Journal of Orthopedics & Rheumatology
Download PDF
Research Article
Clinicians' Preferences and Perspectives on the use of Polmacoxib for Managing Osteoarthritis
Manjula S and Krishna Kumar M
Department of Medical Services, Micro Labs Limited, Bangalore, Karnataka, India
*Address for Correspondence:Dr Manjula S, Department of Medical Services, Micro Labs
Limited, Bangalore, Karnataka, India. Email Id: drmanjulas@gmail.com
Submission: 20 May 2025
Accepted: 17 July 2025
Published: 18 July 2025
Copyright: ©2025 Manjula S, et al. This is an open access article
distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Keywords:Polmacoxib; Osteoarthritis; Nonsteroidal Anti-Inflammatory Drugs; Expert Perspectives
Abstract
Objective:To gather clinicians' preferences and perspectives on
managing osteoarthritis (OA) using polmacoxib in Indian settings.
Methodology:The cross-sectional study was conducted using a 23-item, multiple-response questionnaire to collect perspectives from specialists experienced in managing OA in routine clinical practice in India. The study included questions on current prescription practices, clinical observations, preferences, and experiences related to polmacoxib use in OA treatment. Descriptive statistics were used to analyze the data, and categorical variables were presented as percentages to provide a clear understanding of their distribution.
Results:The study included responses from 281 clinicians. Among them, 45% reported prescribing polmacoxib to 26–50% of their OA patients. Additionally, 48% indicated that polmacoxib is more commonly used in OA patients with comorbid conditions. According to 54% of respondents, the key advantages of polmacoxib include its novel tissue-specific transport mechanism that ensures sustained drug delivery to inflamed tissues, lack of COX-2 inhibition in calcium rich tissues, and a favorable tolerability profile, particularly in terms of cardiovascular, renal, and gastrointestinal safety. Nearly 86% of participants highlighted several benefits of polmacoxib over etoricoxib, such as greater potency at a lower dose (2 mg/day versus 60–120 mg/ day for etoricoxib), a lower risk of gastrointestinal side effects, and tissue selectivity. Approximately 86% of experts identified the unique features of polmacoxib as including a faster onset of symptom relief, convenience of once-daily dosing, the lowest recommended NSAID dose (2 mg/day), significantly improved gastrointestinal safety, and enhanced cardiovascular safety due to its tissue-selective COX-2 inhibition.
Conclusion:The study highlights the preference among Indian clinicians for the use of polmacoxib in OA management, especially in patients with comorbidities. Clinicians cited its tissue-selective action, low effective dose, improved gastrointestinal and cardiovascular safety, and once-daily dosing as key advantages. Overall, polmacoxib is perceived as a potent, well-tolerated alternative to traditional NSAIDs in clinical practice.
Methodology:The cross-sectional study was conducted using a 23-item, multiple-response questionnaire to collect perspectives from specialists experienced in managing OA in routine clinical practice in India. The study included questions on current prescription practices, clinical observations, preferences, and experiences related to polmacoxib use in OA treatment. Descriptive statistics were used to analyze the data, and categorical variables were presented as percentages to provide a clear understanding of their distribution.
Results:The study included responses from 281 clinicians. Among them, 45% reported prescribing polmacoxib to 26–50% of their OA patients. Additionally, 48% indicated that polmacoxib is more commonly used in OA patients with comorbid conditions. According to 54% of respondents, the key advantages of polmacoxib include its novel tissue-specific transport mechanism that ensures sustained drug delivery to inflamed tissues, lack of COX-2 inhibition in calcium rich tissues, and a favorable tolerability profile, particularly in terms of cardiovascular, renal, and gastrointestinal safety. Nearly 86% of participants highlighted several benefits of polmacoxib over etoricoxib, such as greater potency at a lower dose (2 mg/day versus 60–120 mg/ day for etoricoxib), a lower risk of gastrointestinal side effects, and tissue selectivity. Approximately 86% of experts identified the unique features of polmacoxib as including a faster onset of symptom relief, convenience of once-daily dosing, the lowest recommended NSAID dose (2 mg/day), significantly improved gastrointestinal safety, and enhanced cardiovascular safety due to its tissue-selective COX-2 inhibition.
Conclusion:The study highlights the preference among Indian clinicians for the use of polmacoxib in OA management, especially in patients with comorbidities. Clinicians cited its tissue-selective action, low effective dose, improved gastrointestinal and cardiovascular safety, and once-daily dosing as key advantages. Overall, polmacoxib is perceived as a potent, well-tolerated alternative to traditional NSAIDs in clinical practice.
