Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
*Address for Correspondence: Farshad Shirazi, Arizona Emergency Medicine Research Center, College of Medicine, University of Arizona, Tucson, AZ, USA , Tel:(520) 626-5510; Email: mshirazi@aemrc.arizona.edu
Citation: Malekzadeh P, Hu J, Sandweiss AJ, Ameli N, Bierny P, et al. Effect of Centruroides Antivenom on Reversal of Methamphetamine-Induced Hyperkinesis and Hyperthermia in Rats. J Pharmaceu Pharmacol. 2017;5(1): 5.
Copyright & copy; © 2017 Malekzadeh P et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology | Volume: 5, Issue: 1
Submission: 16 February, 2017 | Accepted: 04 April, 2017 | Published: 20 April, 2017
Effect of Centruroides Antivenom on Reversal of Methamphetamine-Induced Hyperkinesis and Hyperthermia in Rats
Pouran Malekzadeh1 Jackie Hu2,3 Alexander J. Sandweiss2 Nina Ameli1Philippe Bierny1 Tally M. Largent-Milnes2 Todd W. Vanderah2 and Farshad Shirazi3,4*
- 1 College of Medicine, University of Arizona, USA
- 2Department of Pharmacology, University of Arizona, USA
- 3Arizona Poison & Drug Information Center, College of Pharmacy, University of Arizona, USA
- 4Arizona Emergency Medicine Research Center, College of Medicine, University of Arizona, USA
*Address for Correspondence: Farshad Shirazi, Arizona Emergency Medicine Research Center, College of Medicine, University of Arizona, Tucson, AZ, USA , Tel:(520) 626-5510; Email: mshirazi@aemrc.arizona.edu
Citation: Malekzadeh P, Hu J, Sandweiss AJ, Ameli N, Bierny P, et al. Effect of Centruroides Antivenom on Reversal of Methamphetamine-Induced Hyperkinesis and Hyperthermia in Rats. J Pharmaceu Pharmacol. 2017;5(1): 5.
Copyright & copy; © 2017 Malekzadeh P et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology | Volume: 5, Issue: 1
Submission: 16 February, 2017 | Accepted: 04 April, 2017 | Published: 20 April, 2017
Abstract
Context: Methamphetamine (MA) toxicity is a major health concern causing agitation, hyperkinesia, hyperthermia, and even death, affecting 24.7 million people worldwide. It has been observed that MA generates movement disorders in children similar to that of scorpion envenomation. Four cases have been reported where MA intoxication in children were both subjectively and objectively improved as indicated by the reversal of nystagmus and movement disorders following administration of Centruroides antivenom (AV) therapy.
Objectives: Here, we aimed to demonstrate the reversal of MA induced movement disorders and hyperthermia by scorpion AV equine immune F(ab’)2 in rats.
Materials and Methods: Baseline core temperature and locomotor activity in adult male Sprague-Dawley rats (200-220 g) were evaluated prior to acute administration of AV (20 mg/kg, intraperitoneally, i.p.) + MA (10 mg/kg, i.p.) or control. Core body temperature was reassessed 10, 50, and 80 min post injection while locomotor activity was reassessed 20-35 and 60-75 min post injection.
Results: At 20-35 min, Saline + MA and BSA + MA groups showed a significant increase in the number of fine events compared to their respective control groups Saline + Saline and BSA + Saline, which indicates an increase in paw movements of animals in situ (p = 0.008, p = 0.006, respectively). In contrast, AV + MA demonstrated a nonsignificant increase in fine activity compared to the control group AV + Saline). At 60-75 min, the AV + MA treatment group were less likely to engage in locomotor activity indicated by the significant decrease in exploratory events compared to BSA + MA control group (p = 0.041). No significant percent change in core body temperature was observed in the AV+MA treatment group compared to the control groups, AV + Saline and BSA + MA.
Discussion: Here, we provide evidence for some aspects of MAinduced hyperkinesia but not hyperthermia reversed by scorpion AV. Further preclinical studies involving adolescent rodents may be necessary to completely mimic the reversal of MA toxicity seen in children in the clinic.
