Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
Aticaprant (Clinically Developed Kappa-Opioid Receptor Antagonist) Combined With Naltrexone Prevents Alcohol “Relapse” Drinking
Zhou Y*, Zhou DC and Kreek MJ
Laboratory of the Biology of Addictive Diseases, The Rockefeller
University, 1230 York Avenue, New York, NY 10065, USA
Address for Correspondence;
Zhou Y, Laboratory of the Biology of Addictive Diseases, The
Rockefeller University, 1230 York Avenue, New York, NY
10065, USA; Phone (212) 327 7228 / Fax: (212) 327-8574;
E-mail: zhouya@rockefeller.edu
Submission: 11 April 2022
Accepted: 11 May 2022
Published: 16 May 2022
Copyright: © 2022 Zhou Y, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Alcohol relapse is the treatment target for medications
development for alcohol dependence. Aticaprant, a selective and
short-acting kappa-opioid receptor (KOR) antagonist, has recently
been under development for new clinical implications (depression or
anhedonia). Recent studies have also found that aticaprant reduces
alcohol intake and prevents stress- triggered alcohol seeking in rodents
via a KOR-mediated mechanism. Here, we further investigated
whether aticaprant alone or in combination with naltrexone (muopioid
receptor [MOR] antagonist) altered alcohol relapse-like drinking
using a mouse alcohol deprivation effect (ADE) paradigm to mimic
the relapse episodes in human alcoholics. A long-acting and selective
KOR antagonist nor-BNI was used as a reference compound for the
effects of the KOR antagonism on the ADE. After 3-week intermittentaccess
alcohol drinking (two-bottle choice, 24-h access every other
day), male and female mice displayed excessive alcohol intake and
then pronounced ADE after 1-week abstinence. Aticaprant alone
decreased alcohol ADE in a dose- dependent manner (1-3 mg/kg) in
both males and females. Aticaprant at a lower dose (0.3 mg/kg) than
the effective one (3 mg/kg) combined with a low dose of naltrexone
(1 mg/kg) reduced the ADE in both sexes, and the combination was
effective after a multi-dosing regimen (5 daily injections during the
abstinence) without development of tolerance, suggesting synergistic
effects of the combination. In contrast, nor-BNI alone or with naltrexone
had no effect on the ADE in either sex. Our present study suggests that
a combination of clinically developed, short-acting KOR antagonist
aticaprant with low-dose naltrexone has therapeutic potential in
alcohol “relapse” treatment.
Keywords
Combination therapy; Aticaprant; KOR; Naltrexone; Alcohol
deprivation effect; Relapse, Nor-BNI
Introduction
It has been well established that classic KOR agonists induce
alcohol-seeking behavior and promote alcohol relapse-like drinking
[1-3]. Classic KOR agonists induce aversion, anxiety-like or
depression-like behavior that may be responsible for alcohol seeking
or relapse- like drinking or dysphoria in humans [4-7]. During acute
alcohol withdrawal, pharmacological blockade of KOR attenuates
alcohol seeking or drinking in rodents, as well as anxiety- or
depression- like behaviors [8-16]. Withdrawal from alcohol activates
KOR systems and induces negative mood states and symptoms, which
is a part of negative reinforcing aspects of alcohol addiction [17,18].
After certain period of abstinence, a transient increase in alcohol
intake is observed in both humans and rodents, which is characterized
as the alcohol deprivation effect (ADE). The ADE has been widely
used in rodent studies and been proved as suitable animal models for
studying alcohol relapse [1,19,20]. Specifically, in our mouse model,
after 1-week abstinence from 3-week intermittent-access alcohol
drinking with excessive alcohol intake, both male and female mice
show significant increases in alcohol consumption in the first 4 hours of alcohol access when alcohol is available again [21]. Our particularly
relevant question was whether there was an upregulated KOR activity
after prolonged withdrawal or abstinence and during alcohol relapselike
drinking, and if so, whether blockade of KOR could reduce
the ADE or not. To date, however, there is no study investigating
effects of the novel KOR antagonist aticaprant on alcohol “relapse”
in rodent ADE models. Aticaprant (LY-2456302, CERC-501 or JNJ-
67953964) is a selective and short-acting KOR antagonist and has
recently been under development for the potential treatment of major
depressive disorder [11,22,23]. Based on recent studies showing that
aticaprant attenuates alcohol self-administration, reduces alcohol
intake escalation, and prevents stress-induced seeking in rodents
[11,12], we proposed a new hypothesis that aticaprant could prevent
alcohol relapse-like drinking in ADE model. As alcohol relapse is
an important target for medications development for alcoholism,
we specifically investigated the pharmacological effects of aticaprant
on mouse ADE in both sexes, to ascertain its potential as an antirelapse
compound. As nor-binaltorphimine (nor-BNI) is a selective,
but long-acting, KOR antagonist, we purposely used nor-BNI as a
reference compound and compared its effect with aticaprant.
Alcohol increases mu-opioid receptor (MOR)-mediated signal
which is responsible for alcohol’s positive reinforcing and motivational
properties and is highly involved in alcohol relapse episodes. MOR
antagonist naltrexone decreases alcohol craving and relapse in
humans [24], and relapse-like drinking in rodent ADE models [1,18].
Among multiple actions of alcohol in the CNS, both KOR and MOR
activities are profoundly changed by alcohol exposure in humans
[25,26], We further hypothesized that by targeting on both KOR and
MOR, the combination of the clinically developed aticaprant and
naltrexone would improve efficacy over the single-receptor approach
on preventing relapse [18]. Therefore, the present study examined
whether the appropriate combination of aticaprant and naltrexone
could be more effective in preventing ADE than each alone.
Materials & Methods
Animals:
Both male and female C57BL/6J mice (8-week-old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA),
housed in a temperature-controlled (21 ºC) facility, and acclimated
on a 12-hour reverse light-dark cycle (lights off at 7:00 am) for at
least one week prior to the experiments. The mice freely accessed
to food and water and individually housed. All the animal care and
experimental procedures were conducted according to Guide for
Care and Use of Laboratory Animals (Institute of Laboratory Animal
Resources Commission on Life Sciences 1996) and approved by
the IACUC (Institutional Animal Care and Use Committee) of the
Rockefeller University.Materials:
Ethanol solution was prepared from 190 proof absolute ethyl
alcohol (Pharmco- AAPER, Brookfield, CT, USA) and dissolved in
tap water. Aticaprant was purchased from MedChemExpress, and
suspended in 5% DMSO, 5% Cremophor and saline. Naltrexone
hydrochloride from Sigma-Aldrich and nor-BNI from the NIDA
Division of Drug Supply and Analytical Services were both dissolved
in saline.Procedures:
Alcohol deprivation effect (ADE) following intermittent-access
alcohol drinking (Table S1). Intermittent-access alcohol drinking
model is a 2-bottle free choice paradigm with alcohol drinking every
other day for 3 weeks as modified based on previous studies [21,27].
