Journal of Pharmaceutics & Pharmacology
Download PDF
Butterworth Roger F
Address for Correspondence:
Butterworth Roger F, Professor of Medicine, University of Montreal, 45143
Cabot Trail, Englishtown, NS, B0C 1H0, Canada; E-mail: rb@enceph.com
Review Article
Repurposing of Adamantanes for the Treatment of COVID-19: Rationale and Perspectives
Butterworth Roger F
University of Montreal, Canada
Submission: 14 August 2020;
Accepted: 10 September 2020;
Published: 16 September 2020
Copyright: © 2020 Butterworth Roger F. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Intensive efforts are underway in the search for novel antiviral agents
and in the repurposing of existing antivirals with the potential to
mitigate the effects of COVID-19. Amantadines represent a large
family of tricyclic agents some of which are known to manifest efficacy
against a range of viruses including influenza A and several human
and animal coronaviruses including SARS-CoV and HCoV-OC43 with
neuroinvasive characteristics. The adamantane derivative memantine
improves clinical scores and motor disabilities while reducing HCoVOC43
viral replication in a dose-dependent manner. Anti-viral actions
of memantine against HCoV are independent of the agent’s action
as a non-competitive NMDA receptor antagonist. Amantadine and
the novel spiroadamantane amine possess significant activity against
coronavirus 229E. Mechanisms proposed to date to account for the anti-
SARS CoV-2 actions of adamantanes include blocking of the viroporin
channel of the virus E protein preventing release of viral nucleus into
the host-cell cytoplasm and down-regulation of the host protease CTSL
and lysosomal disruption leading to decreased viral replication. Further
investigations are now required including the assessment of other
adamantanes as antivirals in the experimental setting and controlled
clinical trials to assess their safety and efficacy for the prevention and
treatment of COVID-19.
Keywords
COVID-19; Adamantanes; Amantadine; Rimantadine;
Bananins; Memantine; Coronavirus; Repurposing; Antiviral; E protein;
Viroporin channel; Proteases; Cathepsin L; Lysosomotropic action of
amantadine
Introduction
In view of the time required for the discovery, safety/efficacy
testing and eventual large-scale production of vaccines for
COVID-19, large numbers of commercial laboratories and clinical
research institutions in various world centers are now actively
concentrating their efforts on the discovery of novel antiviral agents.
In many cases, the repurposing of existing antiviral agents with the
potential to mitigate the effects of COVID-19 is well under way.
At least 15 potential COVID-19 treatments are currently under
evaluation in clinical trials and they include not only established
antivirals but also anti-malarial and newly-synthesized compounds
shown to exhibit antiviral potential in animal studies. Antibody-rich
plasma preparations from patients recovering from COVID-19 are
also under evaluation. As part of the race to find effective treatments
for COVID-19, WHO launched a major trial to test repurposed drugs
and experimental drug candidates that included the experimental
antiviral remdesivir, the antimalarial drugs [chloroquine and
hydroxychloroquine], a combination of the HIV drugs lopinavir and
ritonavir alone or together with interferon-beta, an immune system
messenger [1].
Repurposing of the adamantanes for COVID-19:
The antiviral activity of the adamantanes has a long and chequered
history. To quote from an article published in Science in 1964, “1-Adamantanamine [amantadine] causes a selective, reproducible,
dose-related inhibition of influenza infections in tissue culture, chick
embryos and mice. The compound is not viricidal and appears to act
by interfering with the penetration of the host cell by the virus” [2].
This avenue of research remains active today.From a molecular structural standpoint, the adamantane
molecule consists of three condensed cyclohexane rings fused in an
armchair configuration, chemical formula C6 H16 with a functional
group characteristic of each individual adamantane family member
substituted at one of the four methyne positions. Names and
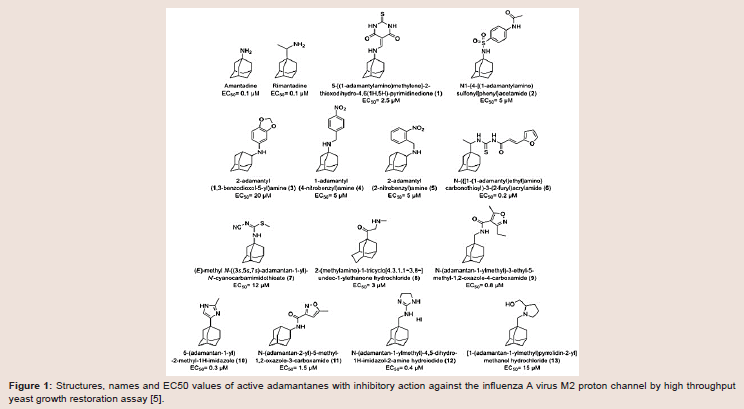
structures of currently-available adamantanes are depicted in (Figure 1).
