International Journal of Otorhinolaryngology
Download PDF
Case Report
Primary Nasal MeningiomaMasquerading as an Antrochoanal Polyp
Kaipuzha RR1 Pulimoottil DT2* and Karthikeyan P3
- 1Department of Otorhinolaryngology and Head and Neck Surgery, Kuwait
- 2Department of ENT, India
- 3Department of Otorhinolaryngology, India
*Address for correspondence: Pulimoottil DT, Assistant Professor, Department of ENT, Al Azhar Medical College and Super Specialty Hospital, Ezhalloor, Thodupuzha, Idukki District, Kerala, India, E-mail: davisthomasp@yahoo.in
Citation: Kaipuzha RR, Pulimoottil DT, Karthikeyan P. Primary Nasal Meningioma Masquerading as an Antrochoanal Polyp. Inter J Otorhinolaryngology. 2019;6(1): 5.
International Journal ofOtorhinolaryngology | ISSN: 2380-0569 | Volume: 6, Issue: 1
Submission: 07 January, 2019| Accepted: 13 February, 2019 | Published: 15 February, 2019
Copyright: © 2019 Kaipuzha RR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
An eleven year old female presented to the Outpatient Department of ENT and Head and Neck Surgery of a tertiary care centre, with a history of right sided nasal obstruction and nasal discharge of two months duration. Examination of the external framework of the nose revealed widening of the bridge of the nose with hyperpigmentation of the skin over the nose and columella. Anterior rhinoscopy showed a pale greyish-white glistening mass which was insensitive to touch on probing and was pedunculated and appeared to arise from the area of the middle turbinate. It did not bleed on touch and it had pushed the septum to the opposite side. Diagnostic nasal endoscopy showed a pale greyish white mass completely occupying the right nasal cavity and pushing the septum to the left side. Computed tomography of the nose and paranasal sinuses showed a soft tissue density lesion filling the right maxillary and ethmoid sinuses, and extending into the right nasal cavity causing widening of the ipsilateral osteomeatal complex and nasal cavity with left sided deviation of the nasal septum with compression of left nasal cavity and there was evidence of mild rarefaction of the septum and extension of the lesion into the right choana. These radiological features were more in favour of a right antrochoanal polyp, and with this diagnosis in mind, the patient was taken up for Transnasal Endoscopic Polypectomy. Intraoperatively, a pinkish slough-covered smooth mass was seen filling the entire right nasal cavity with an atrophied inferior turbinate. The mass was excised and the tissue was sent for histopathological examination. Histopathological examination revealed features consistent with meningioma. Awareness of this diagnosis in an unexpected location will help to avoid potential difficulties associated with the diagnosis and management of these tumours.
Keywords
Nasal obstruction; Nasal cavity; Antrochoanal polyp; Primary extracranial meningioma
Introduction
Meningioma is the most common nonglial intracranial neoplasm, arising from meningocytes (arachnoid cap cells) of arachnoid granulation tissue and constitutes 24-30% of all intracranial tumours and has an incidence of 1.4-4.5/100,000 worldwide [1]. Most commonly extra-neuraxial meningiomas are found overlying the surface of the brain or at the skull base. Uncommonly, meningiomas occur in intraventricular, intraparenchymal, or intraosseous locations. In rare instances (1-2%), they appear as an extracranial tumour, most often in the head and neck region, and specifically in the sinonasal tract, ear and temporal bone, and scalp [2]. Primary extracranial meningiomas are rare neoplasms, frequently misdiagnosed, resulting in inappropriate clinical management. Primary extracranial meningiomas are histologically identical to intracranial meningiomas and usually occur in 40-60 year old patients and are rare in the pediatric age group. Menigiomas rarely occur in children, and if so, they may be present in association with von Recklinghausen neurofibromatosis (Type I). A genuine extracranial primary meningioma is to be confirmed after a CT scan, to rule out intracranial mass or any underlying bony erosion of the skull base. FNAC of the lesion can be deceptively mistaken and final diagnosis is usually made on the basis of histological examination of the excised mass [3]. Meningiomas behave as slow-growing neoplasms with a good prognosis, with longest survival associated with younger age and complete resection. Prognosis is poorer in disease recurrence; however, this is often due to incomplete excision rather than true recurrence [4]. Awareness of this diagnosis in an unexpected location will help to avoid potential difficulties associated with the diagnosis and management of these tumours.
