International Journal of Otorhinolaryngology
Download PDF
Research Article
Clinicians' Insights on the use of Oral Second-Generation Antihistamine Bilastine in Allergic Rhinitis
Manjula S* and Krishna Kumar M
Department of Medical Services, Micro Labs Limited, Bangalore, Karnataka, India
*Address for Correspondence:Dr Manjula S, Department of Medical Services, Micro
Labs Limited, Bangalore, Karnataka, India E-mail Id: drmanjulas@gmail.com
Submission: 01 August, 2025
Accepted: 26 August, 2025
Published: 29 August, 2025
Copyright: © 2025 Manjula S, et al. This is an open access
article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is
properly cited.
Abstract
Objective: To assess expert opinion on the clinical use of bilastine
in the management of allergic rhinitis (AR) in Indian settings.
Methods: The cross-sectional study was carried out by using a 24- item questionnaire which gathered insights from clinicians practicing in India on key aspects, including clinical observations, treatment preferences, and experiences with bilastine monotherapy. The collected data were analyzed using descriptive statistics.
Results: The survey included responses from 557 medical experts. Nearly half of the physicians (49%) reported that allergic rhinitis is most frequently diagnosed in adults. About 63% of respondents identified dust mites as the leading environmental trigger. According to 75% of participants, asthma was the most commonly associated comorbidity. For managing mild allergic rhinitis, 72% of physicians selected oral second-generation antihistamines as their first-line treatment. Over half (56.55%) acknowledged that immunotherapy can modulate immune responses and provide long-term relief when pharmacological treatment alone is inadequate. Additionally, 42% supported the continued use of immunotherapy for sustained benefit in allergic rhinitis cases. The majority (90%) favored bilastine as the antihistamine of choice for allergic rhinitis, with approximately 90% preferring it specifically for patients with renal impairment.
Conclusion: This study highlights current practices in the management of allergic rhinitis, with bilastine favored for its safety, efficacy, and suitability in special populations. Immunotherapy is recognized for its long-term benefits, although diagnostic practices vary.
Methods: The cross-sectional study was carried out by using a 24- item questionnaire which gathered insights from clinicians practicing in India on key aspects, including clinical observations, treatment preferences, and experiences with bilastine monotherapy. The collected data were analyzed using descriptive statistics.
Results: The survey included responses from 557 medical experts. Nearly half of the physicians (49%) reported that allergic rhinitis is most frequently diagnosed in adults. About 63% of respondents identified dust mites as the leading environmental trigger. According to 75% of participants, asthma was the most commonly associated comorbidity. For managing mild allergic rhinitis, 72% of physicians selected oral second-generation antihistamines as their first-line treatment. Over half (56.55%) acknowledged that immunotherapy can modulate immune responses and provide long-term relief when pharmacological treatment alone is inadequate. Additionally, 42% supported the continued use of immunotherapy for sustained benefit in allergic rhinitis cases. The majority (90%) favored bilastine as the antihistamine of choice for allergic rhinitis, with approximately 90% preferring it specifically for patients with renal impairment.
Conclusion: This study highlights current practices in the management of allergic rhinitis, with bilastine favored for its safety, efficacy, and suitability in special populations. Immunotherapy is recognized for its long-term benefits, although diagnostic practices vary.
Keywords:Allergic rhinitis; dust mites; asthma; immunotherapy;
bilastine
Introduction
Allergic rhinitis (AR) is a prevalent chronic respiratory condition
that significantly affects quality of life, work productivity, and
healthcare systems worldwide. The prevalence varies across regions
and age groups, ranging from 10% to 30% in adults and exceeding
40% in children.[1] In India, AR has emerged as a significant public
health issue, affecting roughly 20% to 30% of the general population,
including about 22% of adolescents currently experiencing symptoms.
[2]
Second-generation oral antihistamines are widely recommended
as the first-line treatment for mild-to-moderate allergic rhinitis,
owing to their superior safety profile, minimal sedation, and longer
duration of action compared to first-generation antihistamines.
[3] Among these, bilastine stands out as a highly selective, nonsedating
histamine H1 receptor antagonist with rapid onset and
sustained efficacy. By selectively binding to peripheral H1 receptors
and inhibiting their activation, bilastine effectively suppresses the
cascade of allergic symptoms. Additionally, bilastine does not readily
cross the blood–brain barrier, making it less likely to cause central
nervous system side effects such as drowsiness, which is a significant
advantage in maintaining daily functioning and quality of life [4].
Given the substantial burden of AR in India and the need
for therapies that offer both clinical efficacy and promote patient
adherence, this study aims to gather expert opinion on the clinical
utility, effectiveness, safety, and tolerability of bilastine in routine
practice within Indian settings.