Both the water and alcohol (15%) solution sipper tubes were provided
3 hours after lights off, and the locations (left and right ones of the
cage) of the bottles were randomly positioned to prevent from the
development of side preference. The alcohol bottle filled with fresh
15% alcohol was kept for 24 h before being switched by the water
bottle. Both water and alcohol intakes were recorded after 4, 8 and
24 hours of alcohol access in the drinking days and then calculated
as consumed alcohol intake (g⁄kg) and relative preference for alcohol
(alcohol intake ⁄ total fluid intake).Injection of aticaprant or aticaprant plus naltrexone after
excessive drinking. After 3 weeks, alcohol (15%) was presented 30
min after a single injection of aticaprant (0.1, 0.3, 1 or 3 mg/kg, i.p.) or
vehicle, and then alcohol and water intakes were recorded. The doses
of aticaprant were based on recent publications [12]. The aticaprant
plus naltrexone dose chosen was based on the above experiments and
our recent studies [21,28], and mice were pretreated with aticaprant
(0.3 mg/kg, i.p.) or vehicle 30 min before the test, followed by the
second one of naltrexone (1 mg/kg) in saline 10 min before the test.
Both male and female mice were randomly assigned into
the drug treated and vehicle groups with similar alcohol intake on
day 21. On day 23, the experimenters [blinded to the drug codes]
administered the drugs and vehicle before the test. In the vehicle
control groups, the mice received one or two vehicles; and the mice
in the drug groups received one (aticaprant) or two drugs (aticaprant
plus naltrexone). Then, the alcohol and water bottles were presented,
and their intakes were recorded. Of note, we did not observe
significant sex differences with aticaprant or its combinations with
naltrexone at the doses tested in our experiments, suggesting that
the estrous cycle and associated hormones might not be important
factors in the response to these treatments in females.
Injection of aticaprant alone or aticaprant plus naltrexone in
ADE (Table S1). On day 21, at the end of the 3-week intermittentaccess
alcohol drinking, 30% alcohol and water bottles were provided
and their intakes at 4, 8 and 24 hours were recorded as the ones in
the Baseline session. Then, the alcohol bottle was not offered for the
following week. On day 28, after the 1-week abstinence, the alcohol
(30%) bottle was provided once again 3 h after lights off and the
alcohol and water intakes were recorded as the ones at 4, 8 and 24 h
in the ADE session.
Both male and female mice were randomly assigned into the drugtreated
and vehicle groups with similar alcohol intake in the Baseline
session on day 21. On day 28, the experimenters who were blinded to
the drug codes administered the drugs and vehicle before the ADE
test. In the vehicle control groups, the mice received one or two
vehicles; and the mice in the drug groups received one (aticaprant) or
two drugs (aticaprant plus naltrexone). Then, the alcohol and water
bottles were presented, and their intakes were recorded. (a) The doses
of aticaprant were based on the above experiments in 3.2 section:
mice received aticaprant (0.1 to 3 mg/kg, i.p.) or vehicle 30 min before
the ADE test; (b) The doses of naltrexone were based on our recent
study [28]: mice received naltrexone (0.3 or 1 mg/kg, i.p.) or vehicle
10 min before the ADE test; and (c) The aticaprant plus naltrexone
dose was chosen after the above aticaprant alone and naltrexone
alone experiments: mice received the first injection of aticaprant at
low- dose (0.1 or 0.3 mg/kg) 30 min before the ADE test, followed
by the second one of naltrexone (0.3 or 1 mg/kg) 10 min before the
ADE test.
In the following experiment, the procedures were identical to
the above experiment, with the following exceptions: mice received
5 consecutive injections of vehicles or aticaprant (0.3 mg/kg) plus
naltrexone (1 mg/kg) during the 1-week abstinence. On the ADE test
day, alcohol was presented 1 day after the last combination injection,
and then alcohol and water intake values were recorded after 4, 8 and
24 hours in the ADE session.
Injection of nor-BNI alone or with naltrexone in ADE (Table
S1). Using the above same paradigm with an exception: the mice
were pretreated with nor-BNI (30 mg/kg, i.p.) alone or vehicle on day
27 (1 day before the ADE test). The nor-BNI dose was based on our
early publication in the intermittent-access drinking model [28,29].
