Journal of Orthopedics & Rheumatology
Download PDF
Research Article
Validation of Incorporating Standardized Formula with Phytoconstituents in Knee Osteoarthritis: A Randomized, Placebo-Controlled Study
Shridhar Pandya1, Chetan Savaliya2, Amol Gothwad3, Tanuja Panchabhai4, Gayatri Ganu5 and Dheeraj Nagore6*
1Director, Gplife Healthcare Pvt Ltd, 705-706, Orbit- 1 Building,
Punagam-Saroli Rd, Near RRTM Market, Surat, 395010, India
2Director, Gplife Healthcare Pvt Ltd, 705-706, Orbit- 1 Building,
Punagam-Saroli Rd, Near RRTM Market, Surat, 395010, India
3Consultant, Lokmanya Medical Research Centre Lokmanya Hospital,
Floor 4, OPD 1, 314/B Telco Road, Chinchwad, Pune, 411033
Maharashtra, India
4Consultant, Atharv multispecialty Research center, Krishna chowk,
Kirti Nagar, New Sanghavi, Pune 411027 Maharashtra, India.
5Director, Mprex Healthcare Pvt Ltd, 501, 514, Crossroads building,
Bhumkar Chowk, Wakad, Pune 411057, India
6Director, Mprex Healthcare Pvt Ltd, 501, 514, Crossroads building,
Bhumkar Chowk, Wakad, Pune 411057, India
*Address for Correspondence:
Dr. Dheeraj Nagore, Mprex Healthcare Pvt Ltd, 501, Crossroads
building, Bhumkar Chowk, Wakad, Pune 411057, India.
Email: dheeraj@mprex.in
Submission: 19 October 2022
Accepted: 08 February 2023
Published: 13 February 2023
Copyright: © 2023 Pandya S, et al. This is an open access article
distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Abstract
Background: Osteoarthritis (OA) is the most common joint disease
with a prevalence of 22% to 39% in India and being foremost causes
of nonfatal burden. The use of conventional medication can be
associated with insufficient clinical management, serious side effects.
The present research validates using Joint Support Product (JSP) a
standardized, specially designed herbal extract-based formula with
phytonutrients which are useful in the management of symptoms as
well as regeneration in OA.
Method: Clinical trial was randomized placebo-controlled
involving 150 patients with knee osteoarthritis included in two parallel
groups equally. JSP and placebo were provided for 90 days. The
celecoxib tablets up to 200 mg as rescue was allowed and recorded.
The objectives of the study were to assess the effectiveness and safety
of JSP in osteoarthritis by evaluating pain, stiffness, symptoms and antiinflammatory
activity to improve quality of life.
Results: Treatment of JSP in patients with osteoarthritis led to
reduction in pain, stiffness and other related parameters to provide the
symptomatic relief and thus improved quality of life. Treatment with
JSP reduced the inflammatory markers by 50% suggestive potential
joint restorative action. With JSP, most of the subjects showed reduced
dependency on the analgesics as a rescue. There were no evident
side effects of the JSP treatment.
Conclusion: It can be concluded from the study that JSP is safer
and effective option in the management of OA. With treatment
with JSP, most of the subjects showed reduced dependency on the
analgesics as a rescue in the treatment tenure.
Keywords
Osteoarthritis; WOMAC; Boswellia; curcumin
Introduction
Osteoarthritis (OA) is the common rheumatologic joint disease
with a prevalence of 22% to 39% in India. OA was estimated to be
one of the foremost causes of nonfatal burden [1]. It is a chronic
progressively degenerating disorder with a collective etiology
regarded by the loss of articular cartilage, hypertrophy of bone at
the margins, subchondral sclerosis, and range of biochemical and
morphological alterations of the synovial membrane and joint [2].
The pathophysiological alterations in chronic OA include softening,
ulceration, focal disintegration of the articular cartilage along with
synovial inflammation [3]. The clinical symptoms associated with OA
are pain, with or without activity and weight-bearing; stiffness after
inactivity leading to reduced range of the motion of the joints [4].
