Journal of Pediatrics & Child Care
Download PDF
Case Report
*Address for Correspondence: Pi-Lien Hung, Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, No. 123, Ta-Pei Rd, Niao-Song District, Kaohsiung City, Taiwan, Tel: +886-7-7317123 ext. 8718; E-mail: flora1402@adm.cgmh.org.tw
Citation: Hsu MH, Huang LT, Huang SC, Chang YC, Hung PL. Experience of Intravenous Levetiracetam in Acute Repetitive Seizures in Children: Case Series from a Single Tertiary Center. J Pediatrics Child Care. 2015;1(1): 3.
Copyright © 2015 Hung et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 1, Issue: 2
Submission: 25 May 2015 | Accepted: 12 June 2015 | Published: 17 June 2015
Reviewed & Approved by: Dr. Jennifer Cross, Assistant Professor of Clinical Pediatrics, Weill Cornell Medical College, USA
Experience of Intravenous Levetiracetam in Acute Repetitive Seizures in Children: Case Series from a Single Tertiary Center
Mei-Hsin Hsu, Li-Tung Huang, Song-Chei Huang, Ying-Chao Chang and Pi-Lien Hung*
- Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
*Address for Correspondence: Pi-Lien Hung, Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, No. 123, Ta-Pei Rd, Niao-Song District, Kaohsiung City, Taiwan, Tel: +886-7-7317123 ext. 8718; E-mail: flora1402@adm.cgmh.org.tw
Citation: Hsu MH, Huang LT, Huang SC, Chang YC, Hung PL. Experience of Intravenous Levetiracetam in Acute Repetitive Seizures in Children: Case Series from a Single Tertiary Center. J Pediatrics Child Care. 2015;1(1): 3.
Copyright © 2015 Hung et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 1, Issue: 2
Submission: 25 May 2015 | Accepted: 12 June 2015 | Published: 17 June 2015
Reviewed & Approved by: Dr. Jennifer Cross, Assistant Professor of Clinical Pediatrics, Weill Cornell Medical College, USA
Abstract
Background: Intravenous levetiracetam (LEV) has been reported to be well tolerated and effective in treating status epilepticus and acute repetitive seizures in children. Compared with traditional anticonvulsants, LEV has fewer cardiopulmonary depression and sedative effects. We describe four patients belonging to two epileptic syndromes who were refractory to standard treatment protocols and whose seizures were well controlled by intravenous LEV administration.Method: This was a retrospective chart review of in patients who received intravenous LEV as adjunctive therapy to treat acute repeated seizures from January 2013 to December 2013. Information was collected based on age/gender, underlying disease, concomitant anticonvulsants, major seizure types, seizure frequency before and after intravenous LEV treatment, the dose and the duration of LEV usage, and short-term adverse effects during and after infusion. The paired t test was used to compare seizure frequencies before and after intravenous LEV.
Results: Four patients (mean age 6.3 ± 5.53 years) who received intravenous LEV had acute repetitive seizures, and were clinically diagnosed as intractable epilepsy. Two patients had acute encephalitis with refractory repetitive partial seizures (AERRPS) and the other two patients had infantile spasms (IS). Their seizures were controlled by three or more oral antiepileptic agents. The mean loading dose of LEV was 17.9 mg/kg/dose (range 11.6 to 22.7 mg/kg/dose), and the mean duration of LEV usage was 6.5 days (4 to 11 days). The seizure frequency before intravenous LEV treatment was 9.25 fits per day (5-20 fits per day), which decreased after intravenous LEV treatment to 2.7 fits per day (1-8 fits per day). All of the four patients had favorable responses in seizure frequency reduction after the administration of intravenous LEV. None of the patients reported any side effects after LEV treatment, and all four patients received LEV as oral adjunctive therapy after discharge.
Conclusions: Our results suggest that intravenous LEV can successfully reduce the frequency of seizures in patients with epilepsy syndromes leading to refractory epilepsy. Physicians commonly face the dilemma of controlling seizures and cardiopulmonary depression due to anticonvulsant therapy. Intravenous LEV was well tolerated without any side effects in all of our patients, and therefore we suggest that intravenous LEV should be considered to control repetitive clustering seizures before the use of other traditional intravenous anticonvulsants due to the high efficacy and scanty side effects. However, further studies with a larger number of patients are needed to confirm our findings.