Methodology
We carried out a cross-sectional study among clinicians
actively engaged in AR management across India from June 2024 to
December 2024. The study was conducted after receiving approval
from Bangalore Ethics, an Independent Ethics Committee, which was
recognized by the Indian Regulatory Authority, the Drug Controller
General of India.
A convenient sampling technique was used, and an invitation was
sent to leading clinicians in managing AR in the month of March
2024 for participation in this Indian survey. About 557 clinicians
from major cities of all Indian states, representing the geographical
distribution, shared their willingness to participate and provide
necessary data. The questionnaire booklet titled BEAM (Bilastine and
Montelukast- Expert Assessment in Management of Allergic Rhinitis)
study was sent to clinicians who were interested in participating in
this study. The BEAM study questionnaire consisted of 24 questions,
which covered key areas such as current clinical practices related to
bilastine in routine care, including physician preferences, clinical
indications, perceived efficacy, adverse effects, and patient groups
commonly prescribed bilastine. Reliability, as determined by a splithalf
test (coefficient alpha), was adequate but should be improved
in future versions of the questionnaire. A study of criterion validity
was undertaken to test the questionnaire and to develop methods of
testing the validity of measures of Physicians' Perspectives. However,
the extraneous variables in this include the clinician's experience,
usage of the newer drugs, etc. The two criteria used were the doctors'
perspectives from the clinical practice and the assessment of an
external assessor and statistician. Clinicians had the option to skip
any questions they preferred not to answer. They were instructed
to complete the questionnaire independently, without consulting
their colleagues. Written informed consent was obtained from all
participants before the study commenced.
Statistical Analysis:
Data were analyzed using descriptive statistics, with categorical
variables summarized as frequencies and corresponding percentages.
Visual representations, including graphs and pie charts, were
generated using Microsoft Excel 2013 (version 16.0.13901.20400).Results
The survey included 557 respondents, and 44% of experts reported
that 21–30% of their patients suffer from AR in their clinical practice.
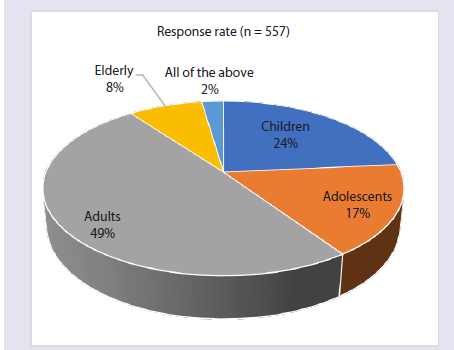
According to 49% of physicians, AR is most commonly diagnosed in
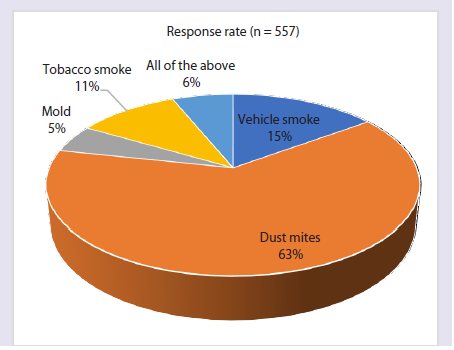
adults [Figure 1]. Approximately 63% of respondents identified dust
mites as the primary environmental trigger for AR [Figure 2].
About 48% of the participants reported that the impact of AR on patients’ quality of life is moderate. Around 30% of physicians
About 48% of the participants reported that the impact of AR on patients’ quality of life is moderate. Around 30% of physicians
Figure 1:Distribution of responses on the age group most commonly
diagnosed with AR in India in clinical practice.
Figure 2:Distribution of responses on primary environmental trigger for AR
in India in clinical practice.
identified sneezing as the most troubling symptom. Regarding
seasonal patterns, 42% of physicians reported winter as the peak
season for AR symptoms. Urban areas showed higher prevalence,
with 44% of physicians reporting a greater occurrence of AR in these
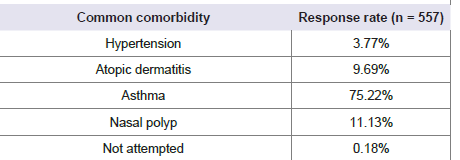
regions. Nearly 75% of respondents identified asthma as the most
common comorbidity associated with AR [Table 1].