Keywords:
Antivenom; Antivenins; Methamphetamine; Rats; Symptomatology; Mechanism
Introduction
According to the National Drug Threat Survey of 2014, 31.8% of state and local agencies consider methamphetamine (MA) to be the greatest drug threat in their areas. MA has become increasingly available and abuse is particularly prevalent in the Western United States [1]. The majority of MA consumed in the U.S. is produced in Mexico. As a consequence of its proximity to Mexico, much of the illicit use of MA takes place in the Southwest U.S., a region incidentally known to have a hospitable climate for Centruroides sculpturatus scorpions.
One of the consequences of increased accessibility of amphetamines to adults is the increased accidental exposure of children. A curious toddler can ingest the unsupervised substance and present with symptoms similar to that of scorpion envenomation: agitation, tachycardia, hyperthermia, excessive salivation, and movement disorders of the upper and lower extremities. Depending on the quantity of ingested MA, children can remain symptomatic for 22 hours or more [2,3]. When caregivers are not forthcoming about their social history or drug use, this can result in misdiagnosis and empiric treatment for scorpion envenomation [4].
Current treatments of MA exposure include benzodiazepines, phenobarbital and charcoal. In scorpion envenomation, mild cases are treated with analgesia, irrigation and tetanus prophylaxis. Severe cases, which are the ones more likely to be confused with MA intoxication, can be managed similarly to MA intoxication with IV benzodiazepines, or with antivenom, but normally not both. Antivenom Centruroides F(ab’)2 (AV), also known as Anascorp, is produced by immunizing horses exposed to scorpion venom [5,6]. This drug was FDA approved in 2011 and effectively resolves the clinical neurotoxic syndrome associated with envenomation. Venomspecific F(ab’)2 fragments of immunoglobulin G (IgG) neutralize venom toxins allowing for their elimination and the alleviation of symptoms.
In a case report by Strommen and Shirazi, a 17-month-old female presented to the emergency department with symptoms of tremors, diaphoresis, and irritability. Additional pertinent positive physical findings were mydriasis with rotary nystagmus, hyper salivation, sinus tachycardia, and hyperthermia. Based on the mother’s account of scorpions around the house and the clinical findings, treatment was initiated with 3 vials of Anascorp [7]. The toddler experienced resolution of her nystagmus and excessive salivation within 30 min of treatment but she continued to have generalized tremors and tachycardia, and she developed a fever of 102.0 °F. The mother’s drug history was revealed upon further questioning and the patient was admitted to a tertiary care center for further observation. Labs later revealed that the patient had serum amphetamine levels approximately 61,000 ng/mL. This reversal of MA-induced symptomology by Anascorp merits further preclinical investigations into the mechanisms of AV therapeutics [7].
Materials and Methods
Animals
Adult male sprague-dawley rats (Harlan, IN, USA) (total of 52 rats, 200-220 g) were housed in a temperature-controlled room on a 12 hour light/dark cycle with food and water available ad libitum. The animals were allowed to acclimate to the testing room enclosure for 30 min prior to all testing. Handling, care, maintenance and testing of the animals were performed in accordance with the policies and recommendations of the international association for the study of pain and the national institutes of health guidelines for the handling and use of laboratory animals. The experimental protocol was approved by the animal care and use committee of the University of Arizona.
Drug preparation
MA (Sigma Aldrich, catalog number: O9512) was prepared with a concentration of 10 mg/ml in 0.9% sterile saline and administered at a dose of 1 ml/kg intraperitoneal (i.p.) injection. This dose of MA was used to elicit hyperactivity as previously described. The scorpion AV F(ab’)2, Alcramyn (Anascorp), was manufactured by Instituto Bioclon S.A de C.V. in Mexico D.F., Mexico and procured by the viper center at the University of Arizona [8]. AV was prepared with a concentration of 20 mg/ml in 0.9% sterile saline and administered at a dose of 1 ml/kg i.p. Bovine serum albumin (BSA; Dextrose USP grade, Sigma Aldrich, catalog number: D8066) (AV placebo) was prepared at 20 mg/ml and injected at a dose of 1 ml/kg i.p. Control animals were injected with 0.9% sterile saline solution, 1 ml/kg i.p.
