Journal of Proteomics & Computational Biology
Download PDF
Research Article
*Address for Correspondence: Jun-Ping Kou, Professor, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, 639 Longmian Road, Nanjing, 211198, P.R. China, Tel & Fax: 86-25-86185158; E-mail: junpingkou@163.com; Bo-Yang Yu, Professor, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, 639 Longmian Road, Nanjing, 211198, P.R. China, Tel & Fax: 86-25-86185158; E-mail: boyangyu59@163.com
Citation: Tan YS, Li F, Lv YN, Zhai KF, Chai CZ, et al. Study on the Multi-targets Mechanism of YiQiFuMai Powder Injection on Cardio-Cerebral Ischemic Diseases Based on Network Pharmacology. J Proteomics Computational Biol. 2014;1(1): 9.
Copyright © 2014 Tan YS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Proteomics & Computational Biology | Volume: 1, Issue: 1
Submission: 30 August, 2014 | Accepted: 29 October, 2014 | Published: 04 November, 2014
The first part of database: All the known targets were derived from the literatures of PubMed Central of the NCBI database (http:// www.ncbi.nlm.nih.gov/pubmed/). The search terms were specified as “compound name (28 compounds identified in YQFM [26]) and key words of diseases”. The key words of diseases were standardized by Mesh term of NCBI database (http://www.ncbi.nlm.nih.gov/mesh/) including: heart or cardio ischemia; cerebral ischemia or cerebral infarction or cerebral and infarction or cerebral and ischemia or brain ischemia or brain and ischemia or stroke. From the obtained literature abstracts, all the proteins or genes conform to the Mesh terms of diseases were text mined for the construction of the network.
Target-Pathway interaction network
Study on the Multi-targets Mechanism of YiQiFuMai Powder Injection on Cardio-Cerebral Ischemic Diseases Based on Network Pharmacology
Yi-Sha Tan, Fang Li, Yan-Ni Lv, Ke-Feng Zhai, Cheng-Zhi Chai, Jun-Ping Kou* and Bo-Yang Yu*
- Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, Nanjing, P.R. China
*Address for Correspondence: Jun-Ping Kou, Professor, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, 639 Longmian Road, Nanjing, 211198, P.R. China, Tel & Fax: 86-25-86185158; E-mail: junpingkou@163.com; Bo-Yang Yu, Professor, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, 639 Longmian Road, Nanjing, 211198, P.R. China, Tel & Fax: 86-25-86185158; E-mail: boyangyu59@163.com
Citation: Tan YS, Li F, Lv YN, Zhai KF, Chai CZ, et al. Study on the Multi-targets Mechanism of YiQiFuMai Powder Injection on Cardio-Cerebral Ischemic Diseases Based on Network Pharmacology. J Proteomics Computational Biol. 2014;1(1): 9.
Copyright © 2014 Tan YS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Proteomics & Computational Biology | Volume: 1, Issue: 1
Submission: 30 August, 2014 | Accepted: 29 October, 2014 | Published: 04 November, 2014
Abstract
YiQiFuMai Powder Injection is a well-known traditional Chinese medicine formula that has been used extensively in clinical treatment of cardio-cerebral ischemic diseases in China. However, the mechanisms underlying its clinical efficacy remain unknown. In this study, a network pharmacology approach was employed to identify the YiQiFuMai Powder Injection’s potential pathways and targets against cardio-cerebral ischemia. The target-pathway interaction network clustered the signaling pathways based on high degree nodes of the drug-target network. The potential protein targets presented in the highly scored clustered pathways were the key network hubs and concentrated on one or limited functional signaling pathways amenable to experimental verification. Twelve main functional annotation clusters and main signaling pathways for YiQiFuMai Powder Injection were established by Biocarta analysis, including the NF-κB signaling pathway, the MAPKinase signaling pathway and the mTOR-signaling pathway and so on. YiQiFuMai Powder Injection is hypothesized to target multiple proteins with a high degree and betweenness of network. In addition, the most related pathways were also confirmed in tumor necrosis factor-alpha (TNF-α) induced human vascular endothelial cell line EA.hy926 by Western blotting. This study elucidates the systematic network and pathway analysis of multitargets in YiQiFuMai Powder Injection. The results provide the possible mechanisms for its mode of action against cardio-cerebral ischemic diseases and may also reveal new clues for its potential application in the inflammatory diseases or tumors.Keywords
YiQiFuMai Powder Injection; Cardio-cerebral ischemic diseases; Network pharmacology; Target-pathway interaction networkIntroduction