About 34% of respondents reported that the lack of effective
treatment is a common challenge in diagnosing AR. Approximately
57% of clinicians stated that 11% to 20% of their patients with AR
experience nasal congestion. Regarding diagnostic approaches,
about 35% of physicians identified increased serum histamine levels
as a key diagnostic criterion. Around 48% of respondents reported
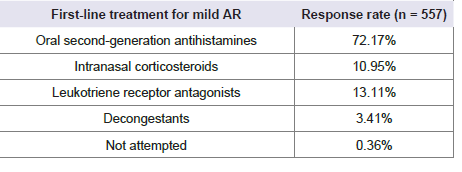
performing skin prick tests in less than 10% of their patients. Nearly
72% of physicians preferred oral second-generation antihistamines as
the first-line pharmacological treatment for mild AR [Table 2]
About 43% of physicians reported that intranasal corticosteroids
in combination with oral second-generation antihistamines is the
treatment approach considered most effective for better symptom
control in cases of moderate to severe AR. Approximately 33% of
participants reported that regular exercise is a recommended nonpharmacological
measure for reducing exposure to indoor allergens
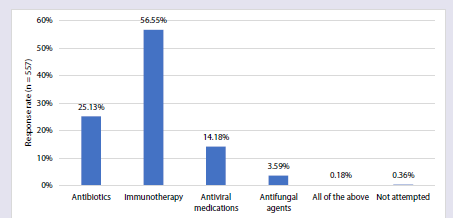
in AR patients. More than half (56.55%) of the experts reported that
immunotherapy is a treatment option that can modify the immune
response and provide long-term relief in AR, particularly in cases
where usual treatment is insufficient [Figure 3].
Around 50% of respondents reported that the nasal mucosa
should be regularly monitored for signs of atrophy in patients on longterm
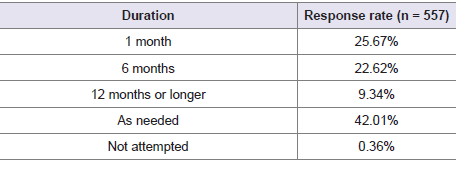
intranasal corticosteroid therapy. Nearly 42% of experts stated
that immunotherapy should be continued as needed for optimal
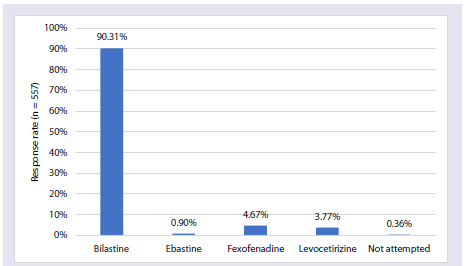
effectiveness in AR patients [Table 3]. The majority of participants
(90%) reported that bilastine is the preferred antihistamine for
patients with AR [Figure 4].
Nearly 33% of participants responded that they preferred bilastine
in routine practice for 31-40% of AR patients whose occupation
involves driving. About 90% of participants reported that bilastine is
the preferred antihistamine for renally compromised patients [Table 4].
Table 2:Distribution of responses on the first-line pharmacological treatment
for mild AR in clinical practice.
Table 3: Distribution of responses on the timing of immunotherapy to be
continued for optimal effectiveness in AR patients in your clinical practice.
About 40% of participants reported that the reduced sedative effect
is the most frequently cited advantage of bilastine. Approximately 49%
of the physicians reported that 21-30% of AR patients are prescribed
bilastine, with or without the addition of montelukast. Around 40%
stated that the combination of bilastine and montelukast is typically
prescribed for 11-14 days in patients with AR.
Discussion
The present survey underscores the role of second-generation
antihistamines, particularly bilastine, as a cornerstone in the
management of AR due to their proven efficacy, tolerability, and
safety, especially in sensitive populations such as drivers and
individuals with renal impairment. A significant proportion of
participants reported that AR is most commonly diagnosed in adults
in clinical practice in India. This observation aligns with findings
from Barne et al., who emphasized that AR poses a substantial health
burden among Indian adults.[5] Similarly, Moitra et al. reported that
approximately 22% of adolescents in India currently suffer from AR.