Study Protocol
Animals were separated into 6 groups of n=8-9. The groups were comprised of (I) AV (20 mg/kg) + Saline (1 ml/kg); (II) AV (20 mg/kg) + MA (10 mg/kg); (III) BSA (20 mg/kg) + Saline (1 ml/kg); (IV) BSA (20 mg/kg) + MA (10 mg/kg); (V) Saline (1ml/kg) + MA (10 mg/kg); (IV) Saline (1 ml/kg) + Saline (1 ml/kg). Baseline core body temperature was probed using a sterilized rectal thermometer. Baseline mobility and activity were determined by placing animals into individual activity chambers (Place Preference system, San Diego Instruments) and analyzed (PAS software, San Diego Instruments). The place preference system records movement with a 4 X 16 photo beam array providing x,y movement. Vertical entries were manually counted each time an animal shifted all weight to the hind paws “to stand”. After obtaining baseline values for core body temperature and activity, animals were administered their first drug at time -10 min (Figure 1) and second drug at time zero. Core body temperature measurements were repeated at 10, 50, and 80 min while activity was measured at 20-35and 60-75 min.
Statistical Analysis
Graph Pad Prism 5 software was employed to plot data and perform statistical analysis. For core body temperature the data was expressed as mean ± standard error of the mean (SEM). A repeated measure two-way analysis of variance (ANOVA) was employed to analyze differences in core body temperature. For activity measurements, data was expressed as mean ± SEM. A one-way ANOVA was with an unpaired t-test with Welch’s correction to determine statistical significance. A p < 0.05 was accepted as statistically significant.
Results
Exploratory activity
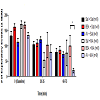
Exploratory movement events were measured to assess the effects of AV pretreatment to MA administration in the animals at baseline, 20-35, and 60-75 min. At baseline, there was no significant difference in the mean number of exploratory events between the six treatment groups. Furthermore, no significant changes were observed at 20-35 min. At time 60-75 min, the AV + MA group were less likely to engage in exploratory activity indicated by the significant decrease in exploratory events (19.1 ± 7.3) compared with BSA + MA (100.3 ± 34.2; P = 0.041) (Figure 1). All other treatment groups exhibited no significant changes in exploratory activity.
Figure 1: Exploratory Activity Timecourse.Exploratory activity in rats at baseline, 20-35, and 60-75 min using individual activity chambers. AV pretreatment to MA administration (20 mg/kg and 10 mg/kg, i.p.) significantly decreased exploratory events(19.1 ± 7.3, n=9) compared with BSA + MA (20 mg/kg and 10 mg/kg, i.p.) (100.3 ± 34.2, n=8) at 60-75 min (*P< 0.05).
Ambulation activity
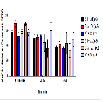
The number of ambulation events was measured to assess the amount of movement in the animals among different treatment groups at baseline, 20-35, and 60-75 min. There were no significant differences in the mean of ambulation events for baseline, 20-35, and 60-75 min (Figure 2). During the first five-minute interval of the 20-35 min time point, BSA + MA had a significant decrease in ambulation events (306.8 ± 110.1) when compared to the BSA + saline control group (654.7 ± 41.5; P = 0.010). In comparison, AV + MA and saline + MA groups did not demonstrate significant differences to their saline control groups, AV + saline and saline + saline (P = 0.998, P = 0.598, respectively) (Figure 3).
Figure 3: Ambulation 20-35 min activity in 5-minute time intervals.Ambulatory activity during the first five-minute interval of the 20-35 min time point. BSA+MA(20 mg/kg and 10 mg/kg, i.p.) had a significant decrease in ambulation events (306.8 ± 110.1, n=8) when compared to the BSA + saline(20 mg/kg and 1 ml/kg, i.p.)control group (654.7 ± 41.5,n=9). In contrast, AV+ MA(20 mg/kg and 10 mg/kg, i.p.,n=9) and saline + MA(1 ml/kg and 10 mg/kg, i.p., n=8) groups did not demonstrate significant differences to their saline control groups, AV + saline(20 mg/kg and 1 ml/kg, i.p., n=9) and saline + saline(1 ml/ kg and 1 ml/kg, i.p., n=9)(**P< 0.01).
Fine activity
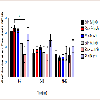
To explore if AV + MA had an effect on the frequency of paw movement in animals in situ, the number of fine movement events were measured at baseline, 20-35, and 60-75 min. At baseline, all treatment groups had no significant differences in the mean number of fine events. At time 20-35 min, saline + MA showed a significant increase in fine events (247.8 ± 19.5) compared to the saline + saline control group (166.0 ± 9.26; P = 0.006). Furthermore, BSA + MA resulted in a significant increase in fine events (257.4 ± 28.9) compared with the BSA + saline control group (177.4 ± 5.15; P = 0.008). In contrast, AV + MA did not have a significant increase in fine events (221.6 ± 9.8) compared with the AV + saline control group (164.6 ± 4.7). At 60-75 min, all MA treatment groups exhibited a significant increase in fine events compared to their respective control saline groups (P < 0.001) (Figure 4).