The common joints affecting includes knees, spine, shoulders and
hips etc. OA of the knee is more prevalent as per the literature
available [5].
The common pharmacological interventions include oral and
topical analgesics, intra-articular corticosteroids, modified release dosage forms of the oral analgesics, hyaluronic acid, glutathione,
chondroitin, vitamin, mineral and collagen supplementation etc
along with non-pharmacological interventions like massage, exercise
and weight loss etc [6].
Due to lack of self-healing capacity of the articular cartilage, OA
is among the most challenging joint diseases and there is currently
no cure for it [7]. The conventional OA medications are limited to
control OA symptoms; moreover, none can reverse the damage in
the OA joint. The newer medicines like biologically derived molecules
and stem cell therapy and tissue engineering are in reassert phase and
it is evident that these newer therapies provide uncertain clinical
outcomes along with the side effects [8]. The use of conventional
medication in OA management can be associated with often
insufficient clinical management as well as serious side effects and
high costs. However, phytotherapy have shown the potential for safe
and effective management of arthritis [9].
Regenerative phytotherapy therapy holds the chance of repairing
and regenerating damaged or lost tissues to restore the original
structure and function. JSP is a standardized, specially designed
herbal extract-based formula with phytonutrients in the management
of symptoms as well as regeneration in OA and comprehensive trial
with actual clinical outcomes will be very useful. The present clinical
trial aims at generating evidences around safety and effectiveness of
incorporating a phytoconstituents based product in the management
of osteoarthritis.
Materials and Methods
Study objectives:
The primary objectives of the study were to assess the effectiveness
and safety of Joint Support Product (JSP) in subjects suffering from
knee osteoarthritis based on changes in inflammatory market status
like CRP, IL-6, performance of patient on pain VAS scale, local
examination, clinical signs assessment of WOMAC from baseline to
day 90 between groups. The secondary objectives of the study were to
evaluate symptom improvement like morning stiffness, tenderness,
tiredness and muscle spasms, along with improvement in quality-oflife
score, reduction in dependency over analgesics from baseline to
day 90 between groups. The tolerability and safety were also evaluated.Inclusion criteria:
Male and/or female volunteers aged between 40 to 80 years
both inclusive were included. The subjects willing to follow the diet,
exercise along with willing to come for regular follow-up visits were
included. Based on the American College of Rheumatology (ACR)
criteria, patients with osteoarthritis of the knee clinical OA of the knee
is defined as knee pain and at least three out of six of the following
criteria: age > 50 years, morning stiffness < 30 min, crepitus, bony
tenderness, bony enlargement, and no palpable warmth.Exclusion criteria:
Patients with congenital arthropathy, rheumatoid arthritis, active
gout, other type of arthritis with/without inflammation e.g. septic,
fibromyalgia or collagen vascular disease were excluded. Patients
with history of major trauma or surgery in the knee joint were not
included in the study. The study excluded obese or diabetic patients,
those with severe cardiac, renal, or hepatic disease, as well as pregnant
or nursing women.Methodology:
Patients between 40-80 years (both inclusive) receiving standard
treatment for knee osteoarthritis were screened for eligibility criteria.
On screening visit, a written informed consent was obtained from
subject for participation in the study. Subjects who were able to
understand and agreed to comply with the planned study procedures
and were available for all study visits.Subject’s demographic details were recorded. Subject underwent
clinical examination. Blood samples of all eligible subjects were
collected for biochemical and hematological investigations. Subjects
were enrolled after meeting all inclusion criteria and not showing
any exclusion criteria. On baseline visit (day 1), subject was recruited
in the study and randomized to one of the two study groups as
per the computer-generated randomization list either in JSP or in
Placebo groups. Subjects were asked for occurrence of any adverse
event during screening period. If subjects had any comorbidity or
concurrent illness, the condition and medication was recorded.
Subjects were screened for any other allergies for the ingredients of
investigational product.
Subjects in both groups received education regarding diet and
lifestyle. For 3 months, both groups consumed two tablets twice a day
after meals, either JSP or placebo.
