Journal of Pediatrics & Child Care
Download PDF
Case Report
*Address for Correspondence: Amanda N. Shaw, Department of Pediatrics, Division of Endocrinology, UT Health - The University of Texas Health Science Center at Houston, 6431 Fannin St. MSB 3.122, Houston, TX 77030, USA, Tel: ++1-713-500-5646; Fax: +1-713-500-0526; E-mail: Amanda.Shaw@uth.tmc.edu
Citation: Shaw AN, Pagan-Pares MI, Yafi M. Hypothyroid Myopathy after Radioactive Iodine Therapy for Graves’ Disease: A Case Report. J Pediatr Child Care. 2016;2(1): 03.
Copyright © 2016 Shaw, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 2, Issue: 1
Submission: 18 March, 2016 | Accepted: 14 April, 2016 | Published: 19 April, 2016
She was evaluated by Pediatric Gastroenterology due to her elevated transaminases. An abdominal ultrasound showed no abnormalities. The gastroenterologist agreed that her presentation was likely related to hypothyroidism with myopathy. She was also evaluated for possible autoimmune liver disorder and found to have weakly positive anti-smooth muscle antibodies. She continues to follow in the gastroenterology clinic as well.
Hypothyroid Myopathy after Radioactive Iodine Therapy for Graves’ Disease: A Case Report
Amanda N. Shaw*, Melissa I. Pagan-Pares and Michael Yafi
- Department of Pediatrics, Division of Endocrinology, UT Health - The University of Texas Health Science Center at Houston, Houston, Texas, USA
*Address for Correspondence: Amanda N. Shaw, Department of Pediatrics, Division of Endocrinology, UT Health - The University of Texas Health Science Center at Houston, 6431 Fannin St. MSB 3.122, Houston, TX 77030, USA, Tel: ++1-713-500-5646; Fax: +1-713-500-0526; E-mail: Amanda.Shaw@uth.tmc.edu
Citation: Shaw AN, Pagan-Pares MI, Yafi M. Hypothyroid Myopathy after Radioactive Iodine Therapy for Graves’ Disease: A Case Report. J Pediatr Child Care. 2016;2(1): 03.
Copyright © 2016 Shaw, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 2, Issue: 1
Submission: 18 March, 2016 | Accepted: 14 April, 2016 | Published: 19 April, 2016
Abstract
Hypothyroid myopathy is a complication of hypothyroidism that is being more commonly recognized by medical providers in more recent years. There are documented cases in both pediatric and adult patients with presentations that are variable and can range from asymptomatic creatine kinase elevation to severe rhabdomyolysis with renal failure. Patients improve with supportive care and thyroid hormone supplementation. Here we present the case of a 14 year old female who was diagnosed with hyperthyroidism and treated with radioactive iodine that who developed hypothyroid myopathy despite the initiation of levothyroxine promptly after her labs indicated that she had developed hypothyroidism.Keywords
Hypothyroid myopathy; Graves’ disease; Radioactive iodine therapy; Hypothyroidism; Rhabdomyolysis; Elevated creatine kinaseIntroduction
Hypothyroidism is a fairly common diagnosis in the pediatric population with a prevalence thought to be as high as 0.135% in previous studies [1]. Hypothyroid myopathy is a known complication of hypothyroidism thought to be rather rare, though it is being recognized more often as more cases are being reported and clinicians are becoming more aware of its presentation. Hypothyroid myopathy presents with a wide range of severity from asymptomatic elevations of creatine kinase to severe rhabdomyolysis with renal failure. Even these laboratory findings can vary; creatine kinase levels can range from only mildly elevated to greater than 25,000 IU/L [2]. Studies have shown varying rates of this creatine kinase elevation, though in general, >55% of patients with symptoms of hypothyroidism also have some degree of creatine kinase elevation [3].Hypothyroid myopathy has been reported in both adult and pediatric patients with varying etiologies of hypothyroidism including Hashimoto’s thyroiditis [4] and chronic acquired hypothyroidism, often after levothyroxine noncompliance and reemergence of symptomatic hypothyroidism [5]. It can also be the presenting symptom in patients with hypothyroidism who have no other symptoms usually found in this condition [6]. Acute hypothyroid myopathy was first reported in an adult patient treated with I-131 radioactive iodine therapy for Graves’ disease in 1987 and since has been recognized in both pediatric and adult patients treated for this condition [7]. The symptoms of hypothyroid myopathy often improve and resolve after treatment with levothyroxine is initiated [8]. We now describe a case of hypothyroid myopathy following treatment with I-131 radioactive iodine for Graves’ disease in which levothyroxine was initiated when laboratory studies showed hypothyroidism, but myopathic symptoms developed despite prompt treatment.