Traditional Chinese medicines (TCMs) are attracting increasing attention due to their reliable curative efficacy and long history of clinical trials [1]. Chinese people have used TCMs for more than two thousand years and continue to do so. During the past half century, the role of TCMs in preventing and treating infectious diseases such as hepatitis, acquired immune deficiency syndrome, and chronic diseases such as cancer, hypertension, diabetes and cardiovascular diseases has become widely accepted [2-7]. Its effectiveness is not questioned and, in fact, its continuous use is commonly presented as proof that it works. In China, TCMs practitioners are using a wide variety of techniques in an effort to treat diseases and promote public health and recently these techniques have drawn much attention in the Western world [8,9]. TCMs formulae are a complex mixture of hundreds or even thousands of chemical components with multiple potential targets. However, the unknown molecular mechanisms are obstacles for their further development and internationalization. Network pharmacology and systems biology are ideal for probing the characteristics of multi-components, multi-targets and multipathways of TCMs, and has facilitated the understanding of the interactions of genes and proteins on a holistic level, thus applying the new methods for uncovering the molecular mechanisms related to the therapeutic efficacy of TCMs [10-12].Drug-target association networks are typically used to elucidate a drug’s mechanism of action [13]. Meanwhile, drug-drug network and protein-disease network were also employed to discover the association between diseases and target proteins [14]. A theoretical algorithm, such as CIPHER, was proposed to identify the pathways and indicate the network-based computational framework [15-17]. Network analysis has become a cornerstone for illuminating TCM mechanisms. Identifying the potential key targets and pathways for the drugs against the diseases by computational methods is an efficient and time-saving approach.
The YiQiFuMai Powder Injection (YQFM) is a traditional Chinese medicine for the treatment of cardio-cerebral ischemia diseases [18]. It has been re-developed based on a well-known TCM formula, Sheng-maisan, which is composed of Ginseng Radix Et Rhizoma Rubra, Ophiopogonis Radix and Schisandrae Chinensis Fructus. Considerable clinical trials have demonstrated that YQFM can be used for the treatment of cardiovascular and cerebrovascular diseases with better efficacy and fewer side effects compared with standard medical treatments [19]. It has also been suggested that the three herbs of YQFM have beneficial effects in the treatment of cardiovascular diseases and the regulation of blood vessel function [20-22]. Although both clinical and experimental studies demonstrate the prophylactic and/or therapeutic effects of YQFM, the key targets and related signaling pathways against the cardio-cerebral ischemia diseases remain unclear. When studying the YQFM pharmacological mechanism, one major challenge is that TCMs have traditionally been administered as an integrated prescription for treating diseases, which implicates a complex and highly dynamic ingredientingredient interaction network underlying the overall clinical effect [23]. Network pharmacology could evaluate the pharmacological effect as a whole, facilitate understanding and predict key YQFM targets and pathways. The predictive results might suggest some clues for further experimental verification. The combined method of network pharmacology prediction and experimental verification are preferred simultaneously to verify the targets and signal pathways of TCMs.
To obtain a better understanding of how YQFM affects biological processes, the target-pathway interaction network was used to interpret the relationship of the YQFM targets and pathways. The compounds in YQFM simultaneously targeted multiple key proteins with a high degree of specificity and with functional contributions to the main pathways, which resulted in the biological system attain a new equilibrium against the disease. The endothelium plays an essential role in vascular tone regulation, leukocyte adhesion, platelet activity and thrombosis, all of which are involved in cardio-cerebral ischemia diseases development [24]. Endothelial cell dysfunction induced by TNF-α, such as endothelial activation and vascular inflammation, also plays a critical role in the cardio-cerebral vascular disease pathogenesis [25]. The most related pathways were verified in EA. hy926 endothelial cells (ECs) stimulated by TNF-α. We expected our findings would shed light on the pharmacological mechanism of YQFM against cardio-cerebral ischemia diseases, and further advance mechanism exploration of the other complex TCM formulae.
Materials and Methods
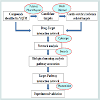
Construction of the protein databaseThe protein database was composed of two parts. In this part, we will introduce how to construct the database. Figure 1 depicts the flowchart of this study.