All the subjects were allowed to consume rescue medication as
celecoxib tablet up to 200 mg in divided dosage as per requirement
and to be terminated as soon as the symptoms subside. Subject were
advised to continue concomitant medication other than protein
supplements, antioxidant agents, vitamins, nutraceutical, Ayurvedic,
herbal medication etc.
Drug compliance was assessed by the investigator on every follow
up visit. If any subject continuously missed dosing for >3 consecutive
days or total missed dose > 6 during the 30 days’period, subject was
treated as drop out. Subjects were assessed for any adverse events
during study period.
The subjects were asked to follow up on every month for 3 months
i.e., day 30, 60 and 90. On each visit day, subject underwent clinical
examination, symptoms, screening for any adverse event, assessment
of pain and flexibility by VAS and WOMAC scoring. On baseline and
day 90 visit all subjects were assessed for quality of life and changes
in biochemical, inflammatory marker and hematological parameters.
Subjects were asked to contact investigator for adverse events
between visits and provide details of rescue medication usage.
After completion of 90 days, the subject was asked to stop the study
medication. Investigator instructions were given for further treatment
to the subjects.
Normality of the data was checked by “The Kolmogorov-Smirnov
Test of Normality’’. Continuous variable i.e. age was summarized by
overall using summary statistics i.e. the number of observations, mean
and standard deviation with 95% CI (among normal distribution)
analyzed by student t test and gender was analyzed using chi square
test. In this study the changes in inflammatory marker status like
CRP and IL-6, was analyzed using student t test and Mann Whitney
U test. whereas, clinical signs assessment of WOMAC symptom,
improvement like morning stiffness, tenderness, tiredness and muscle
spasms, and quality-of-life score was analyzed by student t test. Pain
on VAS scale, and change in pain, stiffness and physical difficulty
domain score of WOMAC scale was analyzed by Mann Whitney
U test. Reduction in dependency over analgesics is documented as
number of events.
Intervention and dosage:
The key ingredients in JSP are standardized and fortified extracts
of Boswellia, Curcumin, Tinospora, Guggul, Nirgundi etc. Subjects
from JSP and placebo group were respectively advised to take two
tablets twice a day after meals for 90 days.Sample size:
