Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
*Address for Correspondence: Himankar Bashiya, Senior Director, Beijing Sciecure Pharmaceutical Co., Ltd., Zhongbei Industrial Park, Beishicao Town, Shunyi District, Beijing, 101301, China, Tel: +861060447688; E-mail: himankar@sciecure.com
Citation: Baishya H, Marihal S, Zibin Z. Prevention of Crystal Formation during Formulation of Acyclovir Powder for Solution for Infusion. J Pharmaceu Pharmacol. 2015;3(2): 4.
Copyright © 2015 Bashiya H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology | Volume: 3, Issue: 2
Submission: 09 September 2015 | Accepted: 21 December 2015 | Published: 28 December 2015
Physical appearance: For the large scale of production, 14,000 vial batch sizes were considered on the tentative basis for further study. It takes almost 24 hrs for processing a batch. Therefore, prepared formulations were visually observed for their physical appearance at initially and after 24 hrs of time interval.
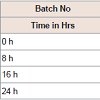
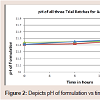
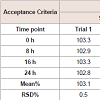
pH measurement: The pH of all the formulations was set initially in the range of 11.2-11.4. pH of the prepared solution initially and after few hours of time interval was measured, which are shown in Table 3.
Crystal growth: After the primary drying, the samples were kept for different time interval to observe crystal growth at room temperature (37 °C) for 5 hrs, the data is given in Table 4 effect of oxygen on stability of formulations
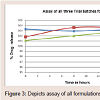
Assay of formulations: Table 5
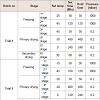
Freeze drying programme: The rate and manner of freezing has been shown to have an effect on cake formed after freezing, which was cracked. So to get uniform cake, freezing programme was modified. Results of freeze dried modification is captured in Table 6.
Related substance: From the above results trial 3 showed comparatively less total impurity compared to trial 1.
Prevention of Crystal Formation during Formulation of Acyclovir Powder for Solution for Infusion
Himankar Baishya*, Sachin Marihal and Zhang Zibin
- Beijing Sciecure Pharmaceutical Co., Ltd., Zhongbei Industrial Park, Shunyi District, Beijing, China
*Address for Correspondence: Himankar Bashiya, Senior Director, Beijing Sciecure Pharmaceutical Co., Ltd., Zhongbei Industrial Park, Beishicao Town, Shunyi District, Beijing, 101301, China, Tel: +861060447688; E-mail: himankar@sciecure.com
Citation: Baishya H, Marihal S, Zibin Z. Prevention of Crystal Formation during Formulation of Acyclovir Powder for Solution for Infusion. J Pharmaceu Pharmacol. 2015;3(2): 4.
Copyright © 2015 Bashiya H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pharmaceutics & Pharmacology | Volume: 3, Issue: 2
Submission: 09 September 2015 | Accepted: 21 December 2015 | Published: 28 December 2015
Abstract
Aims and objectives: The main objective of the present work is to develop acyclovir powder for solution for infusion with clear solution upon half sealing and formation of stable cake after freezing drying programme. During the development, after half sealing stage, a mild turbid color formation in vials was observed followed by crystal formation. This was later investigated due to presence of residual oxygen content in the headspace of the vial and also in freeze drying chamber.Material and method: Aciclovir, sodium hydroxide and cool water for injection was used to prepare solution for injection. Followed by pre-filtration and sterile filtration into colorless vials and semi seal the vials in freeze-drying mode with rubber stoppers. Final freeze-drying programme was performed and cap them with aluminum caps.
Results and discussion: Trial 3 showed stable clear solution after few hours. This was due to lower concentration of oxygen in the headspace, which was obtained due to introduction of nitrogen during filling stage.
Conclusion: Crystal formation was due to presence of oxygen in the head space, which can be avoided by maintaining oxygen level below 0.5% in headspace volume of vials and also stable formed after changing the freeze drying cycles.