Case Report
An 11-year-old female presented to the Department of ENT and Head and Neck Surgery of a tertiary care centre with the complaints of right sided nasal obstruction and nasal discharge of two months duration. The nasal obstruction was insidious in onset and gradually progressive with no aggravating or relieving factors. The patient had no history of epistaxis or trauma. She also had complaints of hyperpigmentation of skin over and around the nose. She had complaints of right sided nasal discharge which was yellowish in colour and foul smelling. There was a history of recurrent upper respiratory tract infections since childhood. There was also no history of headache, nausea or vomiting and there were no visual changes or loss of smell. There was no history of change in personality or seizures. There was no history of any prior nasal surgery and no other remarkable medical history. The patient’s family history was unremarkable.
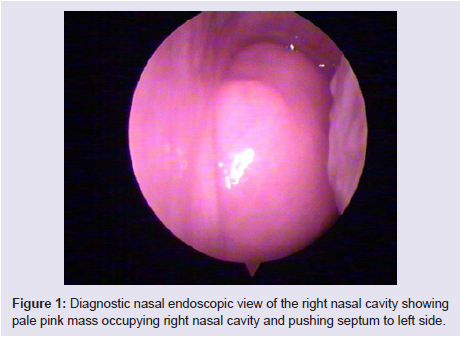
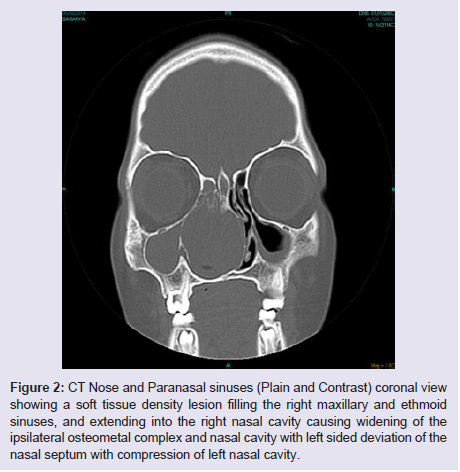
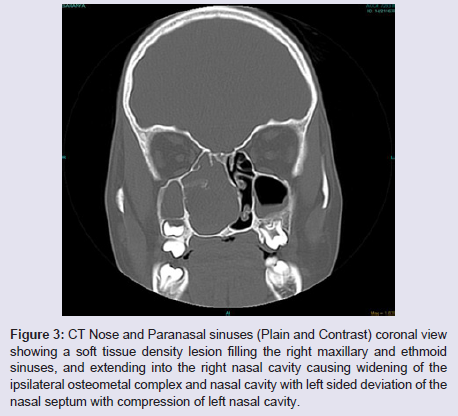
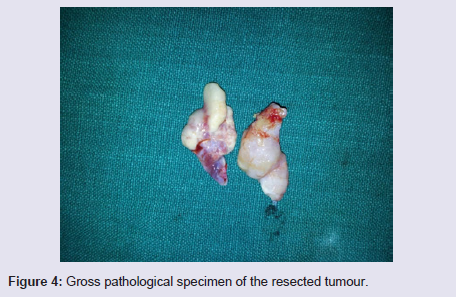
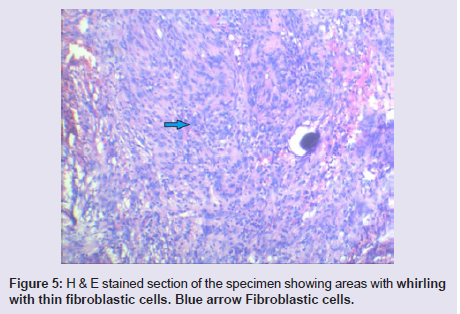
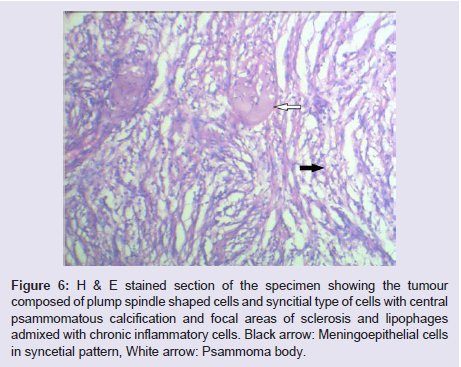
The general and neurological examinations were within normal limits. Examination of the external framework of the nose revealed widening of the bridge of the nose with hyperpigmentation of the skin over the nose and columella. Anterior rhinoscopy showed a pale greyish-white glistening mass which was insensitive to touch on probing and was pedunculated and appeared to arise from the area of the middle turbinbate. It did not bleed on touch and it had pushed the septum to the opposite side causing gross deviation of the septum to left side. Diagnostic nasal endoscopy showed a pale greyish-white mass completely occupying the right nasal cavity and pushing the septum to the left side (Figure 1). Computed tomography of the nose and paranasal sinuses showed a soft tissue density lesion with an attenuation value of ~30-40 HU filling the right maxillary and ethmoid sinuses, and extending into the right nasal cavity causing widening of the ipsilateral osteometal complex and nasal cavity with left sided deviation of the nasal septum with compression of left nasal cavity and there was evidence of mild rarefaction of the septum and extension of the lesion into the right choana (Figure 2 and 3). The osteomeatal unit on the left side was obliterated. These radiological features were more in favour of a right antrochoanal polyp, and with this diagnosis in mind, the patient was taken up for Transnasal Endoscopic Polypectomy. Intraoperatively, a slough-covered smooth mass with soft-to-rubbery consistency about 4 cm in its greatest dimension, was seen arising from the medial surface of the middle turbinate, filling the entire right nasal cavity with an atrophied right inferior turbinate, but the choana was free (Figure 4). The mass was excised and the tissue was sent for histopathological examination. Histopathological examination revealed polypoidal tissue showing tumour composed of plump spindle shaped cells and syncitial type of cells with central psammomatous calcification. Focal areas of sclerosis and lipophages admixed with chronic inflammatory cells were seen. Focal areas showing whirling with thin fibroblastic cells were seen. No atypical features or mitosis or necrosis seen. The features were consistent with meningioma (Figure 5 and 6).
Figure 1: Diagnostic nasal endoscopic view of the right nasal cavity showingpale pink mass occupying right nasal cavity and pushing septum to left side.
