Journal of Parkinsons disease and Alzheimers disease
Download PDF
Review Article
Amantadine for the Treatment of Parkinson’s Disease and its Associated Dyskinesias
Butterworth Roger F*
Department of Medicine, University of Montreal, Canada
*Address for Correspondence: Butterworth Roger F, Professor of Medicine, University
of Montreal, Montreal, Qc, Canada 45143 Cabot Trail,
Englishtown, NS, B0C 1H0, Canada; E-mail: rb@enceph.com
Submission: 1- July- 2020;
Accepted: 5- August- 2020;
Published: 7- August- 2020
Copyright: © 2020 Butterworth RF. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Disturbances of motor function characteristic of Parkinson’s
Disease (PD) are commonly treated with L-Dopa. However, prolonged
treatment commonly results in L-Dopa-Induced Dyskinesias (LIDs)
with high negative impact on patient’s quality of life that seriously
limits the use of L-Dopa. Amantadine, like L-Dopa, is effective for
the replenishment of defective dopamine production in PD by
mechanisms involving increased synthesis and decreased synaptic
reuptake with consequent improvements of the patient’s motor
symptoms. Results of RCTs and meta-analyses continue to support
the claim that amantadine is effective for the treatment of early
or stable PD. Preclinical and clinical studies reveal that LIDs result
from modifications of corticostriatal (glutamatergic) and nigrostrial
(dopaminergic) connectivity resulting from the relative over-activation
of NMDA receptors, a phenomenon shown to occur in patients with
LIDs using Positron Emission Tomography. In addition to its beneficial
actions in restoring dopaminergic function, amantadine is a potent
non-competitive NMDA receptor antagonist and, as such, affords a
potentially effective agent for the treatment for LIDs. Indeed, beneficial
effects of amantadine for the treatment of LIDs have been described
in multiple Randomized Controlled Trials (RCTs) using a range of wellestablished dyskinesia rating scales over the last two decades and
extended-release formulations of amantadine have also been found
to be effective. Confirmation of clinical efficacy of amantadine for the
treatment of LIDs has been complemented by the results of systematic
reviews and meta-analyses that include a Movement Disease Society
(MDS)-commissioned evidence-based update of treatment options.
Treatment of PD patients with amantadine during the COVID-19
pandemic could be advantageous since, in addition to its ability to
correct the movement disorder and dyskinesias, amantadine has the
potential to limit replication of SARS-CoV-2, the virus responsible for
COVID-19.
Keywords
Parkinson’s disease; Amantadine; Dyskinesia;
Nigrostriatal; Corticospinal; NMDA; RCT; Meta-analysis; Systematic
review; Adverse events
Introduction
In 1968, a 58-year-old patient with moderately severe PD reported
that, while taking amantadine to prevent symptoms of the flu, a
remarkable remission of cogwheel rigidity and tremor was found
to occur. The PD symptoms promptly reappeared upon cessation of
amantadine. This serendipitous observation led to a trial in 1969 in
163 patients with PD two thirds of whom showed significant clinical
benefit [1]. These results opened the way for decades of fundamental
and clinical research into the mechanisms involved and evidence base
for the efficacy of amantadine for the treatment of PD and for USFDA approval a short time thereafter.
From the molecular structural standpoint, amantadine has an
adamantine backbone with an amino group substituted at one of the
four methyne positions giving rise to the molecule 1-aminotricyclo
[3.3.1.13,7] decane.
Mechanisms of action of amantadine in the treatment of the motor symptoms of PD:
PD is an age-related neurodegenerative disease characterized
by progressive degeneration of dopaminergic neurons. The
neurodegenerative process in PD is characterized by a loss of
dopamine-secreting cells in the substantia nigra. The most-widely
employed treatment for PD is L-Dopa, the metabolic precursor
for dopamine; the transformation from L-Dopa to dopamine is
catalyzed by the enzyme L-Dopa Decarboxylase (DDC) located in the
presynaptic nerve terminal (Figure 1). L-Dopa serves to replenish the
precursor pool leading to increased dopamine synthesis.Amantadine and the dopamine system: Amantadine, like L-Dopa, is also able to prevent the reduction of dopaminergic synaptic activity in PD and this, via multiple putative mechanisms. Studies in a range of in vitro synaptic preparations reveal that amantadine has the potential to increase dopamine synthesis, turnover and release and similar actions have been reported in vivo [2-5]. An additional proposed mechanism involves the direct inhibition of dopaminereuptake by amantadine that has been demonstrated in both in vitro preparations [6] and in vivo [3].