Clinical Case
This is the case of a 14 year old female with no significant past medical history who was referred to the pediatric endocrinology clinic with a one year history of heat intolerance, weight loss, anxiety, tremors, and left eye changes described as left eye protrusion. She was previously seen by an ophthalmologist who expressed concern for possible hyperthyroidism. Her pediatrician ordered a thyroid function panel which showed her TSH less than 0.01 uIU/mL and her total thyroxine level 17.9 ug/dL. She was then referred to pediatric endocrinology for evaluation of these abnormal lab values.On her initial visit to our clinic, her physical examination revealed a heart rate of 95 bpm, left eye proptosis, a normal sized thyroid gland without nodules or a bruit, generalized tremors, and 5/5 muscle strength throughout. After extensive discussion regarding the different treatment possibilities, she and her parents decided to undergo I-131 radioactive iodine therapy. Her I-123 radioactive iodine uptake scan showed increased homogenous uptake throughout the gland without any hot or cold nodules. Her four hour radioactive iodine uptake was 32% (normal 3-15%) and her 24 hour uptake was 53% (normal 10-30%), suggestive of Graves’ disease. On the following day, she underwent radioactive iodine therapy with oral administration of 10.5 mCi of I-131.
Two and a half weeks after she underwent radioactive iodine therapy, her thyroid function panel showed TSH less than 0.006 uIU/mL, total T4 19.0 ug/dL, and free T4 7.48 ng/dL. She continued to report tachycardia. She was started on propranolol 5 milligrams by mouth twice daily. Her thyroid function panel was monitored weekly. One week later, the propranolol was increased to 5 milligrams by mouth three times daily due to continued tachycardia as well as increasing total and free thyroxine levels.
Seven and a half weeks after she received radioactive iodine therapy, her free T4 had decreased significantly and was concerning for hypothyroidism. At that time, the propranolol was discontinued and she was started on levothyroxine 44 micrograms by mouth daily.
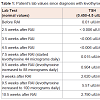
One week after starting levothyroxine therapy, she began to complain of generalized muscle spasms and fatigue. She denied muscle pain and weakness. She had no appreciable muscle weakness on exam. A creatine kinase level was obtained which was elevated to 2923 U/L and CMP revealed elevated transaminases (ALT 57 IU/L, AST 95 IU/L). Her renal function was normal. Due to her symptoms and her abnormal labs, we began to suspect she had developed hypothyroid myopathy. Her dose of levothyroxine was increased to 88 micrograms by mouth daily. Her creatine kinase level was monitored closely and gradually improved as her hypothyroidism improved. Her levothyroxine dose was increased to 100 micrograms by mouth daily due to continued hypothyroidism, and following this dose adjustment, her creatine kinase level improved to 273 U/L (Table 1). Her muscle spasms became less frequent and less severe. We plan to continue to monitor her labs closely to assure that she remains euthyroid and that her creatine kinase level normalizes.
She was evaluated by Pediatric Gastroenterology due to her elevated transaminases. An abdominal ultrasound showed no abnormalities. The gastroenterologist agreed that her presentation was likely related to hypothyroidism with myopathy. She was also evaluated for possible autoimmune liver disorder and found to have weakly positive anti-smooth muscle antibodies. She continues to follow in the gastroenterology clinic as well.
Discussion
Hypothyroidism has long been known to have consequences in muscle tissue such as was seen in infants with untreated congenital hypothyroidism who often showed diffuse muscular hypertrophy with proximal muscle weakness [9]. The exact pathogenesis of hypothyroid myopathy is not well understood. Some believe it is due to impaired muscle function from abnormal glycogenolysis, mitochondrial oxidative metabolism, and triglyceride turnover related to T4 deficiency [10,11]. Subsequently, there appears to be selective atrophy of Type II fibers, which are more dependent on glycolysis for energy. Type I hypertrophy, possibly a compensatory response, then occurs, though Type I fiber atrophy can also be seen. These fiber changes and oxidative damage may lead to cell injury and myopathy [12]. Others believe that the elevation of creatine kinase due to increased capillary permeability related to the local hypothyroid state in the muscle is also a factor [13].Hypothyroid myopathy responds well to treatment with thyroid hormone replacement and creatine kinase levels usually respond and normalize over a few weeks, even before TSH levels have normalized [14]. Clinical symptoms, including muscle pain and, most commonly, muscle weakness can persist for months to years [15]. There are no other routinely recommended treatments for hypothyroid myopathy, though some providers also incorporate physical and occupational therapy into their patients’ treatment plans, especially if their symptoms limit their activities of daily living.