Keywords
Intravenous levetiracetam; Acute repetitive seizures; AERRPS; Infantile spasmIntroduction
Intravenous LEV is approved by the United States Food and Drug Administration as an alternative therapy to treat status epilepticus and acute repetitive seizures in children 4 years of age and older, for whom it has been shown to be safe and effective [1,2]. The safety and efficacy of LEV have also been reported in adults with epilepsy, with similar findings reported in children, including neonates [1]. Intravenous LEV is widely used to treat children with epilepsy of various etiologies including perinatal hypoxic ischemic encephalopathy (HIE), post-head injury, and congenital brain malformation-metabolic encephalopathy, infection, and brain tumors [2]. However, few studies have reported AERRPS or IS. Herein, we report our experience with the use of intravenous LEV in children from a tertiary center hospital in Taiwan.Patients and Methods
We retrospectively reviewed the charts of patients younger than 18 years admitted to Kaohsiung Chang Memorial Medical Center from January 2013 to December 2013. Four patients were enrolled, all of whom presented with acute repetitive seizures refractory to multiple anticonvulsants information was collected on age/gender, underlying diseases, concomitant anticonvulsants, seizure frequency before and after intravenous LEV treatment, the dose and the duration of LEV use, and short-term adverse effects during and after infusion.Acute repetitive seizures were defined as repeated myoclonic, clonic or tonic-clonic seizures (each seizure lasting less than 5 min with recovery of consciousness between each seizure) that had persisted for at least 30 min irrespective of whether they had been treated with any emergency medication [2]. Intravenous LEV was administered in a single 15 min infusion every 12 h until the seizures were controlled.
The side effects of LEV including behavioral abnormalities, psychotic symptoms and serious dermatological reactions were recorded by caring nursing staff three times per day. We calculate the mean seizure fits during LEV administration. In addition, we reviewed chart retrospectively by calculation the mean daily seizure fits with traditional AEDs.
Statistical analyses were done using SPSS 17.0 statistical software (SPSS Inc.). Paired t test was used to compare seizure frequencies before and after intravenous LEV treatment.
Results
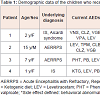
The mean age of the four patients was 6.3 ± 5.53 years. The mean loading dose of LEV was 17.9 mg/kg/dose (range 11.6 to 22.7 mg/kg/dose), and the mean duration of LEV administration was 6.5 days (4 to 11 days). The seizure frequency before intravenous LEV treatment was 9.25 fits per day (5-20 fits per day), which decreased to 2.7 fits per day (1-8 fits per day) after intravenous LEV treatment. All the patients had a favorable response in reducing the frequency of seizures after intravenous LEV treatment, and none reported any side effects after LEV administration. All four patients received oral LEV after discharge. The demographic data of these four patients are listed in Table 1.Patient 1 was a 4-year-old girl born at 36 weeks of gestational age after a cesarean section. Her clinical diagnosis was Aicardi syndrome presenting as corpus callosum agenesis accompanied with retinal lacuna and coloboma. The first seizure attack was IS at the age of 3 months which was poorly responsive to vigabatrin (VGB), however, the seizure pattern transformed into clustering myoclonic epilepsy when she was 1 year of age. Her seizures were poor controlled despite the use of adjuvant anticonvulsants. She was admitted repeatedly with acute repetitive seizure attacks. The mean seizure frequency was 6 times per day. She received intravenous LEV (22 mg/kg over 15 min) for 4 days, and the seizure frequency decreased to around once per day thereafter.
Case 2
Patient 2 was a 14-year-old boy who was born after an uneventful pregnancy. He experienced AERRPS at the age of 12 years. He had been prescribed many anticonvulsants including VGB, LEV, clonazepam, clobazam, topiramate (TPM) and a ketogenic diet. When he was 14 years and 8 months old, he was hospitalized in the pediatric intensive care unit due to status epilepticus. His maximum seizure frequency reached up to 50 times a day. He underwent intubation with continuous infusion of intravenous midazolam. Intravenous LEV (11 mg/kg over 15 min) was applied for 11 days, and finally the frequency of seizures decreased to 8 times a day. His seizure type shifted from major faciobrachial dystonic seizures to minor eye blinking.