The nor-BNI plus naltrexone dose chosen was based on the above
experiments, and mice were pretreated with nor-BNI (30 mg/kg, i.p.)
or vehicle on day 27, followed by the second one of naltrexone (1 mg/
kg) in saline 10 min before the drinking test on day 28.
Sucrose (caloric reinforcer) drinking. The specificity of the
action of aticaprant on alcohol intake was further examined on
sucrose drinking behavior after the ADE at 3 mg/kg dose (the most
effective dose tested on alcohol intake). The sucrose preference
test in mice is sensitive to the function of brain rewarding systems
and is widely used to measure the expression of anhedonia during
alcohol abstinence [21] [30]. In these experiments, the ADE exposure
was identical to the above one as described in section 3.1. During
the 1-week abstinence, a sucrose solution (2%) was provided for 3
sessions with stable intakes. The mice were assigned to the vehicle- or
aticaprant- treated groups with similar sucrose intakes before the test
day. On the test day (after 1 week of alcohol abstinence), aticaprant (3 mg/kg) or vehicle was given 30 min before the sucrose tube was
presented, and both sucrose and water intakes were recorded after 4,
8 and 24 hours of sucrose access.
Data Analysis:
Based on the levels of the mouse individual differences in
our previous alcohol studies [21,28], power analyses were made
to determine the number of mice required to reach statistical
significances in the present studies. If similar effects of each treatment
with no sex differences were found, data of each sex were analyzed
separately. The group differences were analyzed using multiple-way
ANOVAs for treatment (vehicle vs drug) and/or for sessions (Baseline
vs ADE) in each sex, with a priori hypothesis that there were effects
of ADE or drug treatment, based on the early publications and our
new hypothesis in this study [1,,19,21]. The multiple-way ANOVAs
were followed by Newman-Keuls post-hoc tests, and the accepted
significance was p<0.05 in Statistica 5.5 (StatSoft Inc, Tulsa, OK).Results
Aticaprant alone:
Excessive drinking. At the 4-hour time point, the dose responses
of aticaprant (0.1, 0.3, 1 and 3 mg/kg) in terms of alcohol intake,
water intake, alcohol preference and total fluid intake are presented
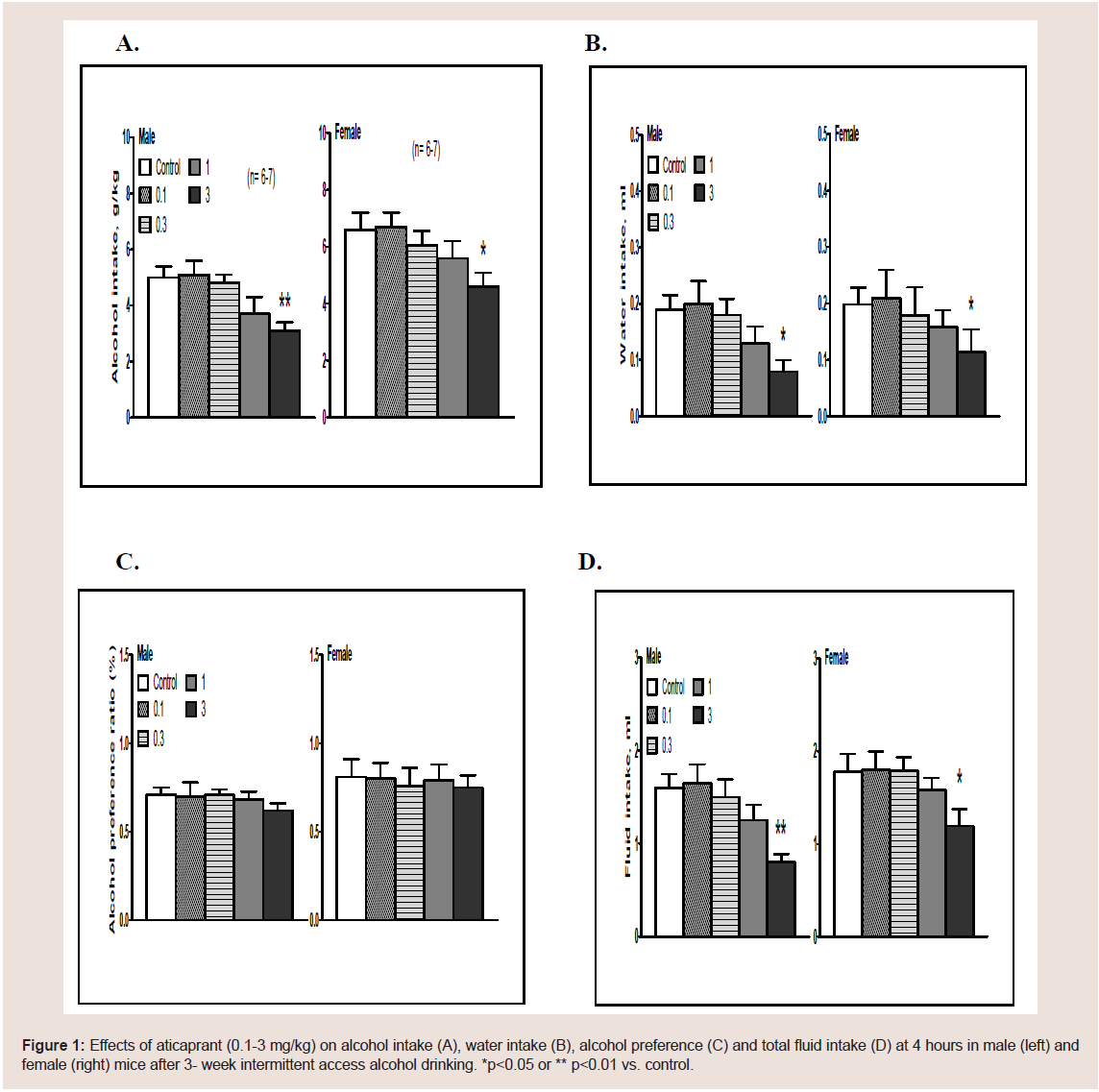
in Figure 1.
Figure 1: Effects of aticaprant (0.1-3 mg/kg) on alcohol intake (A), water intake (B), alcohol preference (C) and total fluid intake (D) at 4 hours in male (left) and
female (right) mice after 3- week intermittent access alcohol drinking. *p<0.05 or ** p<0.01 vs. control.
A. Males: For alcohol intake (Figure 1A, left), one-way ANOVA
revealed a significant effect of aticaprant [F(4,28)=8.6, p<0.005],
and post hoc analysis showed that in comparison with the vehicle
group, the aticaprant-treated mice had less alcohol intake than the
vehicle- treated ones at 3 mg/kg [p<0.01], with a marginal effect at 1
mg/kg [p=0.05]. For water intake (Figure 1B, left), one-way ANOVA
showed a significant effect of aticaprant treatment [F(4,28)=5.2,
p<0.01], and at 3 mg/kg, there were less water intake than the vehicletreated
control [p<0.05]. For preference ratio (Figure 1C, left), there
were not any effect of aticaprant at any doses. For total fluid intake
(Figure 1D, left), one-way ANOVA revealed a significant effect of
aticaprant [F(4,28)=5.4, p<0.01], and post hoc analysis showed that
in comparison with the vehicle group, the aticaprant-treated males at
3 mg/kg had less fluid intake [p<0.01].
B. Females: For alcohol intake (Figure 1A, right), there was
a significant effect of aticaprant [one-way ANOVA, F(4,28)=7.0,
p<0.01], and there was a less alcohol intake than the vehicle-treated
ones at 3 mg/kg [p<0.05]. For water intake (Figure 1B, right), there
was a significant effect of aticaprant treatment [F(4,28)=4.2, p<0.01]
and less water intake at 3 mg/kg than the control [p<0.05]. For
preference ratio (Figure 1C, right), there was not any effect at any
doses. For total fluid intake (Figure 1D, right), one-way ANOVA
revealed a significant effect of aticaprant [F(4,28)=4.9, p<0.05], and
the reduction at 3 mg/kg was significant [p<0.05].
Furthermore, after 8 or 24 hours or during the entire 24-hour
drinking sessions, there were not any changes after aticaprant at
any doses on either alcohol intake or water intake in either sex, as
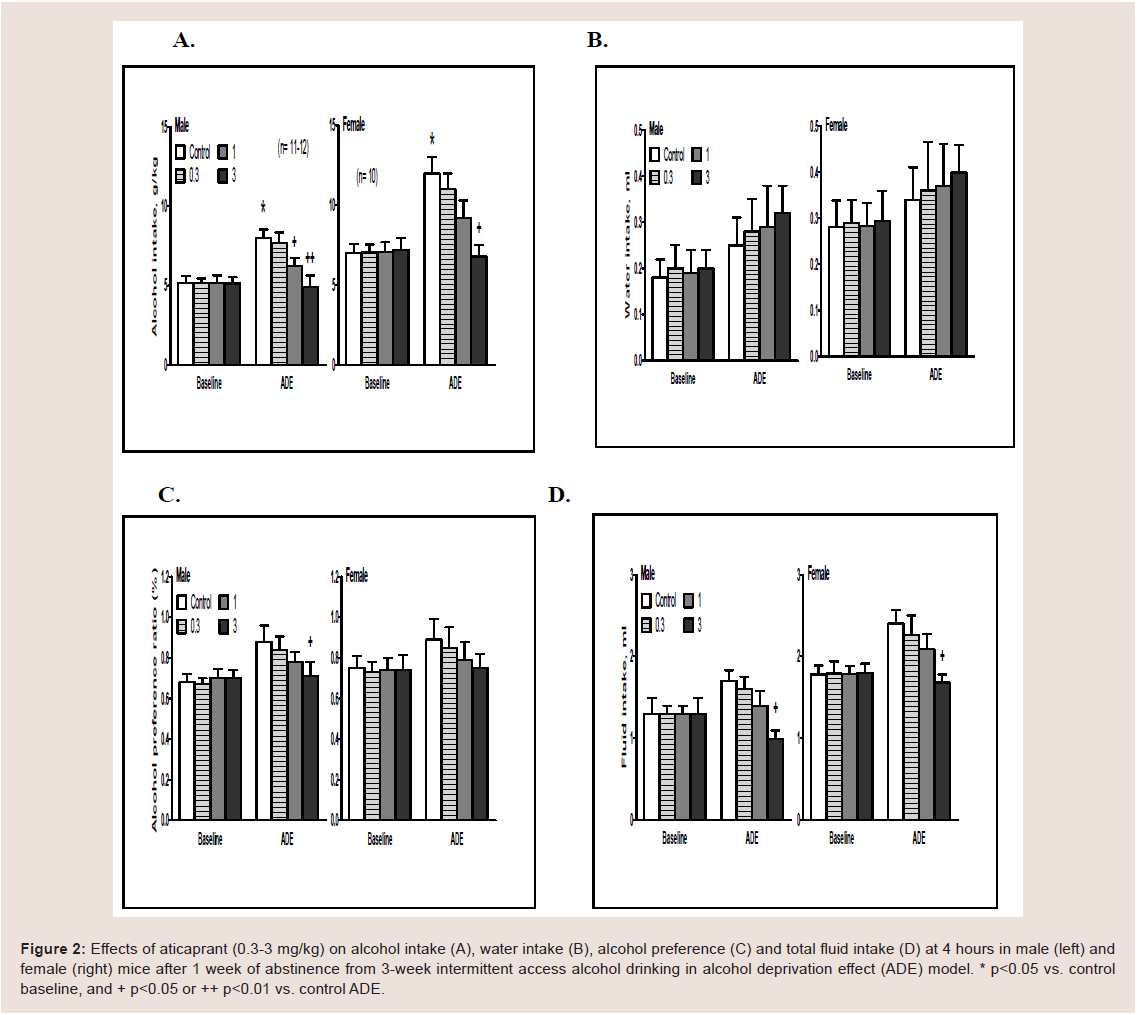
presented in Table 1 at 3 mg/kg of aticaprant.