The best characterized adamantane, amantadine, is widely
prescribed for the treatment of Parkinson’s Disease [PD] where it has
proven efficacy for the treatment of motor disturbances as well as for
the dyskinesias resulting from long-term treatment of PD patients
with L-Dopa [2]. Amantadine is increasingly providing benefit for the
management of disorders of cognition and consciousness in patients
with traumatic brain injury [3].
Several members of the adamantane family have established antiviral
properties. The M2 proton channel of the influenza A virus is
the target for the anti-influenza drugs amantadine and rimantadine
that inhibit the endosomal uncoating of the virus [4]. However,
the efficacy of these agents became limited by the advent of drugresistant
mutations in the pore of the channel. Consequently, a
search is underway for the discovery of additional agents. Making
use of a high throughput yeast growth restoration assay, several other
adamantanes were shown to possess inhibitory potential at the M2
channel of influenza A virus [5]. Moreover, a novel adamantane
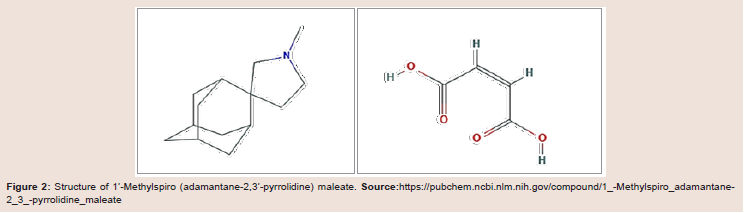
derivative spiroadamantane amine was found to be a highly potent
inhibitor of the amantadine-resistant V27A mutant but was also a
3-fold more potent inhibitor of the WT M2 channel compared to
amantadine or rimantadine making it a potential candidate for
antiviral drug development. This novel agent had previously been
reported to possess significant activity against the coronavirus strain
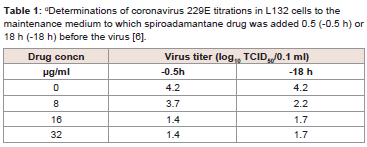
229E [6] (Figure 2 and Table 1).
Tromantadine is an effective inhibitor of both early and later events in Herpes Simplex virus type 1 replication [7]. More recently,
in a case of severe AH1N1 viral pneumonia causing CNS disorder
and multi-organ failure, amantadine was found to be neuroprotective
while also appearing to result in cessation of viral shedding that
contributed to positive outcome and discharge from the ICU [8].
Importantly, a series of novel 2-aminoadamantanes have been
synthesized and shown to manifest persistent in vitro efficacy against
H1N1 [2009] Influenza A where addition of as little as one CH2
group to the methyl adduct of the amantadine/rimantadine analogue
2-methyl-2-aminoadamantane resulted in activity of a range of H1N1
viral strains [9].
Figure 1: Structures, names and EC50 values of active adamantanes with inhibitory action against the influenza A virus M2 proton channel by high throughput yeast growth restoration assay [5].
Figure 2: Structure of 1’-Methylspiro (adamantane-2,3’-pyrrolidine) maleate. Source:https://pubchem.ncbi.nlm.nih.gov/compound/1_-Methylspiro_adamantane-
2_3_-pyrrolidine_maleate
For coronaviruses, modest antiviral effects have been reported
for amantadine, rimantadine and the structurally-related memantine
and the adamantine-derived bananins* for bovine coronavirus,
human coronavirus HCoV-OC43 and SARS-CoV-1 although
amantadine had no inhibitory effect on replication of the coronavirus
Feline Infectious Peritonitis [FIPV] virus [10-13]. Screening against clinical isolates by neutralization tests with confirmation by plaque
reduction assay revealed that rimantadine showed antiviral activity
against SARS-CoV-1 [14]. Human coronaviruses [HCoVs] are wellrecognized
respiratory pathogens that manifest neurotropic and
neuroinvasive actions. The OC43 strain [HCov-OC43] activates
mechanisms implicated in neuroinflammatory and neurodegenerative
processes in susceptible animals [12]. Moreover, treatment with the
adamantane derivative memantine led to improvements in both
clinical scores and motor disabilities in [HCoV-OC43]-infected mice.
Memantine treatment also resulted in attenuation of body weight
loss and mortality rates while reducing HCoV-OC43 replication in
a dose-dependent manner. It was concluded that the antiviral action
of memantine was independent of its NMDA receptor antagonist
properties. Rather, it was proposed that memantine could act by
inhibition of viral replication following viral attachment to the host
cell receptor or by inhibition of the ATPase activity of HCoV-OC43
helicase as was previously shown for other adamantane derivatives [12].