Figure 2: CT Nose and Paranasal sinuses (Plain and Contrast) coronal view showing a soft tissue density lesion filling the right maxillary and ethmoid sinuses, and extending into the right nasal cavity causing widening of the ipsilateral osteometal complex and nasal cavity with left sided deviation of the nasal septum with compression of left nasal cavity.
Figure 3: CT Nose and Paranasal sinuses (Plain and Contrast) coronal view showing a soft tissue density lesion filling the right maxillary and ethmoid sinuses, and extending into the right nasal cavity causing widening of the ipsilateral osteometal complex and nasal cavity with left sided deviation of the nasal septum with compression of left nasal cavity.
Figure 5: H & E stained section of the specimen showing areas with whirling with thin fibroblastic cells. Blue arrow Fibroblastic cells.
Figure 6: H & E stained section of the specimen showing the tumour composed of plump spindle shaped cells and syncitial type of cells with central psammomatous calcification and focal areas of sclerosis and lipophages admixed with chronic inflammatory cells. Black arrow: Meningoepithelial cells in syncetial pattern, White arrow: Psammoma body.
Following the diagnosis of meningioma, the patient was referred to the neurologist who found no localizing signs or symptoms. Electroencephalogram was normal. The patient was followed up regularly postoperatively for the past 1 year and there was no recurrence.
Discussion
Meningiomas are considered to be one of the most common tumours of the central nervous system. These tumours are rarely found extracranially, primarily in the head and neck region. The majority of reported extracranial meningiomas have demonstrable connection with an intracranial tumour. Extracranial meningiomas have been found in the orbital cavity, ear, nose, paranasal sinuses, parotid, neck, pharynx, mandible and skin. Extracranial meningiomas account for less than 2% of all meningiomas and occur most commonly in females with a median age of 45. The mean age for diagnosis of nasal fossa meningiomas is 28 with 40% of patients under 20 years [4].
Primary extracranial meningiomas can be defined as those not connected with an underlying meningioma of the neuraxis. Due to their rarity, one must exclude an extension from a central meningioma [5].
Arachnoid cells (arachnoid granulations, meningiocytes, meningothelial cells, pacchionian bodies) are thought to arise from the neural crest. They normally line the inner aspect of the arachnoid membrane, and fill the cores of the arachnoid villi that project into the lumens of dural veins and venous sinuses. More and more evidence is appearing that supports the development of meningiomas from arachnoid cap cells, with different mechanisms to suggest how extracranial meningiomas arise: 1. Arachnoidal cells are present in the sheaths of nerves or vessels where they emerge through the skull foramina. These arachnoidal cells may be apparently carried out with the nerves as the latter extend outward during embryonic development, 2. Displaced pacchionian bodies become detached, pinched off, or entrapped during embryologic development in an extracranial location, 3. A traumatic event or cerebral hypertension that displaces arachnoid islets, or 4. An origin from undifferentiated or multipotential mesenchymal cells, such as fibroblasts, Schwann cells, or a combination of these, perhaps explaining the diverse pathologic spectrum found in meningiomas [4].