Anti-Parkinson activity of amantadine: Role of glutamate (NMDA) receptors: The potential for amantadine to increase dopamine synthesis from L-Dopa has been attributed, at least in part, to the drug’s effect as an antagonist of NMDA receptors [7]. Support of the novel concept of a dynamic functional interaction between NMDA receptor activation and the release of dopamine in striatum was demonstrated directly in experimental animals using the technique of in vivo cerebral micro-dialysis in which perfusion of amantadine (0.1-1 mM) via the micro-dialysis probe resulted in increases in Ca2+ dependent release of dopamine. Moreover, iv administration of the NMDA receptor antagonist MK 801 significantly attenuated the amantadine-induced increase in striatal dopamine release [5]. These findings support the concept of an interaction between dopaminergic and glutamatergic transmission in the regulation of striatal dopamine release (Figure 1).
Translation of the hypothesis that stimulation of DDC activity occurs as the result of NMDA receptor antagonism by amantadine to
the human condition made use of the technique of Positron Emission
Tomography (PET) and the ligand 6-[18F]-Fluoro-L-Dopa [7]. The
study involved the measurement of radioactivity in brains of normal
human volunteers following iv administration of the 18F PET ligand
under baseline conditions and again following three consecutive days
of treatment with amantadine (100 mg/d po). Data from several brain
regions of interest were obtained and coefficients of in situ 18F-L-Dopa
decarboxylation were calculated. Amantadine treatment resulted in
significant 12%, 28% and 27% increases in caudate nucleus, putamen
and ventral striatum respectively. These findings are consistent with
stimulation of DDC activity in striatum of healthy human brain
secondary to NMDA receptor antagonism by amantadine.
Other proposed mechanisms: Serotonin (5HT) neurons express
the genetic and metabolic machinery necessary for dopamine
synthesis and synaptic release. Chronoamperometric studies in
rodent models of PD suggest a central role for the 5HT system in
L-Dopa-derived dopamine synthesis and the potential for L-Dopainduced deterioration of 5HT function to reduce the clinical efficacy
of L-Dopa and to promote motor side effects [8].
Additionally, based on studies in primary cultures of dopaminergic
neurons, microglia and astroglia, it has been suggested that clinicallyrelevant concentrations of amantadine have the potential to manifest
neuroprotective properties resulting from a dual mechanism of
action involving a reduction in release of proinflammatory factors
from activated microglia together with increased expression of the
neurotrophic factor GDNF [9].
Evidence for the efficacy of amantadine for treatment of motor symptoms of PD:
Evidence from systematic reviews and meta-analyses: A
Cochrane Review of RCTs [10] selected 6 trials that compared
amantadine to placebo with or without L-Dopa or anticholinergic
drugs. The studies included 215 patients treated for periods of
from 6 to 64 weeks and amantadine doses of 100-200 mg/day.
Exclusion criteria included patients with non-idiopathic forms of
PD, patients who had previously undergone stereotactic surgery
as well as uncontrolled or non-randomized trials. Unfortunately,
methodological limitations together with potential sources of bias
meant that it was not possible to draw firm conclusions regarding the
efficacy or safety of amantadine for the treatment of idiopathic PD.On the other hand, the MDS published an evidence-based report of 7 studies on amantadine monotherapy and 14 studies on the efficacy of amantadine as an adjunct therapy to anticholinergics or L-Dopa concluded that amantadine was likely to be efficacious at controlling PD symptoms [11]. The MDS subsequently commissioned a review by way of update on treatments for the motor symptoms of PD that covered the findings from trials published in the period ending 31 December 2016 [12]. They concluded that amantadine was likely efficacious and clinically useful as symptomatic monotherapy as well as symptomatic adjunct therapy in early or stable PD. However, there was insufficient evidence for the effective treatment of motor fluctuations.