Case 3
Patient 3 was a 9-year-old girl-born after an uneventful pregnancy. Her seizure types included eye staring, drop attack, generalized tonic-clonic and versive seizures with secondary generalization. Various anticonvulsants including phenobarbital (PB) LEV, phenytoin and a ketogenic diet were applied. Intravenous LEV (16 mg/kg in 15 min) was administered for 5 days during acute repetitive seizures, and the frequency of seizures decreased from 5 times per day to once per day.
Case 4
Patient 4 was a 2-year-old girl born at 35 weeks of gestational age after a cesarean section. Cryptogenic infantile spasm was diagnosed, and she was then given adrenocorticotropic hormone treatment. Her seizure type transformed into tonic, dialeptic and myoclonic seizures in clusters. She received several anticonvulsants such as PB and LEV, and a ketogenic diet. She once suffered an exacerbation of her seizures with repetitive tonic spasm at 1 year old. She received intravenous LEV (20 mg/kg over 15 min) for 6 days, and the seizure frequency declined from 6 times per day to once per day. She was discharged uneventfully with oral LEV without any side effects.
Discussion
Studies on the use of intravenous LEV for acute repetitive seizures in pediatric patients are limited. We reported our experience with the use of intravenous LEV in patients with two groups of intractable epilepsy, IS and AERRPS. We found beneficial effects of intravenous LEV as adjunctive therapy in both groups, with significant reductions in the average frequency of seizures after treatment.Rosser et al. reported seizures in 92% of patients with Aicardi syndrome, and that these seizures occurred daily in 67% of the patients [3]. Other alternative treatments including valproic acid, nitrazepam, pyridoxine, topiramate, zonisamide, lamotrigine, levetiracetam, felbamate, ganaxolone, liposteroid, thyrotropin releasing hormone, intravenous immunoglobulin and a ketogenic diet have also been reported to be beneficial in treating IS LEV has also been reported to be effective in controlling IS [4]. In our case 1 and case 4, oral LEV was chosen as an add-on therapy for IS, however the effects were limited. Previous studies on the use of intravenous LEV in IS with acute exacerbations are scanty. The findings in our cases revealed that intravenous LEV was a good choice for acute seizure attacks of IS and that it is easy to shift to the oral form for use at home. To the best of our knowledge, this is the first report to use intravenous LEV to control refractory epilepsy successfully in patients with Aicardi syndrome with IS.
The main clinical features of AERRPS are refractory and repetitive partial seizures accompanied by fever. Patients with AERRPS are commonly refractory to conventional anticonvulsants and can only be successfully controlled with the administration of high dose intravenous barbiturates [5]. However, the side effects of barbiturates on cognition deterioration and sedation are unavoidable. One of our patients (case 2) developed a phenobarbital allergic skin rash. We found that intravenous LEV was very successful taking place of PB in patients with repetitive seizures in the acute phase of AERRPS as adjuvant therapy.
In summary, our findings show that intravenous LEV was safe and effective in treating patients with two intractable epilepsy syndromes: infantile spasms and AERRPS. However, our sample size is too small to reach a definitive conclusion, and studies with more patients are necessary to confirm our findings.
References
- Weinstock A, Ruiz M, Gerard D, Toublanc N, Stockis A, et al. (2013) Prospective open-label, single-arm, multicenter, safety, tolerability, and pharmacokinetic studies of intravenous levetiracetam in children with epilepsy. J Child Neurol 28: 1423-1429.
- McTague A, Kneen R, Kumar R, Spinty S, Appleton R (2012) Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children's hospital. Seizure 21: 529-534.
- Rosser TL, Acosta MT, Packer RJ (2002) Aicardi syndrome: spectrum of disease and long-term prognosis in 77 females. Pediatr Neurol 27: 343-346.
- Mikati MA, El Banna D, Sinno D, Mroueh S (2008) Response of infantile spasms to levetiracetam. Neurology 70: 574-575.
- Sakuma H, Awaya Y, Shiomi M, Yamanouchi H, Takahashi Y, et al. (2010) Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand 121: 251-256.