The first part of database: All the known targets were derived from the literatures of PubMed Central of the NCBI database (http:// www.ncbi.nlm.nih.gov/pubmed/). The search terms were specified as “compound name (28 compounds identified in YQFM [26]) and key words of diseases”. The key words of diseases were standardized by Mesh term of NCBI database (http://www.ncbi.nlm.nih.gov/mesh/) including: heart or cardio ischemia; cerebral ischemia or cerebral infarction or cerebral and infarction or cerebral and ischemia or brain ischemia or brain and ischemia or stroke. From the obtained literature abstracts, all the proteins or genes conform to the Mesh terms of diseases were text mined for the construction of the network.
The second part of database: This part included high affinity and novel related proteins for the compounds. The targets were derived from PharmMapper database (http://59.78.96.61/pharmmapper/),which was designed to identify potential target candidates for the given small molecules via a reverse pharmacophore mapping approach. PharmMapper is backed up by a large, in-house repertoire of pharmacophore database covering over 7,000 receptor-based pharmacophore models, and 459 of which are human protein targets [27]. In this work, structures of YQFM compounds were constructed using Sybyl 6.9 (Tripos, Inc) and were applied to all hydrogens and Gastiger-Huckel charges in Sybyl 6.9 in the tripos force field under an implicit solvent environment. The minimization steps included 1000 cycles of steepest descent until satisfying the convergent threshold of 0.05 kal-1•Å-1. These molecules were saved to a mol format for PharmMapper Server analysis. The target set was only limited to the human targets and all parameters were kept as default. Finally, the top ranking 100 reserved matched proteins were determined as the targets.
The combined two parts of the protein database were filtered based on their relationship with the cardio cerebral ischemia in the PubMed central of NCBI database, with the search terms “protein/ gene name AND key words of diseases”. The proteins/genes that have relationships with the diseases were retained in the database. These proteins or genes were standardized as official name in the NCBI database using GEO files function (http://www.ncbi.nlm.nih.gov/ geoprofiles/). The search terms were specified as “protein/gene name AND Homo sapiens”.
Construction and analysis of the network
Drug-Target interaction network: In this work, Cytoscape 2.8.3 was developed for network reconstruction and visualization [28,29]. Cytoscape is a desktop Java application available for download from http://cytoscape.org. The drug-target network was constructed by linking the compounds and proteins if they share one or more target proteins. We analyzed the degree and betweenness of the compounds in the network using the Centiscape module. Those nodes which have a higher degree (≥ 3) and larger betweenness (≥ 1000) of nodes were hypothesized to be the most important [10,30].
Target-Pathway interaction network: Based on the high degree nodes from drug-target interaction network, we excluded the proteins with little biological meanings in functional annotation chart by Biocarta analysis [31]. Pathway analysis plays an essential role in discovering biological processes that input list genes participate in. So, those remained higher degree nodes sets were identified based on pathway interaction network collected from Biocarta, including cellular processes, metabolism and diseases, such as Biocarta, KEGG and GeneGo [32]. Then we grouped the functional annotation clusters into groups of similar annotations according to the enrichment scores using Biocarta functional annotation tool, which used a novel algorithm to measure relationships among the annotation terms based on the degree of their co-association genes. We took full advantage of the well known Biocarta pathways and displayed genes to facilitate biological interpretation in a network context. The clustered pathway required enriched functions for genes when Fisher’s exact test p-value ≤ 0.01. The functional annotation cluster was constructed with the pathways representing similar pathways according to the enrichment scores.
Cell culture and drug treatments: The endothelial cell line EA.hy926 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies, California, USA) supplemented with 10% fetal bovine serum (Wisent, Nangjing, China), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were plated at an appropriate density to give drug treatments when grown to anastomose. Before the experiments, EA.hy926 ECs were cultured in a supplement-free medium for 1 h. The cells were treated with 25-400 μg/mL YQFM (Tasly, Tianjin, China) for 12 h before the treatment with 10 ng/mL TNF-α (Sigma, St. Louis, MO, USA) for 30 min. The control groups were treated without YQFM/TNF-α.