Introduction
Globally, osteoarthritis (OA) affects approximately 7.6% of
the population, and its burden is projected to increase by 60%
to 100% by 2050. [1,2] It is the most common joint disease, with a
prevalence ranging from 22% to 39%, and is the second most frequent
rheumatologic condition in India.[3] OA is more prevalent in women
than in men, with the incidence rising significantly with age. Knee
OA is a major contributor to mobility impairment, especially among
females. Additionally, OA ranks as the 10th leading cause of nonfatal
disease burden worldwide. [4,5]
The management of OA typically involves a combination of
non-pharmacologic approaches, such as physical therapy and
lifestyle modification, and pharmacologic interventions, including
acetaminophen and nonsteroidal anti-inflammatory drugs
(NSAIDs).[6,7] However, long-term NSAID use is associated with
adverse gastrointestinal, renal, and cardiovascular effects, especially
in elderly patients, highlighting the need for safer and more tolerable
therapeutic alternatives and newer NSAIDs with improved safety
profiles for OA treatment.[8]
Polmacoxib, a novel NSAID, has emerged as a promising
therapeutic option for the management of OA. It is a selective
cyclooxygenase-2 (COX-2) inhibitor that functions through a unique
dual mechanism by inhibiting COX-2 and binding strongly to
carbonic anhydrase (CA), an enzyme responsible for regulating pH
balance in the body. [9] This dual mechanism is intended to reduce
the cardiovascular risks typically associated with selective COX-2
inhibition, while potentially enhancing its anti-inflammatory efficacy
at sites of joint inflammation. In conditions where both COX-2
and CA are co-expressed, polmacoxib’s strong affinity for CA may
modulate the extent of COX-2 inhibition. Studies have shown that its
COX-2 inhibitory effect may vary depending on the concentration of
CA in the local environment. Its favorable safety and efficacy profile
supports its long-term use, particularly in patients with comorbid
cardiovascular or gastrointestinal risks, where traditional NSAIDs
may be contraindicated. [10,11]
Polmacoxib is approved for the treatment of OA in South Korea,
Turkey, and across the Middle East and North Africa region, which
includes 19 countries. Most recently, in 2023, it received approval from
the Drug Controller General of India for the treatment of idiopathic
primary OA affecting the hip and knee joints. [11,12] Despite the
availability of several clinical studies, the clinician's perspectives
in actual practice remain limited. This study is intended to gather
expert opinion on the role of polmacoxib in the management of
OA, evaluating their perspectives on its efficacy, safety, and practical
utility in clinical settings.
Methodology
We carried out a cross-sectional study among clinicians across
India who manage OA from June 2024 to December 2024. The study
was conducted after receiving approval from Bangalore Ethics, an
Independent Ethics Committee, which was recognized by the Indian
Regulatory Authority, the Drug Controller General of India.
An invitation was sent to leading clinicians in managing OA in the
month of March 2024 for participation in this Indian survey. About
281 clinicians from major cities of all Indian states, representing the
geographical distribution, shared their willingness to participate and
provide necessary data. The questionnaire booklet titled PRIMER
(Polmacoxib Expert Perspective in Management of Osteoarthritis)
was sent to clinicians who were interested in participating in this
study. The questionnaire consisted of 23 items focused on current
clinical experiences, prescription practices, treatment observations,
and expert feedback related to OA management. Participants were
allowed to skip any questions they did not wish to answer, with
unanswered questions considered unattempt. Clinicians were
instructed to complete the questionnaire independently without
consulting colleagues. Written informed consent was obtained from
all participants prior to the study.
Statistical Analysis:
Data analysis was performed using descriptive statistics.
Categorical variables were summarized as frequencies and percentages
to illustrate their distribution. To facilitate better visualization of the
data, bar graphs were created using Microsoft Excel 2019 (version
16.0.17928.20114).Results
The study included 281 experts, of whom 70% reported that
approximately 11% to 25% of patients with OA presenting to routine
practice are under 50 years of age. More than half (55.52%) of the
clinicians reported that 31% to 40% of patients with OA are men.
About 51% of the clinicians opined that patients screened for OA more
often belong to urban areas. According to 48% of the participants,
the key factor contributing to the increasing disease burden of OA in
India is a sedentary lifestyle. About 50% of the respondents stated that
elderly patients pose unique challenges in OA management.
As reported by nearly half the participants (47.33%), lack of
awareness is the most common limitation in OA treatment. Around
45% of the clinicians stated that about 26% to 50% of patients with
OA have a higher BMI (overweight or obese), while 41% reported
that 11% to 25% of patients have a higher BMI. According to 43% of
the clinicians, about 26% to 50% of patients have knee OA, while 41%
indicated that 11% to 25% of patients have knee OA. Around 42% of
respondents opined that 11% to 20% of patients have hip OA. More
than half (56.94%) of the participants reported that OA of the hand or
shoulder is more common among elderly patients.