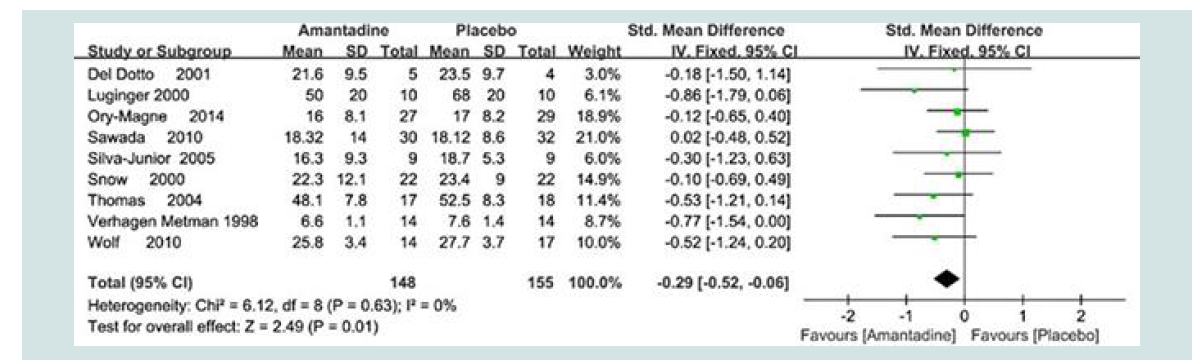
More recently and as part of a systematic review with meta analysis of the efficacy of amantadine for the treatment of the motor symptoms of PD, electronic searches of Medline, PubMed, Cochrane Library and other databases up to May 2016 were performed using appropriate keywords yielding 9 placebo-controlled RCT’s and 303 patients. Results demonstrated that amantadine was of significant benefit for improvement of PD motor symptoms as assessed by UPDRS III scores even at advanced stages of the disorder with [MD:
-0.29 {95% CI: -0.53, -0.06} Z= 2.49, p=0.01]. Details relating to the efficacy of independent trials are shown in the Forest Plot [13] (Figure 2).
Amantadine for the treatment of motor fluctuations in PD: The effect of amantadine on motor fluctuations was investigated in advanced PD [14]. Amantadine significantly lessened the severity of motor fluctuations; diary scores were lower with amantadine versus placebo (n=9; mean, 1.03 ± 0.12 vs 1.62 ± 0.16, p<0.01; variance, 1.3 ± 0.3 vs 3.3 ± 0.5, p<0.01) and the duration of daily ‘OFF’ time declined while on amantadine (n=14; UPDRS IV item 39, 1 [0-2] vs 1.5 [1-3]; p<0.001). Activities of daily living while ‘ON’ and ‘OFF’ (UPDRS II) were also improved with amantadine. The effect of amantadine on motor fluctuations in PD patients was also investigated in an open-label infusion study [15]. Based on patients’ diary notes, there was a reduction of 38% in the mean duration of ‘OFF’ times during amantadine infusion and improvement of 53% (p<0.001).
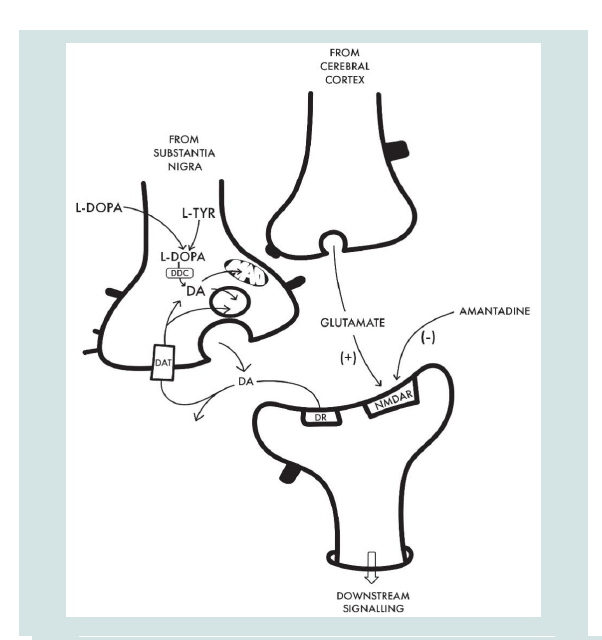
Figure 1: Schematic representation of cellular interactions implicated in the beneficial effects of L-Dopa and amantadine that underpin the beneficial effects of these agents for the treatment of the motor symptoms and dyskinesias associated with PD. On the left is a dopaminergic nerve terminal
of a nigrostriatal fibre with key elements involved in dopamine (DA) synthesis via the two-step process from L-tyrosine (L-TYR) to L-DOPA via the enzyme tyrosine hydroxylase followed by the action of L-Dopa decarboxylase (DDC) to DA that is either stored in the synaptic vesicle prior to release into the synaptic cleft or converted to metabolites via monoamine oxidase (MAO). Released DA then activates its post-synaptic receptor (DR) or is reuptaken via the DA transporter (DAT).