Consequently, by either of these mechanisms, arachnoid cells are identified outside the neuraxis and give rise to meningiomas in extracranial locations. While there are a number of reported cases of “ectopic” or “heterotopic” meningiomas, most of these were reported prior to the modern improvement in radiographic techniques which serves to more definitely exclude intracranial disease. It is important to exclude an intracranial component radiographically or during surgery to yield the best possible management and follow-up. The possibility of an intracranial tumour must always be considered if long term management is to achieve its intended goal [6].
The extracranial meningiomas can be divided into four groups: 1. Extracranial meningiomas representing an extension from a primary intracranial meningioma (Secondary), 2. Extracranial meningioma arising from arachnoidal cell rests of cranial nerve sheathes (cranial nerves I, III, VII, IX, X, XI and XII) (Primary), 3. Extracranial meningiomas without any connection with foramina or cranial nerves (Primary), and 4. Extracranial metastases from intracranial meningiomas (Secondary) [7].
The nasal mucous membrane possesses a unique representation of the meningo-endoneurial junction. This transition from leptomeningeal cells to endoneurium occurs in the neighbourhood of the glio-schwannian junction (Obersteiner-Redlich zone) of both cranial and spinal nerves. Like all such embryonic tissue unions, the meningo-endoneurial junction is thought to be a seat of predilection for malformation or tumour formation (e.g. schwannoma or meningioma of cranial nerve VIII in the internal auditory meatus). The parallel junctions relating to the meningo-endoneurial transitions around the olfactory filaments are less well defined. It is generally presumed however, that the leptomeningeal components extend through the cribriform apertures and establish continuity of CSF and mucosal interstices. It is possible that this continuity accounts for occasional arachnoidal rests on the nasal side of the bony plate. Further, central neural vestiges relating to the embryonic vomeronasal organ or to the nervus terminalis might well provide neuroskeletal intertissue for an embryogenetic tissue back-drop in an isolated case of primary nasal meningioma [8].
Despite its usual benign histologic appearance, the meningioma sometimes demonstrates marked invasive properties. Direct extension of intracranial meningiomas to the calvaria, paranasal sinuses, nasal cavity, middle ear and internal ear have been described. Most of these tumors originate in the anterior cranial fossa, especially over the cribriform plate, orbital cavity, or the medial portion of the sphenoidal ridge. Some can be interpreted as outward extending tumours from the Obersteiner-Redlich junction as in intervertebral or internal auditory meat us tumors [9].
Clinical features are non-specific. Macroscopically, the tumor is solid, well circumscribed and lobulated, but sometimes polypoid. It is pink to gray in colour [6].
The differential diagnosis of extracranial meningiomas in the sinonasal tract include benign and malignant tumors, such as epithelial neoplasms (carcinoma), tumors originating from the neural crest (melanoma, olfactory neuroblastoma) and vascular and mesenchymal tumors (hemangioma, sarcoma, angiofibroma, paraganglioma, aggressive psammomatoid ossifying fibroma) [4].
Radiological imaging can assist in diagnosis, however does not necessarily provide a definitive diagnosis for the nature of the lesion. Therefore, histology remains the gold standard for diagnosis. Important CT and MRI features include the appearance of calcification, intracranial extension and marked contrast enhancement with a typical dural tail. Meningiomas in children tend to present atypical characteristics in imaging examination, including cysts, hemorrhage, aggressiveness and uncommon locations. Computed tomography better demonstrates the presence of intratumoral calcification, the hyperdense aspect of the lesion and homogeneous iodionated contrast enhancement. The MRI supplies more precise information related to the extension and tumoral invasion of adjacent structures, characterized by an increased T1 and T2-weighted signal intensity and with the same pattern of the contrast enhancement of CT [10].
Histologically, meningiomas have been classified into four microscopic patterns according to the predominant cellular morphology: 1. Syncitial (meningiothelial), characterized by uniform sheets of polygonal cells and nuclei which tend to have a typical round to ovoid appearance and contain pseudo-inclusions; 2. Transitional (mixed) or psammomatous, with the typical whorled pattern of polygonal or spindle cells; 3. Fibrous (fibroblastic), manifesting increased collagenous tissue and decreased cellularity, and 4. Angioblastic (angiomatous). The angioblastic type, which is relatively more aggressive, may have a mesenchymal nonmeningothelial origin and is best regarded as a hemangiopericytoma of the meninges. The most common form is the meningothelial meningioma [5].