Table 1: No effect of aticaprant (3 mg/kg) at 8 or 24 hours on alcohol intake or
water intake in an excessive alcohol drinking model in male (A, n=6-7) and female
(B, n=6-7) mice after 3-week intermittent-access alcohol drinking. See Figure 1
at 4-hour time point.
ADE drinking. In a pilot experiment, the effect of aticaprant
at 0.1 mg/kg dose was tested in both males and females, and there was no difference between saline control and aticaprant treatment in
either sex (data not shown). In the following experiment, three doses
(0.3, 1 and 3 mg/kg) were tested, and the results on alcohol drinking
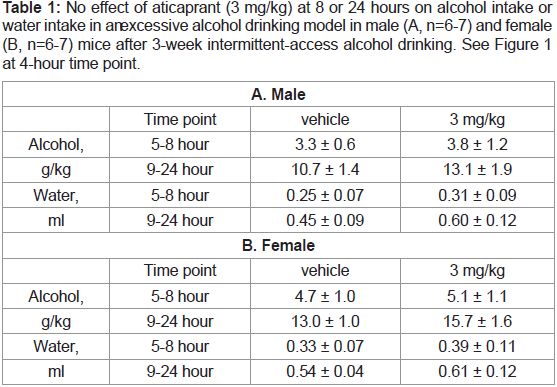
at 4 hours are shown in Figure 2.
Figure 2: Effects of aticaprant (0.3-3 mg/kg) on alcohol intake (A), water intake (B), alcohol preference (C) and total fluid intake (D) at 4 hours in male (left) and
female (right) mice after 1 week of abstinence from 3-week intermittent access alcohol drinking in alcohol deprivation effect (ADE) model. * p<0.05 vs. control
baseline, and + p<0.05 or ++ p<0.01 vs. control ADE.
(A) Males. For alcohol intake (Figure 2A, left), two-way ANOVA
revealed significant effect
ts of aticaprant treatment [F(1,84)=3.8, p<0.05], session
[F(1,84)=9.2, p<0.01], and interaction between session and treatment
[F(1,84)=3.2, p<0.05]. Post hoc analysis showed that:
(1) there was more alcohol intake in the ADE session on day 28
than the baseline on day 21 [p<0.05]; and (2) at 1 mg/kg and 3 mg/
kg, there were less alcohol intakes than the vehicle control in the ADE
session [p<0.05 and p<0.01, respectively]. For water intake (Figure 2B,
left), there was no effect of ADE or aticaprant at any doses. For alcohol
preference, two-way ANOVA showed significant effects of aticaprant
[F(1,84)=3.3, p<0.05], session [F(1,84)=5.1, p<0.05], and interaction
between session and aticaprant treatment [F(1,84)=3.0, p<0.05]. Post
hoc analysis showed that at 3 mg/kg, there was a less preference than
the vehicle control in the ADE session [p<0.05]. For total fluid intake
(Figure 2D, left), two-way ANOVA revealed significant effects of
aticaprant [F(1,84)=4.3, p<0.05], session [F(1,84)=6.0, p<0.05], and
interaction between session and aticaprant [F(1,84)=3.5, p<0.05], and
that at 3 mg/kg, there was a less fluid intake than the vehicle control in
the ADE session [p<0.05].
(B) Females. For alcohol intake (Figure 2A, right), there were
significant effects of aticaprant [two-way ANOVA, F(1,72)=3.3,
p<0.05], session [F(1,72)=7.6, p<0.01], and interaction between
session and aticaprant [F(1,72)=2.9, p<0.05]. There was more alcohol
intake in the ADE session than the baseline [post-hoc test, p<0.05];
and the aticaprant-treated females at 3 mg/kg had less alcohol intake
than the vehicle one in the ADE session [p<0.05], with a marginally
significant decrease at 1 mg/kg [p=0.07]. For water intake (Figure 2B,
right), there was not any effect of ADE or aticaprant at any doses. For
alcohol preference, there were significant effects of aticaprant [twoway
ANOVA, F(1,72)=2.9, p<0.05] and session [F(1,72)=4.6, p<0.05],
and the aticaprant-treated females at 3 mg/kg had a slight less
preference than the vehicle one in the ADE session [p=0.07] (Figure 2C, right). For total fluid intake (Figure 2D, left), two-way ANOVA
revealed significant effects of aticaprant [F(1,72)=3.3, p<0.05], session
[F(1,72)=4.2, p<0.05], and interaction between session and aticaprant
[F(1,72)=3.0, p<0.05]. At 3 mg/kg, there was a less fluid intake than
the vehicle control in the ADE session [p<0.05].
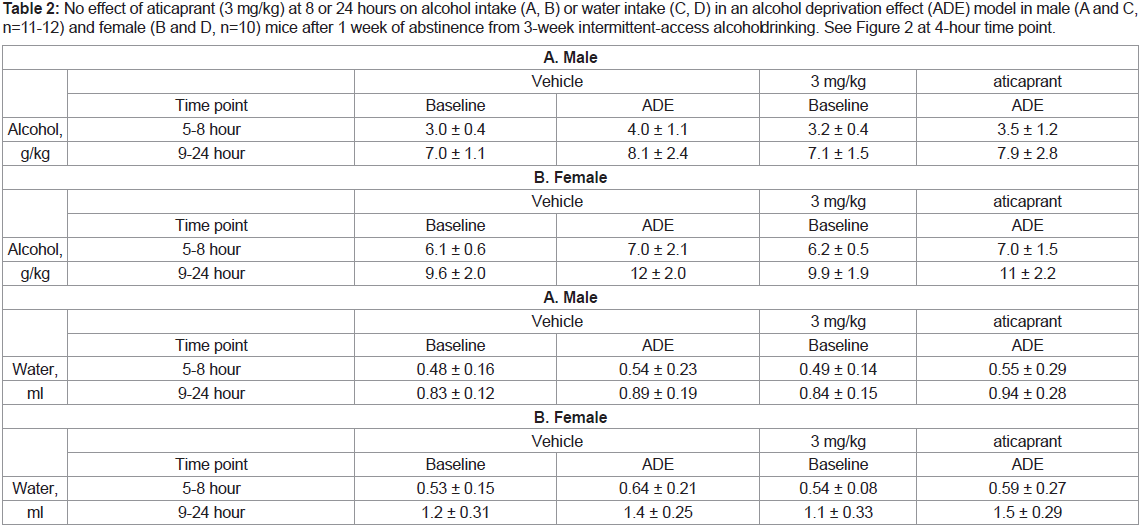
After 8 or 24 hours, there was not any change after aticaprant at
any doses on either alcohol or water intake in either sex, as presented
in Table 2 at 3 mg/kg of aticaprant.