Western blot analysis: For Western immune blotting, EA.hy926 endothelial cells (ECs) were lysed at indicated time points with 40 μl RIPA ice-cold lysis buffer (Vazyme Biotech, Nanjing, China) supplemented with protease inhibitor (Vazyme Biotech, Nanjing, China) for 30 min. As reported [29], the whole cell lysates were centrifuged at 12,000 rpm for 10 min at 4°C and protein concentrations were analyzed by the BCA (Shanghai Beyotime Institute of Biotechnology, China) method. Equal amounts of proteins (30 μg) were separated using 12.5% SDS-PAGE and transferred to a PVDF membrane (Millipore Corporation, Billerica, MA, USA). The membranes were blocked with 3% BSA in TBS/T and stained with primary antibodies (1:1000) and anti-GAPDH (1:8000) overnight at 4°C. Membranes were then probed with peroxidase conjugated secondary antibody at a 1:8000 dilution. The antigen-antibody complexes were detected with an ECL reagent (Vazyme Biotech, Nanjing, China) and visualized by Quantity One software 4.6.2. Primary antibodies used for Western blotting were rabbit antibodies (Cell Signaling Technology, Boston, MA, USA). Secondary antibodies used were peroxidase conjugated goat anti-rabbit antibodies and goat anti-mouse antibodies (Bioworld Technology, Nanjing, China).
Statistical analysis: All experiments were performed in triplicate and data were expressed as the mean ± standard deviation. Statistical significance was determined using a one-way ANOVA, followed by Student’s two-tailed t-test for comparison between two groups andDunnett’s test when the data involved three or more groups. P < 0.05 was defined as significant.
Results
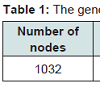
Drug-Target interaction networkThe general network properties of the drug-target network were shown in Table 1 and Figure 2. Apparently, the drug-target network was dense and the YQFM targeted a great deal of proteins. This drugtarget interaction network consisted of 1032 nodes and 2683 edges, with 28 compounds and 1004 targets. The 28 compounds displayed a total of 2683 interactions with their targets. As shown in Figure 2, most compounds targeted only a few candidate targets while some had many targets. 20(R)-ginsenoside Rg3 and 20(S)-ginsenoside Rg3 were the most highly connected nodes with 280 edges and 278 edges, followed by ginsenoside Rb1 with 258 targets, ginsenoside Rg1 with 249 targets and schizandrin with 204 targets, suggesting that they were the hubs in the interaction network and therefore had an important role in the network functionality. However, ginsenoside Rk3 had the least number of targets (only twenty-eight), followed by ginsenoside F4, Rg6, Rk1 and Notoginsenoside R2.
Target-Pathway interaction network
The twelve main functional annotation clusters ranked by the Biocarta functional annotation cluster tool are shown in Figure 3 and Table 2. A functional annotation cluster classified the groups of similar annotations according to the enrichment scores. The relevant protein terms in the highest scored cluster were associated with the activation of transcription nucleus factor signaling pathway, including NF-κB activation by non-typeable Haemophilus influenzae, influence of Ras and Rho proteins on G1 to S Transition, NF-κB signaling pathway and acetylation and deacetylation of RelA in the nucleus pathways, respectively. The second main pathways related to transcription factor CREB and its extracellular signals. The rest clusters on comprised pathways related to apoptosis, MAPK, cytokines and the mTOR signaling pathway. According to the enrichment scores, the NF-κB signaling pathway was the most likely candidate for YQFM action against cardio-cerebral ischemia diseases.
Evaluation of the important proteins
The 118 key proteins (Table S1) in the drug-target interaction network which have a higher degree (≥4) and larger betweenness (≥1750) were selected as a key protein group, including IKK alpha, p65, toll like receptor 4, IL-1, IL-8, all of which were correlated with NF-κB, oxidative stress and cytokine signaling pathways. We identified key proteins including tyrosine kinase, transcription factor CREB related proteins, ribosomal protein, phosphoinositide 3 kinase, nerve growth factor, calcium-dependent protein kinase, colony stimulating factor and E2F1 transcription factors, which were involved in transcription enhancement factor signaling pathway, cell cyclin, neutrophils, B cell activation and transcription factor E2F1 signaling pathways. The pathways contained in the highest scored clusters might have the most potential interaction pathways where the YQFM exerted its effects against the cardio-cerebral ischemia diseases. Analysis based on functional annotation clusters represented important proteins in the signaling pathways. Based on these proteins, we constructed the YQFM key proteins-signaling pathway network (Figure 4). These findings may provide the basis for further study on the YQFM mechanism for preventing and treating cardio-cerebral ischemia diseases.