However, the lack of comprehensive and robust epidemiological data,
particularly from rural and suburban regions, suggests that the actual
burden of AR may be underestimated.[2]
A significant number of participants in the present survey
identified dust mites as the primary environmental trigger for AR
in India. This finding is supported by a study conducted by Krishna
et al. in Eastern India, which found that 96% of patients with
naso-bronchial allergy were sensitized to house dust mites, with
Dermatophagoides pteronyssinus, Dermatophagoides farinae, and
Blomia tropicalis being the predominant species.[6] Similarly, a study
by Ranjana and Maheshwari found that house dust mites were the
most common allergen among patients with allergic rhinitis, ranking
above other environmental triggers such as pollen, cockroach, and
mold. [7]
The majority of survey respondents identified asthma as a
common comorbidity associated with AR. This observation is
consistent with findings from Narasimhan et al. and an expert panel
consensus for India, both of which strongly recommend routine
screening for asthma in AR patients and vice versa, due to the high
rate of co-occurrence. The consensus reports that over 80% of asthma
patients also suffer from comorbid AR, while 17-38% of individuals
with AR concurrently experience asthma. Furthermore, severe AR
has been shown to adversely affect asthma control, with some studies
citing a co-prevalence rate of up to 65% in adult asthmatics.[8]
These findings are further supported by Indian data from Pawankar
et al., as well as international guidelines and multicenter studies
from the Asia-Pacific region, which indicate that 60–80% of asthma
patients exhibit rhinitis symptoms—underscoring the strong clinical
interrelationship between these two conditions.[9]
Most survey respondents indicated that oral second-generation
antihistamines are the first-line pharmacological treatment for mild
AR. This preference aligns with findings by Abdullah et al., who
reported that these agents are non-sedating, effective, and generally
well-tolerated, making them the optimal choice for managing mild
cases.[3] Supporting this, Recto et al. noted a rising prevalence of
allergic diseases across the Asia–Pacific region, and reaffirmed that
second-generation antihistamines continue to serve as the first-line
treatment for both AR and urticaria.[10]
Many respondents recognized immunotherapy as a valuable
treatment option capable of modifying the immune response
and providing long-term relief in patients with AR. Studies by
Sahiner et al. and Akdis and Akdis have demonstrated that allergen
immunotherapy (AIT) can induce long-lasting immune tolerance,
resulting in sustained clinical benefits even after discontinuation of
therapy. The underlying mechanisms involve early desensitization of
mast cells and basophils, modulation of T- and B-cell responses, and
the induction of regulatory T cells, which suppress allergen-specific
Th2 responses. Additionally, AIT promotes a shift in antibody
production from allergen-specific IgE to blocking antibodies such
as IgG4 and IgA, which inhibit allergen-IgE binding and subsequent
effector cell activation. These immunologic adaptations help reduce
allergic inflammation and contribute to prolonged symptom
improvement that may last for years following treatment cessation.
[11,12]
Many participants in the survey reported that immunotherapy
should be continued as needed to achieve optimal effectiveness
in patients with AR. This aligns with findings by Penagos et al.,
who demonstrated that three years of subcutaneous or sublingual
immunotherapy resulted in significant clinical improvement and
immunological changes indicative of allergen-specific tolerance, with
benefits persisting for at least 2-3 years after discontinuation. Based
on such evidence, international guidelines recommend a minimum
of three years of immunotherapy to ensure sustained, long-term
efficacy.[13] Kouzegaran et al. demonstrated that subcutaneous
immunotherapy reduces clinical symptoms and promotes immune
tolerance in patients with AR, highlighting the role of continued
treatment in sustaining symptom relief and modulating the immune
response.[14]
The majority of participants reported that bilastine is the
preferred antihistamine for use in patients with renal impairment.
This preference is supported by pharmacokinetic studies, including
one by Lasseter et al., which demonstrated that a 20 mg dose
of bilastine is safe and well tolerated across all levels of renal
dysfunction, including moderate to severe impairment. Despite
elevated plasma concentrations in individuals with renal impairment,
no dose adjustment was necessary, as bilastine exhibits a favorable
safety profile, minimal central nervous system penetration, and a low
potential for drug interactions. These characteristics make bilastine
a particularly suitable option in patients with renal comorbidities,
where safety and tolerability are paramount.[15] Moreover, the recent
recommendations endorsed that newer antihistamines, including
bilastine, are effective in improving AR symptoms as they block
peripheral H1 receptors without crossing the blood–brain barrier,
which prevents central nervous system side effects. [16]
This large-scale survey, involving a significant number of
clinicians across various Indian settings, provides valuable real-world
insights into the management of AR in India. The comprehensive
questionnaire addressed key areas such as epidemiology, diagnostic
practices, and treatment trends, highlighting a strong preference
for bilastine and growing recognition of immunotherapy’s longterm
benefits. However, the study’s reliance on self-reported data
introduces potential for recall and response bias. Furthermore, the
lack of stratification by physician specialty or geographic location
limits the scope for subgroup analysis. The absence of patient-level
data and objective clinical outcomes also restricts the ability to directly
correlate physician perceptions with actual clinical effectiveness.
Conclusion
This cross-sectional survey highlights the high burden of AR
in adults, with dust mites identified as the primary trigger. Experts
preferred bilastine, especially for patients requiring minimal sedation
or those with renal impairment. Immunotherapy is valued for its longterm
benefits, though diagnostic tools like skin prick tests remain
underutilized. These findings emphasize the need for standardized,
evidence-based approaches to optimize AR care in Indian settings.
Acknowledgement
We would like to thank all the clinicians who were actively
participating in this study.
Author contributions:
Both authors have contributed equally to the development of the
manuscript.Disclosure of compliance with ethical principles:
The study was conducted after receiving approval from Bangalore
Ethics, an Independent Ethics Committee, which was recognized by
the Indian Regulatory Authority, Drug Controller General of India.