Figure 4: Fine Activity Timecourse.Fine activity in rats at baseline, 20-35, and 60-75 min. At time 20-35 min,saline + MA (1 ml/kg and 10 mg/kg, i.p.)had significantly increased fine events (247.8 ± 19.5, n=8) compared to the saline + saline (1 ml/kg and 1 ml/kg,i.p.) control group (166.0 ± 9.26, n=9). BSA + MA (20 mg/kg and 10 mg/kg, i.p.) also resulted in significantly increased fine events (257.4 ± 28.9, n=8) compared with the BSA + saline (20 mg/kg and 1 ml/kg, i.p.) control group (177.4 ± 5.15, n=9). However, AV + MA (20 mg/kg and 10 mg/kg, i.p.) did not have a significant change in fine events (221.6 ± 9.8, n=9) compared with the AV + saline (20 mg/kg and 1 ml/kg, i.p.) control group (164.6 ± 4.7, n=9). All MA treatment groups exhibited a significant increase in fine events compared to their respective control saline groups by the 60-75 min time point (**P<0.01, ***P <0.001).
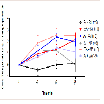
Core body temperature
Core body temperature in vivo was assessed using a temperature probe to measure body temperature at baseline, 10, 50, and 80 min(Figure 5). At baseline, there was no significant difference in body temperature between all treatment groups. The AV + MA group did not demonstrate a significant change in core body temperature at all time points when compared with the AV + saline and BSA + MA treatment groups. However, saline + MA also did not show a significant change in core body temperature at all time points when compared to the control, saline + saline (Figure 6).
Figure 5: Core Temperature Timecourse.Fine activity in rats atbaseline, 20-35, and 60-75 min.At time 20-35 min, saline + MA (1 ml/kg and 10 mg/kg, i.p.)had significantly increased fine events (247.8 ± 19.5, n=8) compared to the saline + saline (1 ml/kg and 1 ml/kg, i.p.) control group (166.0 ± 9.26, n=9). BSA + MA (20 mg/kg and 10 mg/ kg, i.p.)alsoresulted in significantly increased fine events (257.4 ± 28.9, n=8) compared with the BSA + saline (20 mg/kg and 1 ml/kg, i.p.) control group (177.4 ± 5.15, n=9). However, AV+ MA (20 mg/kg and 10 mg/kg, i.p.) did not have a significant change in fine events (221.6 ± 9.8, n=9) compared with the AV + saline (20 mg/kg and 1 ml/kg, i.p.) control group (164.6 ± 4.7, n=9). All MA treatment groups exhibited a significant increase in fine events compared to their respective control saline groups by the 60-75 min time point (**P<0.01, ***P <0.001).
When comparing individual time points for each treatment group, saline + MA had a significant increase in body temperature at 10 and 50 min compared to baseline, but not at 80 min (P = 0.038, P = 0.037, P > 0.05, respectively). AV pretreatment did not prevent a significant increase in MA induced hyperthermia at 50 or 80 min compared to baseline (P = 0.0033 and P = 0.0495, respectively).However, it is important to note that the AV + saline control group also resulted in a significant increase in body temperature at 10, 50, and 80 min compared with baseline (P < 0.05).
Discussion
The present preclinical study investigated the potential of Centruroides AV to prevent MA-induced hyperkinesia, dyskinesia, and hyperthermia. To maximize the peak concentrations of both MA and AV in the circulation, experiments were designed so AV (or control) was administered via i.p. injections 10 min before MA (or control). BSA is a non-specific protein that served as a control group to better evaluate the effects of AV on symptomology using the same concentration (20 mg/ml in 0.9% sterile saline administered at a dose of 1 ml/kg i.p.). Experimental recordings then involved core body temperature probing and measurement of exploratory, ambulatory and fine movements using activity boxes for automatic x,y movement detection and video recordings for manual observations of verticalentries.