To verify the results derived using the target-pathway interaction network, we assessed whether the YQFM protective effect was mediated by the activation of NF-κB signaling pathway (Figure 5). Western blot results showed that phospho-NF-κB/p65 and phospho-IKKβ were up-regulated by treatment of 10 ng/mL TNF-α for 30 min, while pretreatment with 25-400 μg/mL YQFM for 12 h could clearly inhibit the expression and phosphorylation of NF-κB/p65 and IKKβ. With increasing YQFM concentration, the expression of phospho-NF-κB/p65 and phospho-IKKβ decreased.
Figure 5: Effects of YQFM on the expression of IKKβ and NF-κB p65 induced by TNF-α in EA. hy926 endothelial cells. (A) Protein expressions of IKKβ and phospho-IKKβ using GAPDH as the loading control. (B) Protein expressions of NF-κB p65 and phospho-p65 using GAPDH as the loading control. EA. hy926 endothelial cells were pretreated with YQFM at the concentration of 25-400 μg/mL for 12 h and then exposed to 10 ng/mL TNF-α for 30 min. The cell lysates were then subjected to western blotting assay. The band intensities were assessed by scanning densitometry. Results were obtained from three independent experiments and were expressed as mean ± SD. ##P < 0.01 vs. Control group without TNF-α, *P < 0.05, **P < 0.01 vs. group treated with TNF-α alone.
Discussion
As we all know, the complexity of ingredients, unknown targets and the mechanism of action underlying restrict the development of TCMs in the world. Recently, the application of network theory has become a useful tool to visualize and analyze the interaction data to capture the complexity in a simple, compact, and illustrative manner [33]. We proposed drug-target interaction network and targetpathway interaction network to discern the targets and potential pathways of YQFM active components to decipher its mechanism in treating cardio-cerebral ischemia diseases.To construct the protein database, we used twenty-eight compounds identified in YQFM in our laboratory as searching terms [26]. Unfortunately, we did not identify the components in phiopogonis Radix due to their low content and the instrument’s resolution. However, we could not ignore the importance of Ophiopogonis Radix in YQFM and we are currently optimizing the analytical conditions and investigating the role of Ophiopogonis Radix.
In the drug-target network, the most fundamental characteristic of a node is its degree, which is defined as the number of edges incident to the node. Another key property of network nodes is the betweenness, the capacity to be located in the shortest communication paths between different pairs of nodes in the network [34]. The degree and the betweenness are the node topological and central indexes, corresponding to the evaluation of the regulatory relevance of the nodes. In this network, nodes that were scored with a very high degree and betweenness were suggested to have a central regulatory role. Generally, those nodes that have higher degree and larger betweenness in drug-target network would be more important [35]. In this network, of all 28 compounds, the top four key compounds with a high degree and betweenness were ginsenoside Rg3, ginsenoside Rg1, ginsenoside Rb1 and schizandrin, which have widely been reported to play a role in the protection of the heart and brain from hypoxia and oxidative injury [36-38]. If these nodes were removed, the network would be unbalanced. Surely, compounds with a higher degree and betweenness are thought to be key players in YQFM, which implied that ginsenoside Rg3, ginsenoside Rb1, ginsenoside Rg1 and schizandrin were the most important YQFM components for the treatment of cardio-cerebral ischemic diseases and also provided a clue for further effective ingredient combination identification.
We grouped the functional annotation clusters into twelve groups of similar annotations based on the enrichment scores. The proteins in each clusters would be highly relevant to each other. The most important protein terms in the highest scored cluster are associated with the NF-κB signaling pathway. The transcription factor NF-κB plays a key role in the nervous system cell survival and acts as a key regulator of the inflammatory process [39]. Meanwhile, transient focal cerebral ischemia induced activation of NF-κB in neurons and neuroprotective antioxidants may inhibit neuronal death by preventing NF-κB activation [40]. Inhibiting the activity of NF-κB also protected the heart against myocardial ischemia reperfusion injury by reducing lipid peroxidation damage and significantly attenuated Beclin 1 expression and autophagy in the cardiac area at risk for ischemia [41,42]. We have already confirmed that some compounds in YQFM, such as ginsenoside Rg1, protected against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation [43]. The YQFM components were most likely to be made up of a great and interlinked network so that these building blocks could have function as a whole. In the present work, we observed the effect of YQFM on the expression and activation of NF-κB p65 and IKKβ in TNF-α induced EA.hy926 ECs to verify the predictive pathway based on target-pathway interaction network. Western blotting results confirmed the predicted results and provided some explanations for the molecular mechanism of the YQFM for cardio-cerebral ischemia diseases.