Keywords:
Acyclovir; Crystallization; Sodium hydroxide; Headspace; Water for injectionIntroduction
Acyclovir (acyclovir) is a nucleoside antiviral drug with antiviral activity in vitro against members of the herpes group of DNA viruses. As an established treatment of herpes simplex infection, intravenous, oral and to a lesser extent topical formulations of acyclovir provide significant therapeutic benefit in genital herpes simplex and recurrent orofacial herpes simplex. Further treatment of patients with hematologic malignancies frequently includes the use of immunosuppressive agents such ascortico steroids, a stimulus that is known to increase the risk of reactivation of HSV [1]. Injections include a wide variety of therapeutic agents e.g., for the treatment of cancer, infections, cardiovascular diseases, arthritis, inflammatory diseases, diabetes, hormonal deficiencies and many other disease states including life threatening emergency conditions. Formulation scientists have severe restrictions in number and choice of added substances because of safety considerations [2]. Powders for injection (PIs) constitute an important category of dosage forms for active molecules. Because of their instability in the aqueous environment, PIs cannot be marketed as ready-to-use injectables [3]. Instead, they are marketed as dry powders to be reconstituted with a suitable vehicle just before administration. The final form after reconstitution may be either a solution or a suspension [4]. Typical molecules in this category include β-lactam antibiotics, cephalosporin’s, and acyclovir. Injectable products require a unique formulation strategy. The formulated product must be sterile, pyrogen-free, and, in the case of solution, free of particulate matter. No coloring agent may be added solely for the purpose of coloring the injectable preparation [5]. The present work is to develop stable acyclovir powder for solution for infusion by freeze dried technology (Lyophilization). During the development, after semi sealing stage, a haze color was noticed in the vials followed by crystal formation and instable cake formation. Intravenous acyclovir is the treatment of choice in biopsy-proven herpes simplex encephalitis in adults, and has also been successful in the treatment of disseminated herpes simplex in pregnancy and erpes neonatorum [6]. USP specifies that “containers including the losures for dry-powder solids intended for parenteral use, do not interact physically or chemically with the preparation in any manner to alter the strength, quality, or purity beyond the official requirements under the ordinary or customary conditions of handling shipment, storage, sale, and use” [7]. The aim of the present study is to formulate and evaluate the parenteral dosage form containing acyclovir. The objectives of the study are, to study the reason for crystal formation during semi seal stage.Materials
Acyclovir, sodium hydroxide was a gift sample donated from Sciecure Pharmaceuticals; Neutral borosilicate glass vial Aluminum flip-off cap was used as packing material which was also gift from Sciecure Pharmaceutical. All other reagents used were of analytical grade.Pulsation Vacuum Sterilizer 0.36 B & 0.24 B, Air Circulation Oven, Millipore Filter Integrity Test Equipment, Electronic scale, Vertical Ultrasonic Ampoule Cleaning, Vertical Hot Air Circulation Sterilized Oven, dry heat and wet heat sterilization, Lyophilizer.
Methods
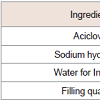
Formulation of powder for Injection for infusion containing 250 mg equivalent acyclovir was prepared by freeze dried technology. The composition details are shown in Table 1.Procedure for preparation of solution:Preparation of bulk solution was done by adding Sodium hydroxide solution into Acyclovir suspension until it is fully dissolved and continue stirring for 30 min for full dissolution under a proper RPM. Transfer the bulk solution to tank, then add water for injections to give desired total weight, check the pH in 11.2~11.4 and if negative, add 10% (w/v) sodium hydroxide solution to adjust the bulk solution pH to 11.2~11.4. Before filtration, check filters for integrity test. Filter through sterile filter of 0.2 μm. After filtration check filters for integrity test. Fill quantity: 4.10 g per vial±0.05 g. After semisealing freeze drying was performed. Before the start of freeze drying, the freeze drying chamber was purged with nitrogen for 6-8 hrs. Place vials in vacuum drying plant and start freeze-drying programme. When secondary drying is nearly finished, flush with sterile filtered (0.2 μm) nitrogen until a rest vacuum of about 0.5 bar, close vials and restores normal pressure. Remove vials and cap them with aluminum caps. Composition details in Table 1. Each trial was done at batch size of 14000 vials.
Evaluation of formulation batches was done by checking physical appearance, crystal growth, pH measurement, assay content and oxygen content in head space. The reconstituted solutions must be assessed for both physical and chemical stability [8,9]. Color absorbance sometimes can be used as a quantification tool during early product stability studies [10], but since it is clear product so color absorbance need not be performed.
Physical appearance: For the large scale of production, 14,000 vial batch sizes were considered on the tentative basis for further study. It takes almost 24 hrs for processing a batch. Therefore, prepared formulations were visually observed for their physical appearance at initially and after 24 hrs of time interval.
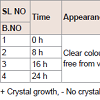
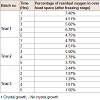
Crystal growth: Each prepared formulations trial before freezing stage were placed at room temperature, respectively for 24 hrs and observed for crystal growth. It is estimated that oxygen content in the headspace will account for crystal growth. The data is given in Table 2.
pH measurement: The pH of the prepared formulations was measured using Thermo Scientific pH meter at 25±1 ºC.