Table 2: No effect of aticaprant (3 mg/kg) at 8 or 24 hours on alcohol intake (A, B) or water intake (C, D) in an alcohol deprivation effect (ADE) model in male (A and C,
n=11-12) and female (B and D, n=10) mice after 1 week of abstinence from 3-week intermittent-access alcohol drinking. See Figure 2 at 4-hour time point.
Sucrose drinking (intake and preference after 1-week alcohol
abstinence). As both alcohol and sucrose are caloric reinforcers, the
specificity of the aticaprant effect on alcohol intake was examined
after aticaprant injection at 3 mg/kg on sucrose drinking after 1 week
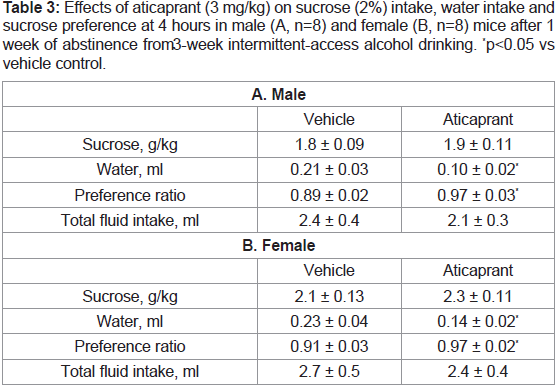
of alcohol abstinence. As shown in Table 3, there was no effect of
3 mg/kg aticaprant on 2% sucrose intake in either males or females
after 4 hours. For water intake, however, the aticaprant- treated mice
had a less water intake than the control mice in both males [Student’s
t-test (1,15)=5.4, p<0.05] and females [Student’s t-test (1,15)=4.9, p<0.05]. For preference ratio, the aticaprant-treated mice had more
preferences than the control mice in both male [Student’s t-test
(1,15)=5.9, p<0.05] and female mice [Student’s t-test (1, 15)=5.1,
p<0.05]. For total fluid intake, there were no effects in either sex.
After 8 or 24 hours, there was not any change by aticaprant on either
sucrose intake or water intake in either sex (data not shown).
Table 3: Effects of aticaprant (3 mg/kg) on sucrose (2%) intake, water intake and
sucrose preference at 4 hours in male (A, n=8) and female (B, n=8) mice after 1
week of abstinence from 3-week intermittent-access alcohol drinking. *p<0.05 vs
vehicle control.
Aticaprant plus naltrexone:
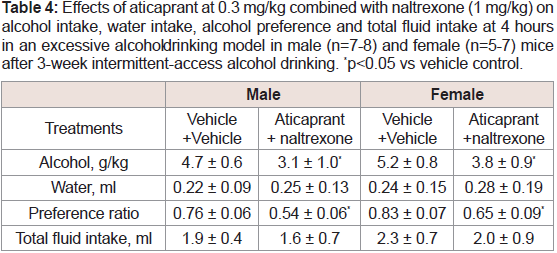
Excessive drinking. In a pilot study, we tested the effect of 0.1
mg/kg aticaprant combined with 0.3 mg/kg naltrexone and found
that the combination did not have a significant effect on excessive drinking in either sex (data not shown). With higher doses of both
compounds: aticaprant at 0.3 mg/kg and naltrexone at 1mg/kg, there
were significant effects after 4 hours of alcohol drinking (Table 4): For
alcohol intake, two-way ANOVA revealed a significant effect of the
combination treatment [F(1,27)=8.9, p<0.01], and the combination
significantly reduced alcohol intake in both males and females
[p<0.05 for both]. For preference ratio, two-way ANOVA also
revealed a significant effect of the treatment [F(1,27)=10.1, p<0.01],
and the combination significantly reduced preference ratio in both
sexes [p<0.05 for both]. For water intake or total fluid intake, there
were not any changes by the combination in either sex. After 8 or 24 hours, there were no significant effects on alcohol intake in either sex
(data not shown).
Table 4: Effects of aticaprant at 0.3 mg/kg combined with naltrexone (1 mg/kg) on
alcohol intake, water intake, alcohol preference and total fluid intake at 4 hours
in an excessive alcohol drinking model in male (n=7-8) and female (n=5-7) mice
after 3-week intermittent-access alcohol drinking. *p<0.05 vs vehicle control.