Both L-Dopa and amantadine have the potential to replenish the nigrostriatal DA deficit characteristic of PD; L-Dopa by supplying the substrate for DDC and amantadine by stimulation of DA synthesis coupled with the blocking of DA re-uptake by mechanisms that have not been completely elucidated.
Benefit of amantadine for the treatment of L-Dopa-induced dyskinesias (LIDs) is generally attributed to the drug’s properties as a non-competitive antagonist of the NMDA subclass of glutamate receptors (NMDAR). Blockade of these receptors results in the modulation of the interactions between corticostriatal
glutamatergic and nigrostriatal dopaminergic inputs leading to improvements in LIDs.
Figure 2: Forest plot of motor symptoms of PD assessed using UPDRS III scores [Mean ± SD by Fixed Odds ± 95% CI] following treatment with amantadine versus placebo. Individual trials are identified by 1st author’s name and year. Full references are available in the reference list [13].
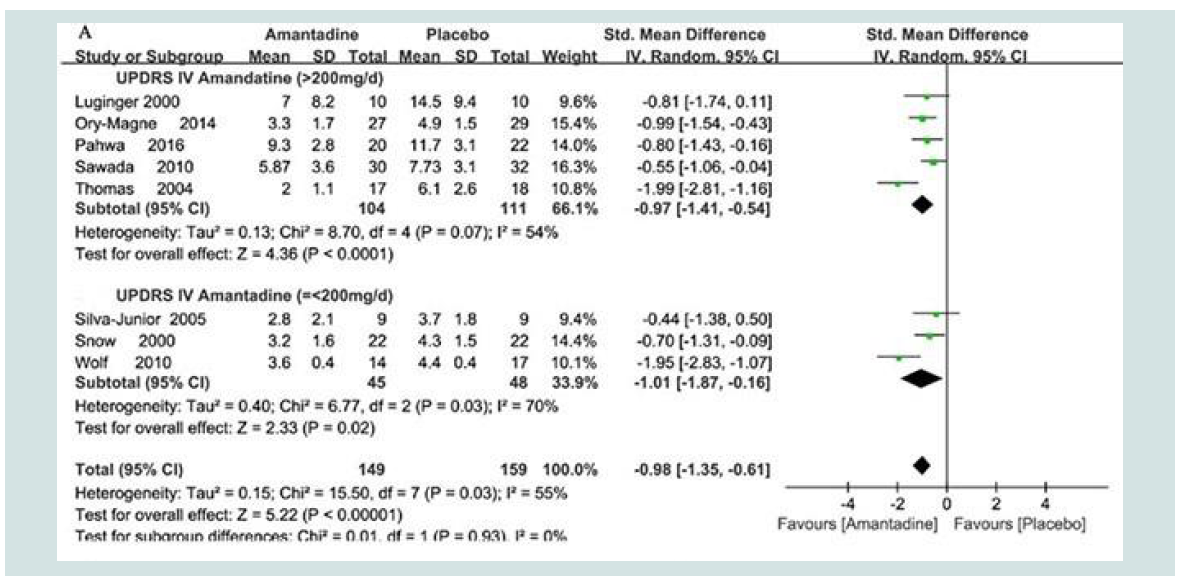
Figure 3: Forest plots of L-Dopa-induced dyskinesias in PD assessed using DRS scores [Mean ± SD by Random Odds ± 95% CI] following treatment with amantadine versus placebo as a function of the dose of amantadine [up to or greater than 200 mg/d]. Data from 9 individual trials are identified by 1st author’s name and year. Full references are available in the reference list [13]
Freezing of gait (FOG) is one of the most disturbing symptoms in advanced PD as well as in some atypical PD syndromes. FOG is often refractory to dopaminergic drugs [16]. There is evidence for the therapeutic use of amantadine for FOG in two RCTs [17,18].
Akinetic crises such as acute akinesia, neuroleptic malignant and neuroleptic malignant-like syndrome, and Parkinsonism hyperpyrexia syndrome can be successfully treated by acute administration of amantadine [19].