Immunohistochemical studies are indispensable in the differential diagnosis of primary extracranial meningiomas from other benign tumours of peripheral nerve origin. The positive expression of EMA (epithelial membrane antigen) and vimentin antigen demonstrated the dual epithelial and mesenchymal characteristics of the cells found in these tumours and agrees with previous studies on antigen expression in meningioma. Meningiomas have a weaker positivitytesting for S-100 protein [7].
Meningiomas are benign in so far as they do not metastasize, but they often show a predeliction for local permeation of crevices and foramina, whilst pressure necrosis may result in spread from one cavity to the other.
In general, the prognosis of extracranial meningiomas appears to be excellent, with an overall median survival of 28 years. This is tempered by the specific anatomic site, histologic type, tumor grade, gender and age of the patient. WHO classification (2007) categorises extracranial meningiomas according to their histological appearance and clinical behavior. WHO grade I tumors generally have an excellent prognosis with infrequent recurrence rates. They are benign with transitional, psammomatous, fibrous, meningothelial, angiomatous or secretory histological patterns. Malignant menigiomas (WHO grade III) are usually fatal with higher rates of recurrence and metastasis. Studies have shown a 5 year mortality rate of 68% with this type. The recurrence rate for meningiomas after total excision varies from 7% to 84% depending upon the number of years of followup. Meticulous surgical extirpation of extracranial meningiomas is important to minimize the recurrence rate, without the necessity of adjuvant therapy [4].
Current treatment of choice for a localized primary extracranial meningioma is complete surgical excision with no documented beneficial effect of radiotherapy or chemotherapy, particularly in benign disease. It is important to consider the location of the tumour and potential disastrous effects to local surrounding structures. Frozen sections are particularly useful in assessing the surgical procedure and should be performed whenever possible to exclude the malignant nature of the lesion and avoid over-treatment. When it is not possible to perform total surgical resection of the lesion as a result of the complex anatomy of both nasal cavity and paranasal sinuses, adjuvant therapeutic options is restricted to radiation therapy andchemotherapy [10].
Conclusion
While primary extracranial meningiomas have no evidence of direct attachment to the brain, the majority of extracranial lesions are a secondary location of a primary intracranial tumour. Primary extracranial meningiomas may arise from arachnoidal cells present in the sheaths of nerves or vessels where they emerge through the skull foramina, displaced pacchionian bodies, displaced arachnoid islets or from undifferentiated or multipotential mesenchymal cells. It is therefore imperative to investigate this once a diagnosis of meningioma has been made. It must be pointed out that it is extremely difficult to suspect a primary extracranial meningioma clinically in the nose other than certain demographic features such as female patients in the 4th decade, and almost certainly not possible to suspect radiologically. Pre-operative biopsy will help to clinch the diagnosis, but only after having ruled out a vascular mass by a contrast enhanced computed tomography. Complete surgical excision is the treatment of choice.
References
- Louis DN, Ohgaki H, Weistler OD, Cavenee WK (2007) World Health Organization classification of tumours of the central nervous system. (4th Edn) Lyon: IARC Press.
- Nichols RD, Knighton RS, Chason JL, Strong DD (1987) Meningioma in the parotid region. Laryngoscope 97: 693-696.
- Chang CY, Cheung SW, Jackler RK (1998) Meningiomas presenting in the temporal bone: the pathways of spread from an intracranial site of origin. Otolaryngol Head Neck Surg 119: 658-664.
- Rushing EJ, Bouffard JP, McCall S, Olsen C, Mena H, et al. (2009) Primary extracranial meningiomas: an analysis of 146 cases. Head and Neck Pathol 3: 116-130.
- Kirshisnik M, Callender DL, Batsakis JG (1993) Extracranial extraspinal meningiomas of the head and neck. Ann Otol Rhinol Laryngol 102: 967-970.
- Batsakis JG (1984) Pathology consultation. Extracranial meningiomas. Ann Otol Rhinol Laryngol 93: 282-283.
- Hoye SJ, Hoar CS, Murray JE (1960) Extracranial meningioma presenting as a tumour of neck. Amer J Surg 100: 486-489.
- Batsakis JG (1984) Pathology consultation. Extracranial meningiomas. Ann Otol Rhinol Laryngol 93: 282-283.
- Boldrey E (1971) The meningiomas. In Pathology of the Nervous System. Minckler J (Eds) New York, McGraw-Hill 2: 2125-2143.
- Taori K, Kundaragi NG, Disawal A, Jathar C, Gaur PP, et al. (2011) Imaging features of extracranial pharyngeal space meningioma: case report. Iran J Radiol 8: 176-181.