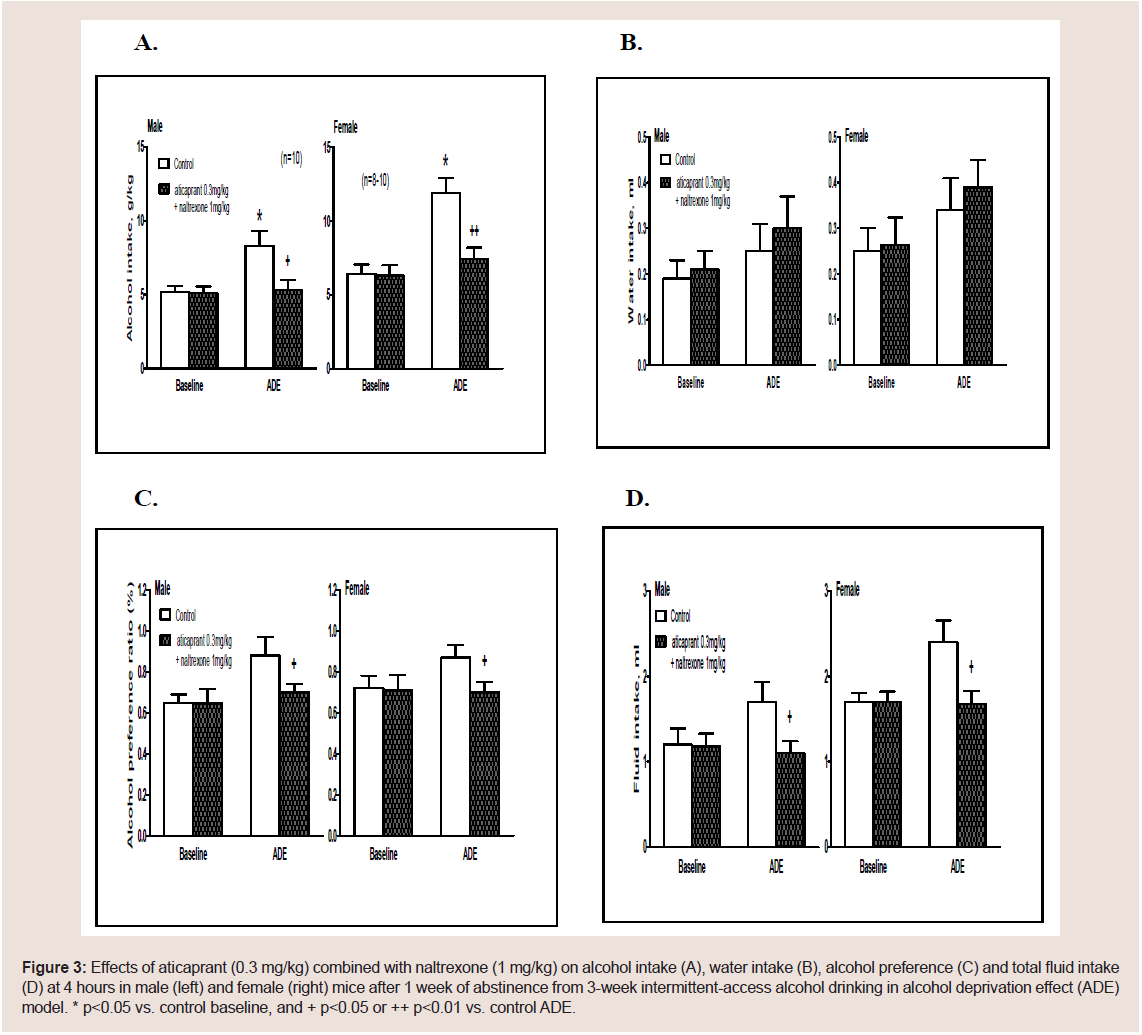
ADE drinking. In a pilot study, we also tested the combination of
0.1 mg/kg aticaprant with naltrexone at 0.3 mg/kg, and there was no
effect on ADE at 4 hours in either sex (Table 5). With higher doses:
aticaprant at 0.3 mg/kg and naltrexone at 1 mg/kg, the ADE alcohol
intake at 4 hours is reduced as shown in Figure 3.
Table 5: No effect of aticaprant (0.1 mg/kg) combined with naltrexone (0.3 mg/
kg) on alcohol intake in an alcohol deprivation effect (ADE) model at 4 hours in
male (A, n=6) and female (B, n=6) mice after 1 week of abstinence from 3-week
intermittent-access alcohol drinking. In the males at 4 hours (A), two-way ANOVA
revealed a significant effect of session only [F (1,33)=6.4, p<0.01], and the males
had more intake in the ADE session than that in the baseline [p<0.05]. In the
females at 4 hours (B), two-way ANOVA revealed a significant effect of session
only [F(1,33)=7.5, p<0.01], and the females had more intake in the ADE session
thant hat in the baseline [p<0.05]. *p<0.05 vs control baseline.
Figure 3: Effects of aticaprant (0.3 mg/kg) combined with naltrexone (1 mg/kg) on alcohol intake (A), water intake (B), alcohol preference (C) and total fluid intake
(D) at 4 hours in male (left) and female (right) mice after 1 week of abstinence from 3-week intermittent-access alcohol drinking in alcohol deprivation effect (ADE)
model. * p<0.05 vs. control baseline, and + p<0.05 or ++ p<0.01 vs. control ADE.
(A) Males. For alcohol intake (Figure 3A, left), two-way ANOVA
revealed significant effects of treatment [F(1,36)=4.9, p<0.05], session
[F(1,36)=5.2, p<0.05] and their interaction [F(1,36)=3.6, p<0.05].
The post-hoc results showed that males had more intake in the ADE
session than that in the baseline [p<0.05]. However, the aticaprant
plus naltrexone-treated males had less intake than the vehicle controls
in the ADE session [p<0.05]. For water intake, there was no effect
of ADE or the combination (Figure 3B, left). For alcohol preference, there was a significant effect of treatment x session interaction [twoway
ANOVA, F(1,36)=4.4, p<0.05], and the males treated with
aticaprant plus naltrexone had a less preference than the vehicle ones
in the ADE session [p<0.05] (Figure 3C, left). For total fluid intake
(Figure 3D, left), two-way ANOVA revealed significant effects of
treatment [F(1,36)=3.4, p<0.05] and treatment x session interaction
[F(1,36)=3.2, p<0.05]. In the males treated with the combination,
there was a less fluid intake than the vehicle-treated control in the
ADE session [p<0.05].
(B) Females. For alcohol intake (Figure 3A, right), two-way
ANOVA showed significant effects of treatment [F(1,32)=6.9, p<0.05],
session [F(1,32)=8.6, p<0.01] and their interaction [F(1,32)=4.5,
p<0.05]. Females had more intake in the ADE session than that in
the baseline [p<0.05]; however, the aticaprant plus naltrexonetreated
females had a less intake than the vehicle controls in the ADE
session [p<0.01]. For water intake, there was no effect of ADE or the combination (Figure 3B, right). For alcohol preference, there was a
significant effect of treatment and session interaction [F(1,32)=4.8,
p<0.05], and the females treated with aticaprant plus naltrexone had
a less preference in the ADE session than the vehicle ones [p<0.05]
(Figure 3C, right). For total fluid intake (Figure 3D, right), twoway
ANOVA revealed significant effects of treatment [F(1,32)=3.6,
p<0.05], and treatment x session interaction [F(1,32)=3.5, p<0.05].
The females treated with the combination had less fluid intake than
the vehicle-treated control in the ADE session [p<0.05].
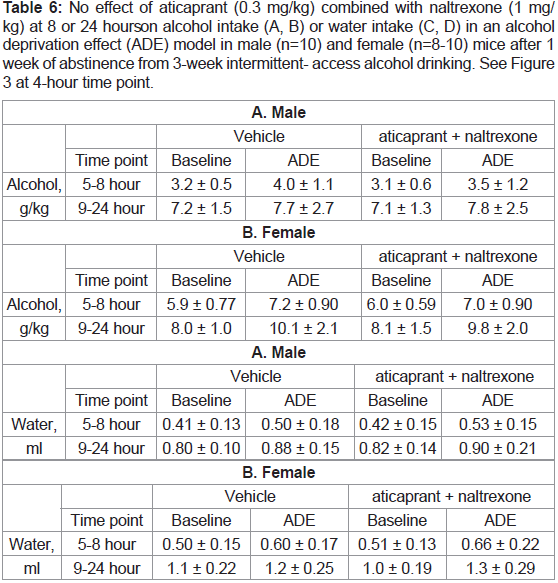
After 8 or 24 hours, there were no changes by the combination on
alcohol intake or water intake in either sex, as presented in Table 6.
Table 6: No effect of aticaprant (0.3 mg/kg) combined with naltrexone (1 mg/
kg) at 8 or 24 hours on alcohol intake (A, B) or water intake (C, D) in an alcohol
deprivation effect (ADE) model in male (n=10) and female (n=8-10) mice after 1
week of abstinence from 3-week intermittent- access alcohol drinking. See Figure 3 at 4-hour time point.