Here, we show that pretreatment with AV prevented MA-induced increases in fine movements 20-35min post-injection, a phenomenon lost by 60-75 min. Fine movements, such as grooming, are defined as rotations in different planes while remaining stationary. In control groups, this behavior was at constant levels both at the 20-35 min and 60-75 min recordings. In contrast, saline-MA and BSA-MA groups had an increase in fine motor activity. Interestingly, AV-MA groups had reversal of fine motor activity levels similar to control groups 20-35 min post injections (Figure 4). These behaviors are also consistent clinically in patients on MA, who exhibit “grooming” behaviors such as picking at skin and scabs.
Our data also support the idea that AV pretreatment prevents a MA-induced decrease in ambulatory behavior in the first 5 min of the 20-35 min time point post-MA administration (Figure 3). Under normal circumstances, animals placed in activity boxes explore their environment maximally within the first 5 min. Thereafter, the rate of exploration and ambulation decreases linearly when the rats acclimate and resume stationary behaviors such as grooming or napping. In the control groups, this trend was consistently observed. It was hypothesized that saline-MA and BSA-MA groups would have increased rates of exploratory and ambulatory activities. However, the saline-MA and BSA-MA groups showed a decrease in ambulatory activity. This was specifically seen at the 20-35 min bin, when the rats placed in the activity boxes within the first 5 min had a significant decrease in ambulation. In contrast, AV-MA groups resumed activities similar to that of controls (Figure 2).
The only difference in core body temperature was between saline only-treated animals and every other group. In fact, any animal that received either a protein (BSA/AV) or MA had an increase in core body temperature while saline only-treated animals remained at baseline (Figure 5). Saline-MA groups, as predicted, had elevated temperature recordings. Interestingly, AV-saline and BSA-saline groups also had elevated temperatures respective to normal values (Sprague-Dawley rats 36.99 °C). This requires further investigation but it is theorized that the elevated temperatures were a result of an inflammatory response to the introduction of the novel proteins into circulation.
It has been proposed that F(ab’)2 binds MA in the blood and thereby decreases the amount that can effectively bind receptors, which correlates clinically with the reduced symptoms observed in close proximity of F(ab’)2 administration. This concept has been utilized before and has clinical applications. MA vaccines have been shown in rodents to decrease the effects of MA in a dose dependent manner. In theory, anti-MA vaccine (SMA-KLH) generates antibodies against MA so that circulating MA in the blood stream can form a complex with the antibodies that then decreases its potential to cross the blood brain barrier (BBB). This can then reduce the effects of MA both centrally and peripherally. In one study, it was shown that acute exposure of antibodies (anti-MAmAbs) to MA decreased the duration of locomotor activity (sniffing, head bobbing, etc.). However, vaccines might be a better approach for reducing MA addiction rather than managing acute exposure [9,10].
Another theorized mechanism is that AV crosses the BBB and hinders MA’s ability to bind to receptors, effectively reducing the symptoms seen clinically. This theory requires a discussion of the BBB and an example of a disease process in which antibodies both cause and reverse pathology. The BBB is produced by the inter connections of non-fenestrated capillary endothelial cells and the supporting astrocyte glial cells, pericytes, and microglia. Endothelial cells create a physical barrier that has the resistance of 1100-1500 Ω cm-2. The passage of molecules is passive for lipid soluble molecules while polar molecules with a surface area greater than 80 Å 2 or molecular weight exceeding 450 Da must utilize different transporters [11]. These include solute carriers (SLC) for small molecules and transcytosis receptor-mediated channels for larger macromolecules. The specific and non-specific transcytosis receptors allow for the movement of proteins and peptides. Vesicular mechanisms also exist in aiding the movement of macromolecules. Receptor-mediated transcytosis(RMT) or adsorptive-mediated transcytosis (AMT) receptors bind to specific macromolecules that trigger endocytosis. Contents can then fuse with a lysosome or may be transported to the opposite pole of the cell [12].
Many disease states have been identified that alter the BBB (Alzheimer’s disease, epilepsy, tumors, etc.). In one disease state, antibodies against N-methyl-D-aspartate (NMDA) receptors cross the BBB and cause encephalitis. These patients present with dyskinesias (orofacial, choreoathetosis), autonomic instability, psychiatric symptoms, memory loss and seizures and are positive for anti-NMDA receptors (NR1-NR2 subunits). NMDA receptors are ligand-gated cation channels (NR1 subunits bind glycine and NR2 subunits bind glutamate) and cause intracellular messaging resulting in distinct pharmacological properties. Over activity of these receptors causes excitotoxicity accounting for the observed clinical symptoms. First line treatment has shown to significantly reverse symptoms within four weeks and includes intravenous immunoglobulin, steroids, and plasmapheresis. As it pertains to our study, it is theorized that antibodies cross the BBB and impede MA’s interaction with receptors in a similar manner [13].