Approximately 56% of clinicians opined that older age is the most
common attributing factor for OA in the majority of patients. As
reported by 56% of the respondents, etoricoxib is the most commonly
preferred NSAID. Nearly 52% of clinicians stated that paracetamol
is prescribed in about 11% to 20% of OA patients. Approximately
43% of respondents reported that 26% to 50% of patients required
additional NSAIDs or other analgesics for pain management, while
an equal proportion indicated that 11% to 25% of patients required
such additional medications. Nearly 41% of respondents each
reported that gastrointestinal side effects from traditional NSAIDs
occur in 11% to 25% and 26% to 50% of OA patients, respectively.
Approximately 44% of participants reported that 11% to 25%
of patients with OA on NSAIDs experience cardiovascular or
renal adverse effects. Nearly 46% of clinicians opined that the risk
of gastrointestinal-related side effects is the drawback of COX-2
selective NSAIDs. According to 45% of respondents, about 26%
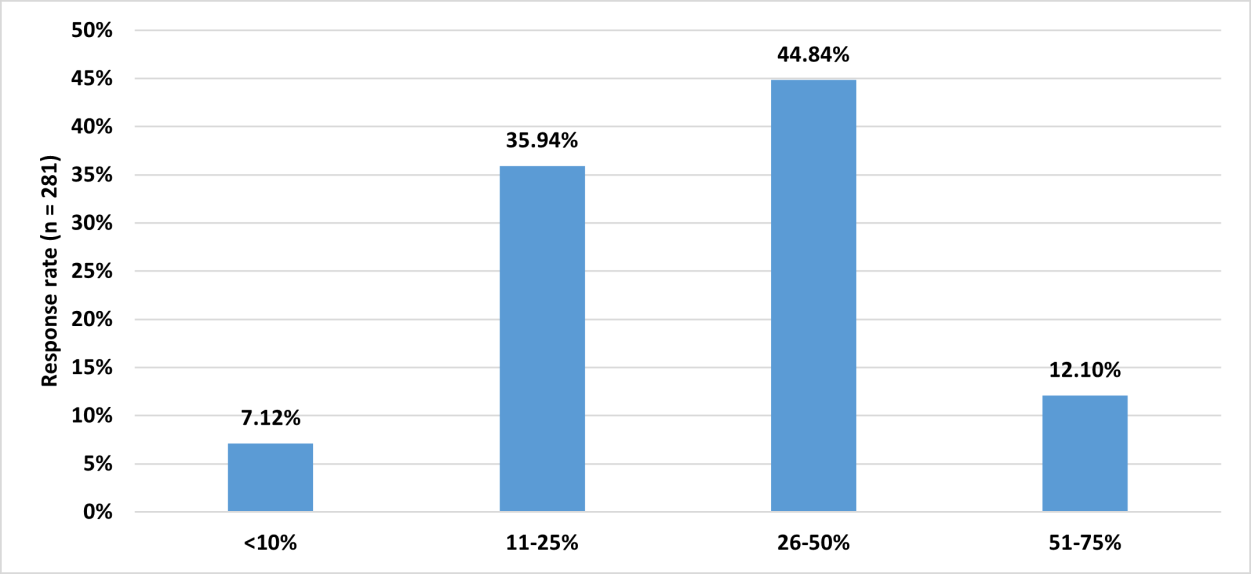
to 50% of patients with OA are prescribed polmacoxib [Figure 1].