Table 1: αDeterminations of coronavirus 229E titrations in L132 cells to the
maintenance medium to which spiroadamantane drug was added 0.5 (-0.5 h) or
18 h (-18 h) before the virus [6].
*Bananins are a class of antiviral compounds constituted of a
trioxa-adamantane moiety covalently bound to a pyridoxal derivative.
At least one family member is an effective inhibitor of the SARSCoV-
1 virus [11].
Mechanisms of anti-viral action of adamantanes repurposed for COVID-19:
Three independent mechanisms of action have been proposed
to explain the anti-SARS-CoV-2 actions of adamantanes in general
and of amantadine and memantine, in particular. These mechanisms,
simply stated, are:i] Blockage of the viroporin channel of the E protein of SARSCoV-
2 preventing the release of the viral nucleus into the host cell
cytoplasm [15].
ii] Down regulation of expression of the host cell protease
Cathepsin L and lysosomal dysfunction leading to protection against
viral entry and, ultimately, its replication [16].
iii] Mechanisms related to non-competitive antagonism of
NMDA receptors.
According to hypothesis [i], amantadine enters the E-channel of
the coronavirus where it prevents release of the viral nucleus into the
cell. Docking studies suggest that amantadine interacts with amino
acids ALA 22 and PHE 26 and, in so doing, blocks the proton channel
[17].
Hypothesis [ii] is predicated on the observation that SARS CoV-
2 entry into the host cell depends upon binding of the viral spike
protein to cellular receptor and upon its subsequent cleavage by host
cell proteases such as Cathepsin [CTSL] located in the lysosomes.
Amantadine, in addition to causing down-regulation of CTSL also
has the capacity to further disrupt the lysosome pathway resulting in
decreased viral replication with the potential to decrease viral load
and improve clinical outcome.
The expression of NMDA receptors in the lungs and airways
provides a solid basis for the notion that signalling via these receptors
is implicated in the pathogenesis of the acute respiratory distress
syndrome [18]. In order to evaluate Hypothesis [iii], experiments
were conducted in mouse primary CNS cell cultures known to
express NMDA receptors compared to those of a human epithelial
cell line commonly employed to amplify HCoV-OC43 that does not
express NMDA receptors. The observation that memantine affected
viral replication in both cell types suggested that the antiviral action
of memantine was independent of its NMDA receptor antagonist
properties. Alternative mechanisms were proposed namely that memantine may act by inhibition of viral replication following viral
attachment to the host cell receptor or alternatively by inhibition
of ATPase activity of HCoV-OC43 helicase as was shown for other
adamantane derivatives [12].
Conclusions and Prospects
Whilst awaiting the availability of an effective vaccine against
SARS-CoV-2, intense efforts have been underway towards the
discovery of novel antiviral agents or the repurposing of existing
compounds known to manifest antiviral properties to be used as
potential novel approaches to the prevention and treatment of
COVID-19. Based upon recent literature citations, calls for the
repurposing of adamantanes have repeatedly been made [19-21].
Members of the adamantine family of agents including amantadine,
rimantadine, tromantadine and bananins and their derivatives
memantine, spiroamantadine manifest antiviral activities against
a range of viruses from Herpes Simplex and influenza A to the
coronaviruses 229E, HCoV-OC43, SARS-CoV-1 and SARS-CoV-2].
Evidence in support of multiple mechanisms of action has been
presented whereby the adamantanes manifest their antiviral effects
that include inhibition of the viroporin channel of the E protein,
down-regulation of expression of the host cell protease Cathepsin
L and lysosomal dysfunction leading to impaired replication and,
thirdly, non-competitive antagonism of brain glutamate [NMDA]
receptors. An appeal is made for further assessment of the potential
of these and other members of the adamantine family of agents for
their capacity to act against SARS-CoV-2.
Alternatively, combination therapies involving the use of
adamantanes could be envisaged. For example, it has been suggested
that the combined use of serine protease inhibitors and CTSL
inhibitors could offer a safer and effective therapy compared to
other available therapeutics to block coronavirus host cell entry and
intracellular replication [22]. Combination therapy with amantadine
[shown to manifest potential benefit against some coronaviruses
with low-dose dexamethasone might offer one such combination for
COVID-19.
Given the relatively short period of time [barely 6 months] since
the arrival of SARS-CoV-2 and its associated disease COVID-19,
clinical assessments of efficacy of adamantanes with established
antiviral properties have been restricted to a handful of Case Reports.
Some suggest the potential for the prevention of COVID-19 as
summarised in the current report. The time has now come for the
creation of an evidence base for these suggested benefits. Randomised
controlled clinical trials for the assessment of efficacy and safety of
these agents for the prevention and/or treatment of COVID-19 are
now required.