Amantadine for the Treatment of L-Dopa-Induced Dyskinesias (LIDs) in PD
Dyskinesias occur in up to 90% of patients with PD treated with L-Dopa for ten years or more. Such dyskinesias manifest as increased spontaneous motricity in the form of hyperkinesias occurring at high plasma levels of L-Dopa and are also known as ‘peak-dose dyskinesias’. Amantadine has been successfully employed in the management of this type of LID.
Mechanisms of action of amantadine in the treatment of LIDs in PD:
LIDs are generally considered to result from biochemical
mechanisms in striatal neurons that result from rapibly-changing
exposure to dopamine [23]. More recent investigations provide
convincing evidence that these changes in the dopamine system are,
in large part, the result of alterations in corticostriatal connectivity
through changes in functional activity of NMDA receptors. Thus,
as depicted in Figure 1, the corticostriatal (glutamatergic) and
nigrostriatal (dopaminergic) input stimuli converge at the striatum
where they have a key modulating influence on neuronal activity
and consequently on motor control. Using the technique of PET
and the ligand 11C-N-methyl-3-(thiomethylphenyl)-cyanamide,
a marker of activated NMDA receptor channels, glutamatergic
function in patients with PD with and without LIDs were compared
[24]. Patients were assessed twice; after taking L-Dopa and again after
withdrawal from it. Striatal uptake of tracer was calculated. Tracer
uptake measured in the ‘ON’ condition following L-Dopa was higher
in dyskinetic patients compared to patients without dyskinesia. These
findings were consistent with those previously observed in animal
studies and suggest that increased glutamatergic synaptic activity
is implicated in the pathogenesis of LIDs. Amantadine is a noncompetitive antagonist of the NMDA receptor [25]. Consequently,
blockade of NMDA receptors by amantadine has the potential to
provide an approach to the control of LIDs in PD.Efficacy of amantadine for the treatment of LIDs; results of systematic reviews and meta-analyses:
Electronic searches of The Cochrane Controlled Trials Register
issue 3 (2001), Medline (1966-2001), Embase (1974-2001),
Clinicaltrials.gov (2001) and other databases as well as manual
searches of reference lists from selected studies/reviews were
examined in order to select RCTs comparing amantadine with placebo
for the treatment of dyskinesias in patients with a clinical diagnosis of
idiopathic PD. Three RCTs satisfied search criteria and were doubleblind crossover trials for a total of 53 patients. Regrettably, as a result
of inadequate trial quality, missing data, lack of washout interval
data, reviewers concluded that evidence was insufficient to determine
safety or efficacy of amantadine for treatment of LIDs in patients with
PD [10].Searches of PubMed (1990-2010), Medline (1966-2010), Embase
(1974-2010) and other databases with appropriate keywords were
used resulting in 11 RCTs for a total of 253 PD patients with peakdose LIDs. The outcome measures were various dyskinesia rating
scales and the Unified Parkinson’s Disease Rating Scale (UPDRS) III
and IV. The analysis showed a significant reduction in Standard Mean
Difference (SMD) for UPDRS IV (SMD -1.45; 95% CI: -2.28 to -0.63)
and UPDRS III (SMD -0.41; 95% CI: -0.69 to -0.12) after treatment
with amantadine. Overall the meta-analysis confirmed the short-term
benefits of amantadine therapy in the treatment of LIDs [26].
In the most recent MDS-commissioned evidence-based medicine
review of treatments for the motor symptoms of PD covering the
results of trials up to December 31, 2016, new conclusions for the
treatment of dyskinesias were reported in which 3 new high-quality studies using amantadine were identified [12,27-29]. In line with
the conclusions from previous MDS recommendations, the use of
amantadine for the treatment of dyskinesias in PD was judged to be
both efficacious and clinically useful.
A contemporary up-dated meta-analysis of the efficacy of
amantadine for the treatment of dyskinesias in PD made use of
electronic searches of Medline, PubMed, Cochrane Library and other
databases revealed a total of 11 eligible RCTs involving 356 patients
with PD and LIDs. Making use of UPDRS IV and Dyskinesia Rating
Scale (DRS) as measures of efficacy outcomes, highly significant
benefits (p<0.0001) were shown for amantadine [13]. Forest plots of
dyskinesia assessment comparisons on UPDRS IV for amantadine
versus placebo as a function of drug dosage [MD: -0.98 {95%CI: -1.35,
-0.61} Z=5.22, p<0.00001] and trial design (parallel vs crossover).