Sucrose drinking. The specificity of the combination of aticaprant
with naltrexone on alcohol drinking was further examined on sucrose
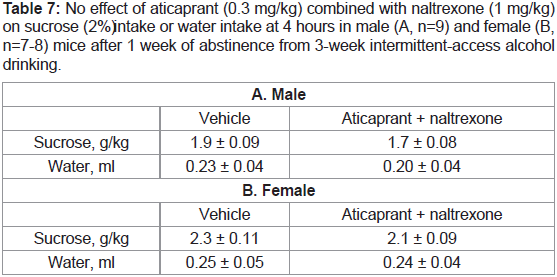
drinking after 1 week of alcohol abstinence. There was no effect of the combination on 2% sucrose intake in either sex after 4 hours (Table 7) or after 8 or 24 hours (data not shown).
Table 7: No effect of aticaprant (0.3 mg/kg) combined with naltrexone (1 mg/kg)
on sucrose (2%)i ntake or water intake at 4 hours in male (A, n=9) and female (B,
n=7-8) mice after 1 week of abstinence from 3-week intermittent-access alcohol
drinking.
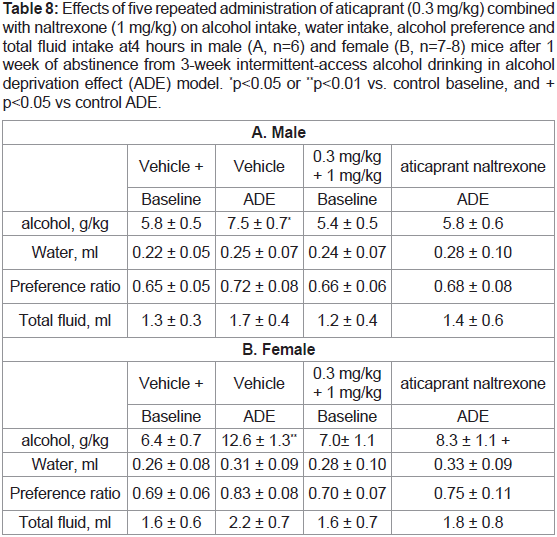
Repeated combination on ADE drinking. In this experiment,
we tested the effect of the repeated combination administrations in
both males and females and found similar reducing effects on the
ADE. As there were no significant sex differences again, the data
of each sex are presented separately (Table 8): [A] In males at 4
hours (Table 8A), two-way ANOVA showed a significant effect of the combination treatment [F(1,20)=5.5, p<0.05], and a significant
interaction between session and treatment [F(1,20)=5.6, p<0.05],
and that the combination- treated males had a less intake than the
vehicle-treated ones in the ADE session [p<0.05]. To test our a priori
hypothesis that there was an ADE, the post-hoc result was included
here: the vehicle-treated males had a significant ADE [p<0.05] (Table 8A), though 2-way ANOVA did not reveal any significant effect of
the ADE session; [B] In females at 4 hours (Table 8B), two- way
ANOVA revealed a significant effect of session [F(1,26)=11, p<0.001]
and post hoc analysis showed that the females had more intake in the
ADE session than the baseline [p<0.01], and the combination-treated
females did not show the ADE [p<0.05]; and [C] However, there was
no significant effect of the repeated combination treatment on either
males or females after 8 or 24 hours (data not shown).
Table 8: Effects of five repeated administration of aticaprant (0.3 mg/kg) combined
with naltrexone (1 mg/kg) on alcohol intake, water intake, alcohol preference and
total fluid intake at 4 hours in male (A, n=6) and female (B, n=7-8) mice after 1
week of abstinence from 3-week intermittent-access alcohol drinking in alcohol
deprivation effect (ADE) model. *p<0.05 or **p<0.01 vs. control baseline, and +
p<0.05 vs control ADE.
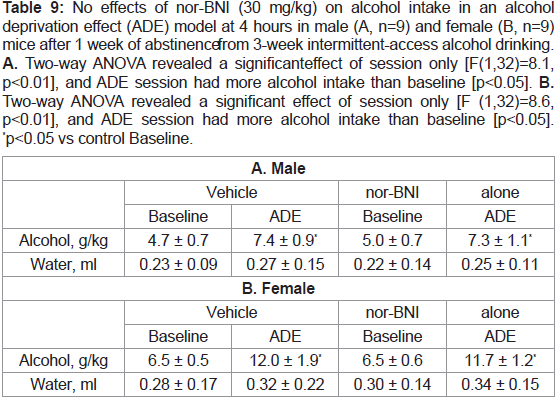
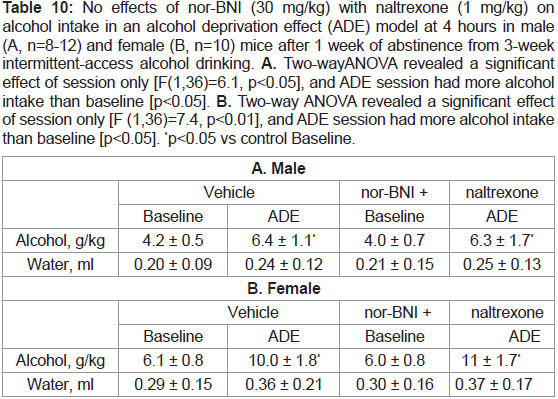
nor-BNI alone or with naltrexone:
The results with 30 mg/kg nor-BNI alone at 4 hours are shown in Table 9, or with 1 mg/kg naltrexone in Table 10. There was not
any effect on ADE in either sex at 4 hours. After 8 or 24 hours, there
were no changes by either nor-BNI alone or with naltrexone (data
not shown).
Table 9: No effects of nor-BNI (30 mg/kg) on alcohol intake in an alcohol
deprivation effect (ADE) model at 4 hours in male (A, n=9) and female (B, n=9)
mice after 1 week of abstinence from 3-week intermittent-access alcohol drinking.
A. Two-way ANOVA revealed a significant effect of session only [F(1,32)=8.1,
p<0.01], and ADE session had more alcohol intake than baseline [p<0.05]. B.
Two-way ANOVA revealed a significant effect of session only [F (1,32)=8.6,
p<0.01], and ADE session had more alcohol intake than baseline [p<0.05].
*p<0.05 vs control Baseline.
Table 10: No effects of nor-BNI (30 mg/kg) with naltrexone (1 mg/kg) on
alcohol intake in an alcohol deprivation effect (ADE) model at 4 hours in male
(A, n=8-12) and female (B, n=10) mice after 1 week of abstinence from 3-week
intermittent-access alcohol drinking. A. Two-way ANOVA revealed a significant
effect of session only [F(1,36)=6.1, p<0.05], and ADE session had more alcohol
intake than baseline [p<0.05]. B. Two-way ANOVA revealed a significant effect
of session only [F (1,36)=7.4, p<0.01], and ADE session had more alcohol intake
than baseline [p<0.05]. *p<0.05 vs control Baseline.