Other underlying YQFM signaling pathways were also found to be associated with cardio-cerebral ischemia diseases. The activation of transcription factor CREB and its extracellular signals led to the expression of neuroprotective molecules, such as the anti-apoptotic protein Bcl-2, and contributed to neuronal survival after ischemia [44]. Multiple anti-apoptotic pathways have been reported to be closely related with stroke, and recent findings indicated that apoptosis occurred in neuronal degeneration after ischemic brain injury in animal stroke models [45]. Granulocyte adhesion participated in the process of acute ischemic stroke [46] and cell cycle signaling pathway was also involved in the cardiac conduction [47]. Moreover, granulocytes and B cells were stimulating factors of endothelial cells Other underlying YQFM signaling pathways were also found tobe associated with cardio-cerebral ischemia diseases. The activation of transcription factor CREB and its extracellular signals led to the expression of neuroprotective molecules, such as the anti-apoptotic protein Bcl-2, and contributed to neuronal survival after ischemia [44]. Multiple anti-apoptotic pathways have been reported to be closely related with stroke, and recent findings indicated that apoptosis occurred in neuronal degeneration after ischemic brain injury in animal stroke models [45]. Granulocyte adhesion participated in the process of acute ischemic stroke [46] and cell cycle signaling pathway was also involved in the cardiac conduction [47]. Moreover, granulocytes and B cells were stimulating factors of endothelial cells
in chronic ischemic heart disease [48,49], and the E2F1 pathwaywas involved in apoptosis [50]. These predicted signaling pathwayswere associated with the YQFM signaling pathways for the treatmentof cardio-cerebral ischemia diseases. These findings indicatedthat YQFMmight exert multiple pharmacological functions onthe signaling pathways. Further study is necessary to confirm whether YQFM exerts its effects via these essential targets in related experiments.
We found that YQFM interacted with cytokine signaling pathways. It has been reported that IL-1 participated in the inflammatory process, while IL-6 played a pivotal role in the host defense against pathogens and acute stress [51,52]. Anti-inflammatory cytokines IL-4 and IL-10 were indicated to have an influence in the neurodegenerative process of Creutzfeldt-Jakob disease [53]. Moreover, the level of caspase-3 expression was higher in LN metastases or primary tumors [54]. Seminal Bax was significantly increased and seminal Bcl-2 was significantly decreased in men associated with varicocele [55]. Additionally, misfolded and aggregated TTRs could decrease overall cellular antioxidant capacity in Schwannoma cells and lead to agerelated diseases such as senile systemic amyloidosis and familial amyloid polyneuropathy [56]. Our findings also indicated that YQFM may be useful for treating cytokines-mediated diseases, which needs further confirmation.
Conclusion
In this study, we used network pharmacology to predict potential molecular drug mechanisms. From this network, we inferred the links between the components of YQFM and cardio-cerebral ischemia diseases through the molecular targets and several key signaling pathways. Such results were partially confirmed by experimental verification in the cell model. The findings demonstrated that the network-based approaches were of great importance for elucidating and predicting the inter-relationship between drug interventions and complex diseases through the network target paradigm estimating. Based on these combined methods, we identified and verified that the NF-κB signaling pathway played an essential role in the YQFM mechanism targeting cardio-cerebral ischemia diseases. Future studies are needed to test the effects of bioactive ingredients on other pathways and their interactions. Our findings could also pave the way for further mechanism exploration of other TCM formulae with multiple bioactive components.Acknowledgements
This research work was supported by the National Natural Science Foundation of China (No. 81274004), 2011’ Program for Excellent Scientific and Technological Innovation Team of Jiangsu Higher Education, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Graduate Student Scientific Research Innovation Plan of Jiangsu Higher Education Institutions (CXLX13_30).References
- Cheung F (2011) TCM: made in China. Nature 480: S82-S83.
- Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ, et al. (2013) Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discov Ther 7: 212-224.
- Liu ZB, Wang X, Liu HJ, Jin YT, Guo HJ, et al. (2013) Treatment of acquired immunodeficiency syndrome with Chinese medicine in China: opportunity, advancement and challenges. Chin J Integr Med 19: 563-567.
- Lee YW, Chen TL, Shih YR, Tsai CL, Chang CC, et al. (2014) Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer. Cancer 120: 1338-1344.
- Xiong X, Yang X, Liu W, Chu F, Wang P, et al. (2013) Trends in the treatment of hypertension from the perspective of traditional Chinese medicine. Evid Based Complement Alternat Med 2013: 275279.
- Wei J, Wu R, Zhao D (2013) Analysis on traditional Chinese medicine syndrome elements and relevant factors for senile diabetes. J Tradit Chin Med 33: 473-478.
- Zhao ZQ, Mao JY, Wang XL, Hou YZ (2013) Application and evaluation of Chinese medicine in treatment of chronic heart failure. Zhongguo Zhong Xi Yi Jie He Za Zhi 33: 1701-1704.
- Dong J (2013) The relationship between traditional Chinese medicine and modern medicine. Evid-Based Complement Alternat Med 2013: 153148.
- Chen X, Pei L, Lu J (2013) Filling the gap between traditional Chinese medicine and modern medicine, are we heading to the right direction? Complement Ther Med 21: 272-275.
- Barabasi AL, Oltvai ZN (2004) Network biology: understanding the cell's functional organization. Nat Rev Genet 5: 101-113.
- Verpoorte R, Choi YH, Kim HK (2005) Ethnopharmacology and systems biology: a perfect holistic match. J Ethnopharmacol 100: 53-56.
- Li S, Zhang B, Zhang N (2011) Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst Biol 5: S10.
- Gu J, Zhang H, Chen L, Xu S, Yuan G, et al. (2011) Drug-target network and polypharmacology studies of a Traditional Chinese Medicine for type II diabetes mellitus. Comput Biol Chem 35: 293-297.
- Jiang M, Chen Y, Zhang Y, Chen L, Zhang N, et al. (2013) Identification of hepatocellular carcinoma related genes with k-th shortest paths in a protein-protein interaction network. Mol Biosyst 9: 2720-2728.
- Liu KQ, Liu ZP, Hao JK, Chen L, Zhao XM (2012) Identifying dysregulated pathways in cancers from pathway interaction networks. BMC Bioinformatics 13: 126.
- Zhao SW, Li S (2010) Network-based relating pharmacological and genomic spaces for drug target identification. PLoS One 5: e11764.
- Wu X, Jiang R, Zhang MQ, Li S (2008) Network-based global inference of human disease genes. Mol Syst Biol 4: 189.
- Xing L, Jiang M, Dong L, Gao J, Hou Y, et al. (2013) Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J Ethnopharmacol 148: 239-245.
- Xu LH (2009) Therapeutic effect of YiQiFuMai in patients with chronic congestive heart failure. Medical Information 22: 2418-2419.
- Lee CH, Kim JH (2014) A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res 38: 161-166.
- Lan S, Yi F, Shuang L, Wang CJ, Zheng XW (2013) Chemical constituents from the fibrous root of Ophiopogon japonicus, and their effect on tube formation in human myocardial microvascular endothelial cells. Fitoterapia 85: 57-63.
- Panossian A, Wikman G (2008) Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol 118: 183-212.
- Zhao J, Jiang P, Zhang WD (2010) Molecular networks for the study of TCM Pharmacology. Brief Bioinform 11: 417-430.
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673-2678.
- Jin HM, Liu QH, Cao X, Wu ZH, Zhang GP, et al. (2000) Dysfunction of microvascular endothelial cells induced by tumor necrosis factor (TNFalpha): cellular and molecular mechanism. Clin Hemorheol Micro 23: 109-112.
- Wang YQ, Liu CH, Zhang JQ, Zhu DN, Yu BY (2013) Protective effects and active ingredients of Yi-Qi-Fu-Mai sterile powder against myocardial oxidative damage in mice. J Pharmacol Sci 122: 17-27.