Details are shown in Figure 3.
Efficacy of amantadine for the treatment of LIDs; results of individual RCTs:
Benefits of oral or intravenous formulations of amantadine have
been evaluated in patients with PD and LIDs in 10 RCTs the key
elements of which are summarized as follows:Sawada et al. [2010]: In a 27-day trial carried out in 36 patients
with PD and LIDs, amantadine treatment (300 mg/d) was associated
with a significant improvement of 64% in the Rush Dyskinesia
Rating Scale versus 16% of placebo patients. The adjusted odds ratio
for improvement by amantadine was 6.7 (95% Confidence Interval
[CI], 1.4-31.5, p<0.016); UPDRS IVa, dyskinesia improved to an even
greater degree following amantadine treatment [27].
Wolf et al. [2010]: The long-term anti-dyskinetic effect of
amantadine was evaluated in 32 patients with PD who were receiving
L-Dopa and who had been on stable amantadine therapy for at least 1
year [30]. In patients who had been switched to placebo at the 3-week
follow-up, there was a significant increase in dyskinesia, as measured
with UPDRS item 32-33, but there was no significant change between
baseline and follow-up in patients who had continued on amantadine.
Da Silva Junior et al. [2005]: The effects of amantadine on LIDs
were assessed in 18 consecutive PD patients in an RCT [31]. The
primary outcomes were improvements in CDRS and UPDRS IVa
scores. Secondary outcomes were improvements in UPDRS II and III
scores. Amantadine did not change the CDRS score for hyperkinesia
or dystonia but decreased the duration of LIDs and its influence on
daily activities (p=0.04) and UPDRS II score (p=0.01) more than
placebo. These findings show that amantadine reduces the duration
of LIDs and improves motor disability in PD.
Thomas et al. [2004]: A trial of amantadine was conducted in 14
PD patients patients treated with L-Dopa for 7.5 ± 2.2 yrs with motor
fluctuations and dyskinesias evaluated by UPDRS IV and blinded
videotape-based ratings using DRS. After 15 days of amantadine
treatment, total dyskinesia scores decreased by 45%, and there was
a reduction in UPDRS IV item 32-34 scores compared with baseline
and placebo (p<0.001). The mean positive duration of the effect in
reducing dyskinesias was 4.9 months for amantadine versus 1.3
months for placebo (p<0.001), [32].
Del Dotto et al. [2001]: Nine patients with PD and severe peak-dose dyskinesias received L-DOPA followed by amantadine (200 mg
iv) or placebo and were assessed by UPDRS, motor exam & AIMS.
Amantadine improved dyskinesias by 50% with no loss of benefit for
the motor symptoms of PD [33].
Luginger et al. [2000]: A 5-week, crossover trial examined the effect of amantadine on LIDs. Ten of 11 patients completed the
study. Dyskinesia severity, following oral L-Dopa challenges, was significantly reduced by 52% after amantadine treatment (baseline score 14.5 ± 9.4 vs post-treatment score 7.0 ± 8.2; p<0.05) but not placebo (baseline score 16.6 ± 11.4 vs post-placebo score 15.5 ± 12.1; p>0.05). Similarly, amantadine was associated with a significant reduction of 53% in the cumulative dyskinesia score in self-scoring dyskinesia diaries (post-amantadine treatment period 11.9 ± 11.4 vs post-placebo period 25.6 ± 16.7; p<0.05). Dyskinesia duration and dyskinesia disability, as measured by UPDRS IV items 32-33, were also significantly reduced by amantadine (baseline score 3.4 ± 0.3 vs post-reatment score 1.7 ± 0.5; p<0.05) [34].
Snow et al. [2000]: A trial examined the effect of amantadine on
LIDs in 22 patients with PD. Patients were treated with amantadine
(up to 200 mg/d for 3 weeks) in addition to other antiparkinsonian
medications. Dyskinesias were evaluated following an oral L-Dopa
challenge. Amantadine treatment significantly reduced the total
dyskinesia score by 24%; this was also reflected in the subjects’
perception that dyskinesias, measured by UPDRS III and IV, were
reduced [35].