Discussion
In several recent studies with different alcohol self-administration
models, the selective and short-acting KOR antagonist aticaprant has
been found to reduce alcohol intake and prevents stress-induced
alcohol seeking [11,12]. To further examine whether aticaprant could
have potential as an anti-relapse compound, the present study utilized
a mouse ADE paradigm to mimic alcohol relapse in human alcoholics
and explored the potential of aticaprant in preventing relapse-like
drinking after alcohol abstinence from excessive intake. The ADE has
been studied as a rodent model of craving for alcohol and alcohol
relapse drinking with good predictive validity [20]. Specifically, we
tested aticaprant with 0.1-3 mg/kg doses and found that aticaprant
at 3 mg/kg significantly reduced alcohol intake in both the ADE
relapse-like model and excessive drinking model after 4 hours in both
males and females. The effect of aticaprant on the mouse ADE intake
may be not due to its general inhibition of consumption behaviors,
as paticaprant at the effective dose 3 mg/kg did not change sucrose
intake. Therefore, aticaprant may provide potential treatments for alcohol relapse. Our finding would constitute additional information
of the aticaprant properties in alcohol, nicotine, or opiate -related
studies [11,12,31,32].
Increased KOR activity might occur after alcohol exposure or
during different phases of alcohol withdrawal and KOR antagonists
might be useful during withdrawal or abstinence [3,10,12,26,33-41].
Recent human PET study found that compared to healthy controls,
alcohol-dependent subjects have altered KOR availability across
multiple brain regions, including the frontal cortex, dorsal striatum,
and amygdala [25,26]. Our new study here suggests that KOR activity
is involved in relapse-like drinking, as pharmacological blockade of
KOR using the short-acting antagonist aticaprant prevented mouse
ADE, though more study is needed.
Previous work has found that KOR antagonists do not alter
sucrose drinking in stress- naïve animals but increases sucrose
preference in stress exposed animals due to “anti- depression”
properties [13,15]. Though aticaprant does not produce any reward
in rodents [14], repeated administration of aticaprant has been
found to correct the reduction in sucrose preference or intake after
chronic stress and is under development for the potential treatment
of major depressive disorder [15,23]. Consistently, our results here
showed that aticaprant at 3 mg/kg (the most effective dose for
reducing alcohol intake) increased sucrose preference in mice after
experiencing repeated stress during 3-week intermittent alcohol
abstinence and after 1-week abstinence, suggesting that the blockade
of KOR activity by aticaprant could modulate sensitivity to sucrose
reward during the alcohol abstinence. Alternatively, aticaprant may
increase preference for more palatable reinforcers, such as sucrose,
with decreased preferences for less palatable reinforcers (15-30%
alcohol), though aticaprant itself is not rewarding. However, we
did observe that aticaprant decreased water intake in the excessive
alcohol drinking experiment, suggesting that aticaprant may have
decreased alcohol intake through off-target effects, such as general
motivation to consume any fluid. Therefore, this question led us
to specifically examine its effect on sucrose drinking behavior and
we found that aticaprant did not change sucrose intake, with water
intake reduction, arguing that the reductions of alcohol drinking
could not be attributed to general inhibition of consumption
behaviors. As reported by other groups, aticaprant as a selective and
short-acting KOR antagonist, does not display any sedative activity
or cause “depression or dysphoria” in rodents as found with classic
KOP-r agonists and has recently been under development for new
clinical implications as anti- depressant [11,15,23]. It is unclear why
aticaprant decreased water intake in some of our experiments.
It was noteworthy that in contrast to aticaprant, the long-acting
KOR antagonist nor-BNI at 30 mg/kg did not prevent the ADE in
either sex. In our previous dose-response experiments, 5- 20 mg/kg of
nor-BNI was tested and all failed to show any effect of the long-acting
KOR antagonist on mouse relapse-like drinking [28]. Consistently,
an early study by another group reported that nor-BNI at 10 mg/kg
did not alter the ADE in male rats with long-term (one and half years)
of alcohol drinking experience in a 4-bottle choice drinking paradigm
[19]. Using an operant alcohol self-administration paradigm, nor-
BNI had no effect on the rat ADE or alcohol intake neither [19,,42].
Nevertheless, the behavioral observations explored in both the rat and mouse studies with nor-BNI suggest that the long-acting KOR
antagonist may represent different actions and mechanisms from
short-acting antagonists [4,22,43].
In the second main objective of our present study, we provide clear
experimental evidence showing that the combination of aticaprant
with MOR antagonist naltrexone is more effective than either single
drug alone. When aticaprant (0.3 mg/kg) with naltrexone (1 mg/kg)
together was tested in both the excessive drinking model and ADE
relapse-like models, there was a profound and synergistic effect of the
combination on decreasing excessive intake and preventing ADE in
both males and females, as each compound at its low dose did not
have any effect alone. The combination did not alter sucrose intake or
water intake, indicating an alcohol- specific effect of the combination.
However, one possible concern about the repeated use of aticaprant
is that its KOR antagonistic activity after repeated administration
could result in the development of tolerance. Therefore, our study
purposely examined the efficacy of a multiple- dosing regimen (5
daily injections before the ADE during the 1-week abstinence) to
mimic the multiple-dosing treatment in the clinic. Like the effect of
single administration of the combination (0.3 mg/kg aticaprant with
1 mg/kg naltrexone), the repeated pretreatments of the combination
during the abstinence effectively prevented the ADE drinking in
both sexes, and the 1-week daily administrations did not show any
development of tolerance in our multiple-dosing regimen, consistent
with the human clinical testing and preclinical studies [15,23,44]. As
the single-receptor pharmacotherapies have been recently found to
have modest therapeutic value on alcohol relapse, there is an obvious
need for better efficacy [18,20], and our new finding here has provided
promising in vivo data demonstrating that the clinically developed
short-acting KOR antagonist aticaprant, in combination with lowdose
naltrexone, may offer a novel strategy to treat alcohol relapse.
Summary
Alcoholism remains a big public health problem with limited
treatment choices. The KOR system has been a potential target for
the development of drug addiction treatments, as many preclinical
studies consistently demonstrated that KOR antagonists decrease
drug- taking and seeking behaviors, as well as drug withdrawalrelated
behaviors in rodents, especially under stress conditions [17].
Consistently, we found that aticaprant (a selective and short-acting
KOR antagonist) alone decreased alcohol intake and prevented
“relapse” drinking in both the excessive alcohol drinking and alcohol
relapse-like (ADE) mouse models respectively, with a paralleled
reduction of water intake. However, several recent clinical reports
have found negative results on drug addiction, showing that aticaprant
treatment is ineffective on nicotine or cocaine (for example, [32]),
though aticaprant has been under development for the potential
treatment of major depressive disorder in humans [23]. Therefore,
more effective treatment strategies need to be further developed
with the KOR antagonist in the preclinical research. As both KOR
and MOR systems interact each other in neurobiological processes
of drug addiction, the present study specifically tested whether the
effectiveness of aticaprant can be improved under MOR antagonism
by naltrexone. Of interest, when aticaprant co-administered with
naltrexone together, aticaprant at sub-effective doses profoundly
reduced excessive alcohol consumption and prevented relapse-like
drinking, without affecting sucrose or water intake, particularly with a multiple-dosing regimen of the combination. Together, our
new study strongly suggests that aticaprant in combination with
naltrexone offers a novel approach for alcoholism treatment.
Acknowledgement
NIH AA021970 (YZ) and Robertson Therapeutic Discover Fund
at the Rockefeller University (YZ). Specifically, we remember Dr.
Mary Jeanne Kreek for her contributions in medical research on drug
addiction diseases.