reaction, spontaneously regressed without the need of local or systemic treatments. A biocompatibility comparable to that of this device has not been reported in the literature for other products [21-24]. Symptoms such as urgency and pain were reported in only one patient who, after a few weeks, showed a recurrence, characterized by functional limitation due to the inability to maintain a sitting position for a long period of time, and discomfort related to the abundant secretions.
In the first 3 months the presence of serous secretions was more abundant (33.3%), but it could be interpreted as a physiological step in the healing process, as it ceased spontaneously without any further complications (Table 3). The only plug dislocation was caused, in our opinion, by a technical error during surgery.
Finally, our preliminary data showed that, for the treatment of complex anal fistulas secondary to chronic fissure, the positioning of the plug resulted in symptomatic improvement in 2 cases out of 3, even in the event of recurrence.In conclusion, the use of the ADM plug could represent a valuable, new, therapeutic “sphincter saving” option, in particular for the treatment of cryptogenic complex transphinteric anal fistulae with linear route. The new developed surgical technique allowed the plug implantation according to the biomechanical requirements for the device incorporation: intimate contact and primary stability.
The product has demonstrated high biocompatibility and minimal post-operative complications.
We believe that the achievement of a successful outcome could be also related to seton positioning and maintenance prior to surgery with the above described modalities. Further multicentric studies and wider patient samples, however, are required to confirm what we described, as well as to establish a standardized approach for the treatment of complex anal fistulas in the era of regenerative surgery.
References
- Parks AG, Gordon PH, Hardcastle JD (1976) A classification of fistula-in-ano. Br J Surg 63: 1-12.
- Whiteford MH (2007) Perianal abscess/fistula disease. Clin Colon Rectal Surg 20: 102-109.
- Deeba S, Aziz O, Sains PS, Darzi A (2008) Fistula-in-ano: advances in treatment. Am J Surg 196: 95-99.
- Malik AI, Nelson RL (2008) Surgical management of anal fistulae: a systematic review. Colorectal Dis 10: 420-430.
- Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD, et al. (2011) Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum 54:1465-1474.
- Van Koperen PJ, Horsthuis K, Bemelman WA, Stoker J, Slors JF (2008) Perianal fistulas: developments in the classification and diagnostic techniques, and a new treatment strategy. Ned Tijdschr Geneeskd 152: 2774-2280.
- Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K (2007) Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai 90: 581-586.
- Zbar AP (2011) “Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure to repair complex analfistulas” by Piercarlo Meinero and Lorenzo Mori. Tech Coloproctol 15: 423-424.
- Prosst RL, Herold A, Joos AK, Bussen D, Wehrmann M, et al. (2012) The anal fistula claw: the OTSC clip for anal fistula closure. Colorectal Dis 14: 1112-1117.
- Safar B, Jobanputra S, Sands D, Weiss EG, Nogueras JJ, et al. (2009) Anal fistula plug: initial experience and outcomes. Dis Colon Rectum 52: 248-252.
- Ratto C, Litta F, Parello A, Donisi L, Zaccone G, et al. (2012) Gore Bio-A® Fistula Plug: a new sphincter-sparing procedure for complex anal fistula. Colorectal Dis 14: e264-e269.
- Cintron JR, Park JJ, Orsay CP, Pearl RK, Nelson RL, et al. (2000) Repair of fistulas-in-ano using fibrin adhesive: long-term follow-up. Dis Colon Rectum 43: 944-949.
- Garg P, Song J, Bhatia A, Kalia H, Menon GR (2010) The efficacy of anal fistula plug in fistula-in-ano: a systematic review. Colorectal Dis 12: 965-970.
- Lenisa L, Espin-Basany E, Rusconi A, Mascheroni L, Escoll-Rufino J, et al. (2010) Anal fistula plug is a valid alternative option for the treatment of complex anal fistula in the long term. Int J Colorectal Dis 25: 1487-1493.
- Badylak SF, Freytes DO, Gilbert TW (2009) Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 5: 1-13.
- Badylak SF (2002) The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol 13: 377-383.
- Inglin R, Brugger L, Candinas D, Eberli D (2011) Bioengineering of Colo-Rectal Tissue, Tissue Engineering for Tissue and Organ Regeneration, (Ed. Prof. Daniel Eberli), ISBN: 978-953-307-688-1, InTech, DOI: 10.5772/25101 Chapter 13.
- Champagne BJ, O’Connor LM, Ferguson M, Orangio GR, Schertzer ME, et al. (2006) Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum 49: 1817-1821.
- El-Gazzaz G, Zutshi M, Hull T (2010) A retrospective review of chronic anal fistulae treated by anal fistulae plug. Colorectal Dis 12: 442-447.
- Ky AJ, Sylla P, Steinhagen R, Steinhagen E, Khaitov S, et al. (2008) Collagen fistula plug for the treatment of anal fistulas. Dis Colon Rectum 51: 838-843.
- Safar B, Jobanputra S, Sands D, Weiss EG, Nogueras JJ, et al. (2009) Anal fistula plug: initial experience and outcomes. Dis Colon Rectum 52: 248-252.
- Chan S, McCullough J, Schizas A, Vasas P, Engledow A, et al. (2012) Initial experience of treating anal fistula with the Surgisis anal fistula plug. Tech Coloproctol 16: 201-206.
- de la Portilla F, Rada R, Jiménez-Rodríguez R, Díaz-Pavón JM, Sánchez-Gil JM (2011) Evaluation of a new synthetic plug in the treatment of anal fistulas: results of a pilot study. Dis Colon Rectum 54: 1419-1422.
- Lawes DA, Efron JE, Abbas M, Heppell J, Young-Fadok TM (2008) Early experience with the bioabsorbable anal fistula plug. World J Surg 32: 1157-1159.
- O’Connor L, Champagne BJ, Ferguson MA, Orangio GR, Schertzer ME, et al. (2006) Efficacy of anal fistula plug in closure of Crohn's anorectal fistulas. Dis Colon Rectum 49: 1569-1573.
- Mitalas LE, van Wijk JJ, Gosselink MP, Doornebosch P, Zimmerman DD, et al. (2010) Seton drainage prior to transanal advancement flap repair: useful or not? Int J Colorectal Dis 25: 1499-1502.