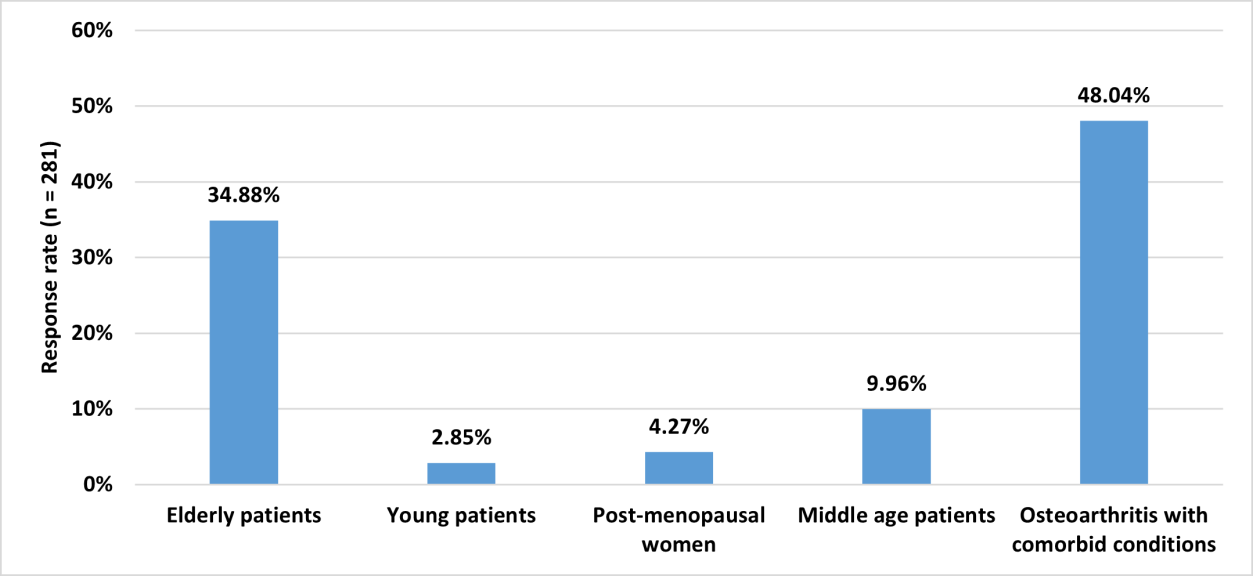
Approximately 48% stated that patients with OA and comorbid
conditions are more likely to use polmacoxib [Figure 2].
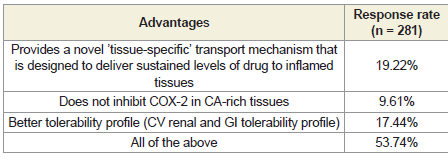
Approximately 54% of respondents reported that the advantages
of polmacoxib include a novel ‘tissue-specific’ transport mechanism
designed to deliver sustained levels of the drug to inflamed tissues,
minimal COX-2 inhibition in calcium-rich tissues, and a better
overall tolerability profile (cardiovascular, renal, and gastrointestinal)
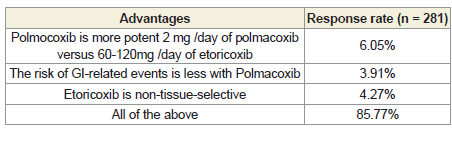
[Table 1]. According to 86% of participants, the advantages of
polmacoxib over etoricoxib are its higher potency at a lower dose (2
mg/day of polmacoxib versus 60-120 mg/day of etoricoxib), lower
risk of gastrointestinal-related events, and the fact that etoricoxib
lacks tissue selectivity [Table 2].
Polmacoxib has high tissue selectivity, whereas naproxen,
ibuprofen, and diclofenac have low tissue selectivity. Additionally,
polmacoxib offers improved cardiovascular safety, while naproxen,
ibuprofen, and diclofenac are associated with a higher risk of
gastrointestinal events. These advantages of polmacoxib over the
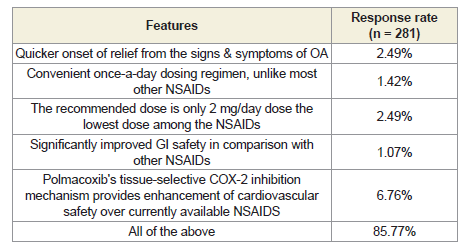
other NSAIDs were reported by 87% of clinicians. The majority of
respondents (85.77%) reported that the unique features of polmacoxib
include quicker onset of relief from OA symptoms, a convenient
Figure 2:Human microbiota composition in different locations (oral cavity,
respiratory tract, skin, gut, and vagina).
once-daily dosing regimen, the lowest recommended dose among
NSAIDs (2 mg/day), significantly improved gastrointestinal safety,
and enhanced cardiovascular safety due to its tissue-selective COX-2
inhibition mechanism [Table 3].
Discussion
The study highlights evolving clinical practices in OA management
across India, with a shift towards individualized treatment, especially
in younger, urban, and comorbid populations. The present study
showed that a significant proportion of clinicians prescribed
polmacoxib to their OA patients. Similarly, a cross-sectional study
conducted in routine clinical settings across India reported that
many clinicians had incorporated polmacoxib monotherapy into
their treatment protocols, reflecting its growing acceptance and
integration into standard OA management.[13] Hussain et al.,
in their review of polmacoxib, concluded that this drug presents a
promising therapeutic option for the management of OA and other
inflammatory conditions.[10]
In the current study, clinicians reported that patients with OA and
comorbid conditions are more likely to be prescribed polmacoxib,
highlighting its perceived safety profile in complex patient subsets.