- Liu X, Ouyang S, Yu B, Liu Y, Huang K, et al. (2010) PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res 38: W609-W614.
- Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, et al. (2007) Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2: 2366-2382.
- Lv YN, Li SX, Zhai KF, Kou JP, Yu BY (2014) Network pharmacology-based prediction and verification of the molecular targets and pathways for schizandrin against cerebrovascular disease. Chin J Nat Med 12: 251-258.
- Opsahl T, Agneessens F, Skvoretz J (2010) Node centrality in weighted networks: Generalizing degree and shortest paths. Social Network 32: 245-251.
- Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44-57.
- Apic G, Ignjatovic T, Boyer S, Russell RB (2005) Illuminating drug discovery with biological pathways. FEBS Lett 579: 1872-1877.
- Berger SI, Iyengar R (2009) Network analyses in systems pharmacology. Bioinformatics 25: 2466-2472.
- Azuaje FJ, Zhang L, Devaux Y, Wagner DR (2011) Drug-target network in myocardial infarction reveals multiple side effects of unrelated drugs. Sci Rep 1: 52.
- Jeong H, Mason SP, Barabási AL, Oltvai ZN (2001) Lethality and centrality in protein networks. Nature 11: 41-42.
- Gao XQ, Yang CX, Chen GJ, Wang GY, Chen B, et al. (2010) Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol 132: 393-399.
- Zhu D, Wu L, Li CR, Wang XW, Ma YJ, et al. (2009) Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem 108: 117-124.
- Xu Y, Liu Z, Sun J, Pan Q, Sun F, et al. (2011) Schisandrin B prevents doxorubicin-induced chronic cardiotoxicity and enhances its anticancer activity in vivo. PloS One 6: e28335.
- Mattson MP (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem Res 30: 883-893.
- Xu M, Yang L, Hong LZ, Zhao XY, Zhang HL (2012) Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain Res 1454: 48-64.
- Zeng M, Wei X, Wu Z, Li W, Li B, et al. (2013) NF-κB-mediated induction of autophagy in cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun 436: 180-185.
- Liang X, Huang J, Lin X, Qin F, Wen Q, et al. (2014) The effect of 17-methoxyl-7-hydroxy-benzene-furanchalcone on NF-κB and the inflammatory response during myocardial ischemia reperfusion injury in rats. J Cardiovasc Pharm 63: 68-75.
- Liu Q, Kou JP, Yu BY (2011) Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int 58: 119-125.
- Kitagawa K (2007) CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J 274: 3210-3217.
- Mattson MP, Culmsee C, Yu ZF (2000) Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 301: 173-187.
- Grau AJ, Berger E, Sung KL, Schmid-Schönbein GW (1992) Granulocyte adhesion, deformability, and superoxide formation in acute stroke. Stroke 23: 33-39.
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, et al. (2007) Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129: 303-317.
- Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, et al. (2006) Effects of granulocyte colony stimulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscl Thromb Vasc Biol 26: 2238-2243.
- Dixon DL, Griggs KM, Bersten AD, De Pasquale CG (2011) Systemic inflammation and cellactivation reflects morbidity in chronic heart failure. Cytokine 56: 593-599.
- Ginsberg D (2002) E2F1 pathways to apoptosis. Febs Lett 529: 122-125.
- Li L, Fei Z, Ren J, Sun R, Liu Z, et al. (2008) Functional imaging of interleukin 1 beta expression in inflammatory process using bioluminescence imaging in transgenic mice. BMC Immunol 9: 49-57.
- Yao X, Huang J, Zhong H, Shen N, Faggioni R, et al. (2014) Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 141: 125-139.
- Stoeck K, Bodemer M, Ciesielczyk B, Meissner B, Bartl M, et al. (2005) Interleukin 4 and interleukin 10 levels are elevated in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Arch Neurol 62: 1591-1594.
- Wang XS, Luo KJ, Bella AE, Bu SS, Wen J, et al. (2014) Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J Gastroenterol 20: 4414-4420.
- Mostafa T, Rashed L, Nabil N, Amin R (2014) Seminal BAX and BCL2 gene and protein expressions in infertile men with varicocele. Urology 84: 590-595.
- Fong VH, Vieira A (2013) Pro-oxidative effects of aggregated transthyretin in human Schwannoma cells. Neurotoxicology 39: 109-113.