Verhagen Metman et al. [1998]: A trial evaluated amantadine for
treatment of dyskinesias in 18 patients with advanced PD. Patients,
who had already been treated with L-Dopa for a mean period of 12
years, were given amantadine (up to 400 mg/day) or placebo for 3
weeks. Signs and symptoms of PD and dyskinesias were recorded at
the end of the 3-week treatment period during intravenous L-Dopa
infusions over 7 h under steady-state conditions. Amantadine
reduced dyskinesia severity by 60% (p=0.0001) compared to placebo
in completers and this was not accompanied by aggravation of the
signs and symptoms of PD [36]. Beneficial effects of amantadine
on motor response complications were maintained in a 1-year
follow-up study in which a 56% reduction in dyskinesia was noted
with amantadine versus 60% in the initial study. Motor symptoms
associated with L-Dopa were still improved [14].
Goetz et al. [2013]: Assessment of the effects of treatment of
patients with PD and dyskinesia with amantadine (up to 300mg/d for
8 weeks) versus placebo were made by comparing the sensitivity to
treatment effects at 4 and 8 weeks using 8 different dyskinesia rating
scales. Four of the eight scales (UDysR, Lang-Fahn, PDys-26 and
CGI-C) demonstrated a significant improvement in dyskinesia after 8
weeks treatment with amantadine vs placebo [28].
Ory-Magne et al. [2014]: The AMANDYSK trial was a 3-month, multicenter, parallel group/wash out in single line RCT in patients with PD. Fifty-seven dyskinetic patients who had received amantadine at a dosage of 200 mg/d for ≥6 months were included in the study. Washout of oral amantadine significantly worsened LIDs where UPDRS IV item 32-33 deteriorated more in patients who switched to placebo (‘discontinuing’ group; +1.7 ± 2.0 units; 95% CI: 0.9-2.4) compared with those maintained on amantadine (‘continuing’ group; +0.2 ± 1.5 units; 95% CI: -0.4 to 0.8; p=0.003). Patients in the discontinuing group also had more dropouts for LIDs, a greater increase in ‘ON’ time with troublesome dyskinesia, and a greater worsening of Abnormal Involuntary Movement Scale scores. No significant effect of amantadine on PD motor symptoms was observed [29].
Efficacy of amantadine (extended release) for the treatment of LIDs: results of RCTs and a meta-analysis:
The last 5 years have witnessed the publication of two RCT’s and
a meta-analysis in which the efficacy of amantadine (extended-release
formulation) for the treatment of LIDs were described. A report of the
efficacy of ADS-5102, a long-acting, extended -release formulation of
amantadine has appeared [37]. In a parallel group phase 2/3 RCT 83
PD patients with LIDs were assigned to one of three doses of ADS5102 or placebo for an 8-week period making use of UDysRS, UPDRS
and other instruments. The drug was well tolerated and resulted in
significant and dose-dependent improvements in dyskinesia. A
follow-up phase 3 trial of the same agent essentially confirmed the
above results showing significant benefit for both dyskinesias and
OFF times [38].To determine the effectiveness and safety of ADS-5102 for LIDs
in patients with PD, a meta-analysis of relevant trials was undertaken.
Electronic databases were searched on or before March 1, 2019 for
relevant trials. Only RCTs using ADS-5102 for LID in PD were
included. ADS-5102 led to a reduction in dyskinesia scores (mean
difference: -9.56: CI: -10.05 to -9.07; p<0.00001) and ’ON’ times
without troublesome dyskinesia (mean difference 2.50: CI 2.38 to
2.63; p<0.00001) [39].
Amantadine treatment of PD during the COVID-19 pandemic:
PD and COVID-19 share multiple common features including
age-dependency of disease severity and an association with comorbidities such as diabetes, respiratory problems and cardiovascular
disorders. Concerns have been raised regarding the potential effects
of COVID-19 on PD severity and, conversely, on the effects of PD
on immune status that could impact disease outcome in COVID-19.