Supporting this observation, Sinha et al. compared polmacoxib 2
mg to celecoxib 200 mg in patients with idiopathic OA of the hip or
knee and concluded that polmacoxib is non-inferior to celecoxib in
terms of safety and efficacy, making it a viable alternative, particularly
for patients where traditional NSAIDs may be contraindicated.[14]
Similarly, Lee et al. reported that polmacoxib 2 mg is relatively well
tolerated and may be preferable for pain relief in OA patients due
to its reduced gastrointestinal side effects compared to traditional
NSAIDs.[15]
The current study respondents highlighted several key advantages
of polmacoxib, emphasizing its unique tissue-specific transport
mechanism, which enables sustained delivery of the drug to inflamed
tissues. Studies have also demonstrated that erythrocytes contribute
to the tissue-specific delivery of polmacoxib, transporting the drug
preferentially to CA-deficient inflamed tissues. While in circulation,
polmacoxib remains bound to CA within erythrocytes, which helps
maintain low systemic exposure and allows focused release at the
sites of inflammation. This targeted delivery is believed to maximize
therapeutic benefit in osteoarthritic joints while minimizing adverse
effects on the cardiovascular, renal, and gastrointestinal systems.
[16,17,11]
A comprehensive review has further supported its superior
tolerability profile, attributing this to its targeted delivery and
selective action at sites of inflammation. These properties position
polmacoxib as a promising alternative to conventional NSAIDs,
offering meaningful improvements in safety and efficacy.[9] Studies
have shown that erythrocytes provide a tissue-specific transport
mechanism delivering sustained levels of the drug to CA-deficient
inflamed tissues. This, in turn, helps maintain low systemic exposure
as polmacoxib is transported in a combined state with CA within
the erythrocytes. Thus, polmacoxib is believed to offer maximum
effectiveness in inflamed osteoarthritic joints while reducing its
effects on the cardiorenal system or the gastrointestinal tract. [16]
In the present study, polmacoxib demonstrated several advantages
over etoricoxib. Notably, it is more potent, with an effective dose of
just 2 mg/day compared to the 60–120 mg/day typically required
for etoricoxib. Additionally, polmacoxib is associated with a lower
risk of gastrointestinal-related adverse events. Lee et al. reported
that polmacoxib 2 mg was well tolerated and showed superior
efficacy to placebo and non-inferiority to celecoxib after six weeks
of treatment in patients with OA.[15] Their findings support the
potential of polmacoxib as an effective analgesic with a reduced GI
side effect profile. Further reinforcing its safety profile, Easwaran
et al. demonstrated that polmacoxib does not significantly affect
blood pressure or heart rate, and clinical trials have not reported
an increase in cardiovascular events, highlighting its favorable
cardiovascular safety profile.[11] Despite NSAIDs, chondroitin sulfate
supplementation and intra-articular injections of hyaluronic acid
have also been used in the management of OA for their effectiveness
on pain symptoms and joint mobility.[18]
The present study provides valuable insights into the use of
polmacoxib in the management of OA within Indian clinical practice.
These findings hold significant relevance, given the limited published
literature on polmacoxib use in the Indian context. One of the
major strengths of the study is the use of a structured and validated
questionnaire. However, several limitations must be acknowledged.
The study relies solely on expert opinion, which may introduce
inherent biases due to variations in individual clinical experience
and preferences. Additionally, the study methodology may not
fully capture emerging trends or newly evolving clinical evidence.
The absence of direct patient data limits the ability to correlate
clinician-reported findings with real-world clinical outcomes. These
limitations should be considered when interpreting the results.
Future research should include prospective observational studies or
real-world evidence to validate clinician perceptions and provide a
more comprehensive understanding of polmacoxib’s role in OA
management.
Conclusion
The study findings indicate that a substantial proportion of
clinicians favor the use of polmacoxib, particularly in patients with
comorbid conditions, citing its favorable efficacy and safety profile.
The key advantages identified include its tissue-selective mechanism,
lower effective dose, improved gastrointestinal and cardiovascular
tolerability, and convenient once-daily dosing. These findings
suggest that polmacoxib is perceived as a potent and well-tolerated
therapeutic option, offering meaningful benefits over traditional
NSAIDs in routine clinical practice.
Acknowledgement
We would like to thank all the clinicians who were actively
participating in this study.
Author contributions:
Both authors have contributed equally to the development of the
manuscript.Disclosure of compliance with ethical principles:
The study was conducted after receiving approval from Bangalore
Ethics, an Independent Ethics Committee, which was recognized by
the Indian Regulatory Authority, Drug Controller General of India.