In our patient’s case, thyroid hormone replacement was started prior to the development of the clinical manifestations of hypothyroid myopathy. Despite this prompt initiation of thyroid hormone replacement, she developed elevated creatine kinase levels and elevated transaminases, and clinically, she developed muscle spasms and fatigue. As her levothyroxine dose was increased and she became euthyroid, she had subsequent improvement of both her creatine kinase level and her clinical symptoms. She is being monitored closely in our clinic and continues to improve.
Acknowledgement
Amanda N. Shaw is a fellow in the Division of Pediatric Endocrinology and her contribution included reviewing the patient’s data, revising the data table, and writing the case report submission. Melissa I. Pagan-Pares is a fellow in the Division of Pediatric Endocrinology and her contribution included reviewing the patient’s data and composing the data table.Michael Yafi is an Associate Professor and the Division Director of the Division of Pediatric Endocrinology and his contribution included reviewing the patient’s data and reviewing the case report.
References
- Hunter I, Greene SA, MacDonald TM, Morris AD (2000) Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child 83: 207-210.
- Muir P, Choe MS, Croxson MS (2012) Rapid development of anterotibial compartment syndrome and rhabdomyolysis in a patient with primary hypothyroidism and adrenal insufficiency. Thyroid 22: 651-653.
- Hekimsoy Z, Oktem IK (2005) Serum creatine kinase levels in overt and subclinical hypothyroidism. Endocr Res 31: 171-175.
- Brito JP, Domecq JP, Prutsky G, Malaga G, Young L, et al. (2013) Rhabodomyolysis and myopathy as the only manifestations of severe hypothyroidism secondary to Hashimoto’s thyroiditis. Rev Peru Med Exp Salud Publica 30: 129-132.
- Comak E, Koyun M, Kilicarslan-Akkaya B, Bircan I, Akman S (2011) Severe rhabdomyolysis and acute renal failure in an adolescent with hypothyroidism. Turk J Pediatr 53: 586-589.
- Madhu SV, Jain R, Kant S, Prakash V, Kumar V (2010) Myopathy presenting as a sole manifestation of hypothyroidism. J Assoc Physicians India 58: 569-570.
- Benavides VC, Rivkees SA (2010) Myopathy associated with acute hypothyroidism following radioiodine therapy for Graves Disease in an Adolescent. Int J Pediatr Endocrinol 2010: 717303.
- Klein I, Mantell P, Parker M, Levey GS (1980) Resolution of abnormal muscle enzyme studies in hypothyroidism. Am J Med Sci 279: 159-162.
- Najjar SS, Nachman HS (1965) The kocher-debr’e-S’em’elanigne syndrome; hypothyroidism with muscular “Hypertrophy”. J Pediatr 66: 901-908.
- Monzani F, Caraccio N, Siciliano G, Manca L, Murri L, et al. (1997) Clinical and biochemical features of muscle dysfunction in subclinical hypothyroidism. J Clin Endocrinol Metab 82: 3315-3318.
- Argov Z, Arnold DL (2000) MR spectroscopy and imaging in metabolic myopathies. Neurol Clin 18: 35-52.
- Riggs JE (1990) Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 155: 171-172.
- Hernán Martínez J, Sánchez A, Torres O, Palermo C, Santiago M, et al. (2014) Abrupt onset of muscle dysfunction after treatment for Grave's disease: a case report. Bol Asoc Med P R 106: 40-42.
- Klein I, Mantell P, Parker M, Levey GS (1980) Resolution of abnormal muscle enzyme studies in hypothyroidism. Am J Med Sci 279: 159-162.
- Torres CF, Moxley RT (1990) Hypothyroid neuropathy and myopathy: clinical and electrodiagnostic longitudinal findings. J Neurol 237: 271-274.