Symptoms of PD are known to deteriorate during systemic infections
and diagnostic features of COVID-19 such as fever, fatigue and
stress are known to not only aggravate tremor, gait disturbances and
dyskinesias in PD but may also compromise the efficacy of L-Dopa
[40].Concerns on the likelihood of interactions between COVID-19
and PD with the potential to result in the clinical worsening of both
conditions described above may be mitigated once treatment with
amantadine is initiated. Recent investigations provide convincing
evidence for significant benefit of amantadine for the treatment of
COVID-19 per se. Two independent mechanisms have been proposed
namely 1. The down-regulation of expression of host-cell proteases
leading to impaired release of the viral genome into the host-cell
cytoplasm and 2. Activation of NMDA receptors has been implicated
in the pathogenesis of the acute respiratory failure characteristic of
COVID-19 [41]. The pertinence of these mechanisms of action of
amantadine in relation to the management of COVID-19 in patients
with PD is worthy of further investigations in future research.
Adverse Events (AEs) associated with amantadine treatment for LIDs in PD
AEs listed in the present review are largely taken from data
provided in the reports of meta-analyses, systematic reviews and
individual RCTs described in the text and relate to AEs using
therapeutically-relevant concentrations of amantadine. Common
AEs included visual hallucinations, confusion, blurred vision, foot
edema and constipation. Behavioral symptoms characteristic of
impulse control disorders including pathological gambling, hyper
sexuality and compulsive spending/eating have been described.
Other AEs include live do reticular is, nausea, dry mouth, dizziness and somnolence, peripheral edema, anxiety and depression [13,29,30,35,36]. Similar AEs for the extended-release formulations of amantadine in the treatment of LIDs in PD have been reported [37,38].
Conclusions
Amantadine is effective for the replenishment of defective synaptic DA turnover by mechanisms involving both increased synthesis and decreased release of the transmitter with consequent improvements of motor control in PD patients. LIDs in such patients, on the other hand, appear to be the consequence of modifications of both corticostriatal (glutamatergic) and nigrostriatal (dopaminergic) neurotransmitter systems leading to impaired striatal connectivity resulting from the relative over-activation of the NMDA subclass of glutamate receptors. Being an effective non-competitive NMDA antagonist, amantadine affords a potentially-effective agent for the treatment of LIDs. The functional interplay between glutamatergic
and dopaminergic systems in striatum and beneficial effects of amantadine have been demonstrated using PET and the ligand 6-[F18]-Fluoro-L-Dopa in human brain.
Evidence in support of the efficacy of amantadine for the treatment
of PD and its associated dyskinesias has been forthcoming in the
form of numerous RCTs, systematic reviews and meta-analyses. The
MDS published an updated evidence-based report of clinical trials
in which amantadine monotherapy was effective for the control of
the motor symptoms of PD and, more recently, a systematic review
with meta-analysis confirmed that amantadine was of significant
benefit for improvement of motor symptoms assessed by UPDRS
even at advanced stages of the disorder. There is evidence to support
the notion that amantadine may also be beneficial for the control of
motor fluctuations in PD such as ON and OFF times as well as FOG
and acute akinesia. Further high quality RCTs with systematic review
and meta-analysis are necessary in order to confirm these reports.
Evidence for the efficacy of amantadine for the treatment of LIDs
in patients with PD is provided by the results of 10 good-to-excellent
quality RCTs published in the last two decades using a range of
doses of amantadine from 100-300mg/d, a variety of well-established
dyskinesia rating scales, and trial design (parallel, crossover) for
treatment periods of up to 1 year. Publication of the successful results
of RCTs and a meta-analysis of the efficacy of an extended-release
formulation of amantadine for the treatment of LIDs will provide a
choice of treatment options; trials comparing this formulation with
the classic immediate-release formulation with flexible increasing
(100-400mg/d) doses of amantadine together with assessments of cost-effectiveness are now required. It appears that both immediaterelease and extended-release formulations of amantadine have
associated AEs that include foot edema, blurred vision, visual
hallucinations and constipation.
Amantadine treatment for PD patients has the potential to
provide a treatment of choice during the COVID-19 pandemic given
the growing body of evidence that, in addition to the well-established
actions on motor performance and dyskinesias summarized in the
present review, amantadine may also limit replication of the SARSCoV-2 virus that is responsible for COVID-19. This issue warrants
further investigation at pace.
Acknowledgement
Research from the author’s Unit including work on Parkinsonism and costs of publication of original articles and reviews was funded in part over the last two decades by The Canadian Institutes of Health Research (CIHR) and The Canadian Association for Study of The Liver (CASL). The author is grateful to Mr Jonas Eric Pilling for the design of Figure 1.