Assay content for acyclovir: Assay of formulationsReference solution preparation: 100 ml of stock reference solutions for each formulation was prepared. The composition of the reference stock solution was similar to that of the respective formulations excluding the drug and also they were diluted similarly as the formulations were diluted using water. This resulting solution is used as reference solution (blank) in comparison with the prepared formulations to measure the % drug content by measuring the absorbencies using Shimadzu UV-Visible spectrophotometer. The amount of acyclovir was determined from standard calibration curve. The assay was determined at different time points to check for stability of formulation. The data is given in Table.
Freezing time programme: Place vials in vacuum drying plant and start freeze-drying programme. Freeze drying was done at different stage like freezing, primary freezing and secondary freezing. The structure of the lyophilized cake is important, since proper cake formation leads to proper pore formation that provides the means for vapor to escape from the product during the drying cycle [11]. The rate and manner of freezing has been shown to have an effect on cake formed, after freezing which was cracked for trial 1 and 3. So to get uniform cake freezing programme should be modified. When secondary drying is nearly finished, flush with sterile filtered (0.2 μm) nitrogen until a rest vacuum of about 0.5 bar, close vials and restores normal pressure. Remove vials and cap them with aluminum caps.
Related substance: Should comply with BP Limits.
Results and Discussion
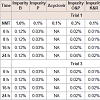
Physical appearance and crystal growth: Physical appearance (color, transparency, precipitation, etc.) of prepared formulations was studied. All batch appeared colorless and clear, initially bur crystal growth was observed in trial 1 and 2 after 16 and 24 hrs of time intervals. The crystallization of sodium salt was happening due to permitted level of oxygen.pH measurement: The pH of all the formulations was set initially in the range of 11.2-11.4. pH of the prepared solution initially and after few hours of time interval was measured, which are shown in Table 3.
Crystal growth: After the primary drying, the samples were kept for different time interval to observe crystal growth at room temperature (37 °C) for 5 hrs, the data is given in Table 4 effect of oxygen on stability of formulations
Assay of formulations: Table 5
Related substance: From the above results trial 3 showed comparatively less total impurity compared to trial 1.
Conclusion
The concept of powder for solution for infusion of parenteral formulations containing acyclovir offers a suitable, practical approach to achieve desired stable parenteral preparation. In present work, stable parenteral formulation of acyclovir was prepared successfully by selection of suitable freeze drying programme and nitrogen purging into freezing chamber for 6-8 hrs prior and also into vials during filling to avoid crystal formation. These formulations were expected to be stable for sufficiently long time (trial 3). The conclusions arrived from the above results indicated that the parenteral formulation containing acyclovir developed was found to be complying satisfactorily with all the evaluation tests performed and was stable for sufficiently longer duration of time.References
- Bubley GJ, Chapman B, Chapman SK, Crumpacker CS, Schnipper LE (1989) Effect of acyclovir on radiation- and chemotherapy-induced mouth lesions. Antimicrob Agents Chemother 33: 862-865.
- Leon L, Deluca P, Akers MJ (1987) Kinetic Principles and Stability Testing. Chapter 26. In: The Theory and Practice of Industrial Pharmacy, Varghese Publishing House, Bombay, pp. 902.1.
- Boylan JC, Fites AL (1979) Parenteral Products. In: Banker GL, Rhodes CT, (Eds). Modern Pharmaceutics Eds, Marcel Dekker Inc, New York, pp. 445.
- DeLuca PP, Boylan JC (1992) Formulation of Small-Volume Parenterals. In: Avis KE, (Ed) Pharmaceutical Dosage Forms: Parenteral Medications, Marcel Dekker Inc, New York, 1: 215.
- Rushil S, Priti M (2014) Freeze dried injectable drug product development: selection of non functional additives. Int J Pharm Pharm Sci 6: 1-5.
- O'Brien JJ, Campoli-Richards DM (1989) Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 37: 233-309.
- U.S. Pharmacopeial Convention (2000) USP-NF 19. pp. 1776.
- Prasad VK, Johns WH, Wingate MW, Mihotic MM, Southard BJ, et al. (1974) Pharmaceutics of cephapirin sodium (Cefadyl), a new semisynthetic cephalosp orin - part III. Curr Ther Res Clin Exp 16: 1214-1237.
- Kaplan MA, Granatek AP (1974) Stability of frozen solutions of cephapirin sodium. Curr Ther Res Clin Exp 16: 573-579.
- Stark G, Fawcett JP, Tucker IG, Weatherall IL (1996) Instrumental evaluation of color of solid dosage forms during stability testing. Int J Pharm 143: 93-100.
- Pikal MJ (1990) Freeze-drying of proteins. Part II: Formulation selection. Bio Pharm 3: 26-30.