Of particular interest is the role of inflammation and BBB permeability. The production of free radicals, IL-6, IL-1 and elevated temperature all affect the permeability of the BBB. MA has also been reported to increase permeability [14]. In one study, MA was shownto increase the BBB permeability by causing oxidative stress and by decreasing the expression of cell membrane-associated tight junction proteins in a time and dose dependent manner. It is plausible that once MA mediated oxidative injury has taken place, AV then readily crosses the BBB and hinders MA interactions on receptors [15]. However, there are presently no published evidence regarding the BBB permeability potential of F(ab’)2.
The results also showed that AV-MA had the greatest reduction of exploratory activity at around 60 min post-injection (Figure 1). This observed pharmacokinetic behavior of the F(ab’)2 can be explained by both theories. In the first theory, the lag in time can be explained by the time it takes F(ab’)2 to neutralize sufficient MA in the blood to observe a physical decrease in movements. In the second theory, the time discrepancy can be suggestive of the time it takes for F(ab’)2 to cross the BBB and accumulate in sufficient amount to hinder the effects of MA on CNS tissue. The remainder of the results showed no remarkable changes in the number of ambulatory movements between the groups. Fine motor activity increased across the MA-exposed groups. This is clinically consistent with patients’ mannerisms in scratching and picking at skin. Furthermore, there were no significant changes observed with the temperature recordings across the groups.
In summary, pediatric MA toxicity is an important topic because of the implications it can have on patients health. It has been postulated that Centruroides a neither specifically reverses channelopathy by MA or due to the presence of AV in the CNS, that the same result is observed by a protein-receptor interaction. Although this study showed that AV does reduce the locomotor activity in MA-exposed rats, which correlates clinically, anascorp is costly and economically not practical. Future projects can compare the efficacy of IVIG in the reversal of movement disorder to that of anascorp in MA-exposed rats. Additionally, the mechanism of the reversal of symptoms is unknown at this time. Future experiments aim to detect a moleculeprotein interaction by obtaining and analyzing blood samples from the test groups. Other objective recordings to consider for future research include measurements of pain, blood pressure, and respirations in test rats.
References
- Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Reid N, et al. (2005) 2004 Annual Report of the American Association Of Poison Control Centers toxic exposure surveillance system. Am J Emerg Med 23: 589-666.
- Nagorka AR, Bergeson PS (1998) Infant methamphetamine toxicity posing as scorpion envenomation. Pediatr Emerg Care 14: 350-351.
- Kolecki P (1998) Inadvertent methamphetamine poisoning in pediatric patients. Pediatr Emerg Care 14: 385-387.
- Kish SJ (2008) Pharmacologic mechanisms of crystal meth. CMAJ 178: 1679-1682.
- Curry SC, Vance MV, Ryan PJ, Kunkel DB, Northey WT (1983) Envenomation by the scorpion Centruroides sculpturatus. J Toxicol Clin Toxicol 21: 417-449.
- Rimsza ME, Zimmerman DR, Bergeson PS (1980) Scorpion envenomation. Pediatrics 66: 298-302.
- Strommen J, Shirazi F 2015 Methamphetamine ingestion misdiagnosed as Centruroides sculpturatus envenomation. Case Rep Emerg Med 2015: 320574.
- Brien JF, Kitney JC, Peachey JE, Rogers BJ (1978) Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res Commun Chem Pathol Pharmacol 22: 313-328.
- Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, et al. (2013) A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend 129: 41-48.
- Gentry WB, Rüedi-bettschen D, Owens SM (2009) Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin 5: 206-213.
- Butt AM, Jones HC, Abbott NJ (1990) Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429: 47-62.
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13-25.
- Punja M, Pomerleau AC, Devlin JJ, Morgan BW, Schier JG, et al. (2013) Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis: an etiology worth considering in the differential diagnosis of delirium. Clin Toxicol (Phila) 51: 794-797.
- Roberts DJ, Goralski KB (2008) A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin Drug MetabToxicol 4: 1245-1264
- Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, et al. (2009) Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab 29: 1933-1945.