Sample size calculation was performed by referring to the research
done by Shep et. Al., 2019, [10] based on a power of 90% and a type I
error rate of alpha of 0.05 (two-tailed), a sample size of 65 participants
per group was required to detect an estimated difference of 1.24 in
the mean pain scores between the treatment arms with a standard
deviation of 2.5. A total sample size of 75 participants per treatment
group was considered in this study.Randomization:
We intended to complete 150 subjects at the end of the study.
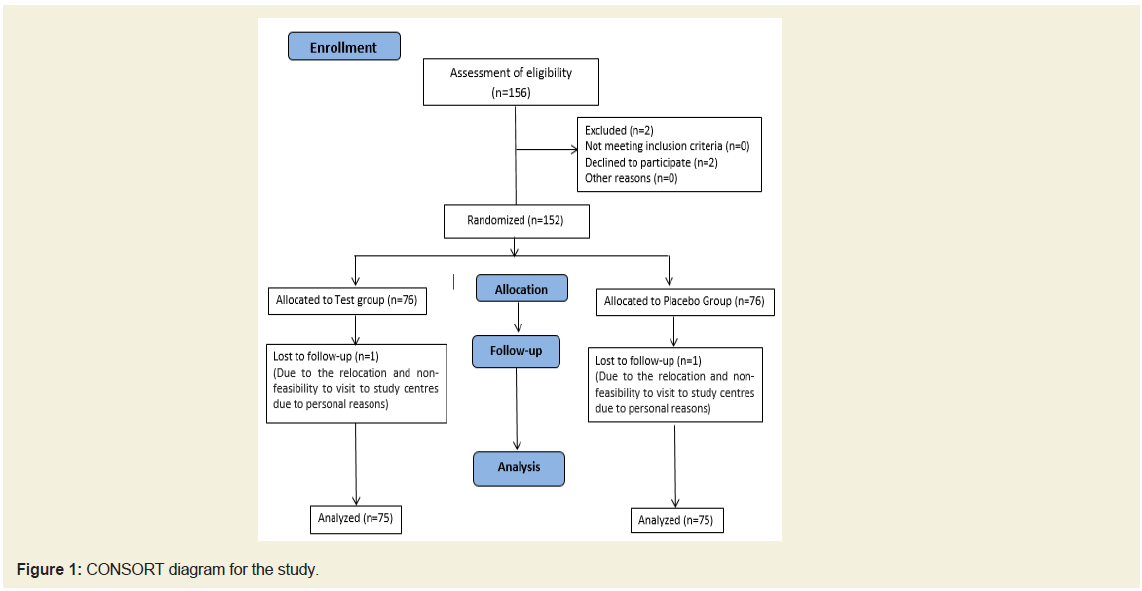
We screened 156 subjects of which four were screen failure. Total of 152 subjects entered the randomization out of which 150 subjects
were considered for the final analysis (75 in each group). The patient
disposition is depicted in Figure 1. This was a randomized study
wherein all the subjects were randomly allocated (as per computer
generated randomization list) to either one of the treatment arms i.e.,
JSP and placebo in 1:1 ratio. We received randomization schedule
from qualified statistician, investigator enrolled the participants to
respective study groups. The informed consent process is followed by
instigator to obtain informed consent documents signed by patients.Identical placebo in terms of color, size, shape, weight was
followed in order to keep both investigator as well as the subject
blind of which medication was being received. The de randomization
process was kept in place in case of adverse event which needs to
know about the treatment received by patient. The concealment of the
investigational products was achieved by numbering the containers
as per the subject’s identity numbers and randomized accordingly.
Statistical analysis has been done by using SPSS version 10.0.
Results
Demographic characteristics:
There were 152 subjects enrolled into study and 150 subjects
completed the study and data is analyzed (Figure 1). There were 75
evaluable subjects in each group. Both groups were comparable in
their gender distribution and mean age. The details are presented in
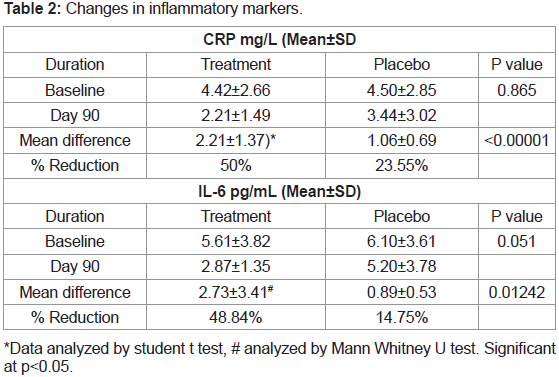
Table 1.Changes in inflammatory markers between groups:
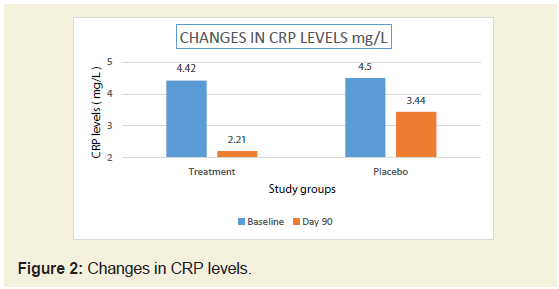
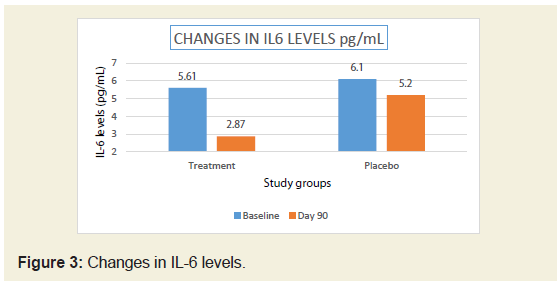
In the present study, there was significant reduction (50%)
in serum CRP levels in JSP treatment group compared to 23.55 %
in placebo group (Figure 2). There was 48.84% reduction in IL-6
levels in JSP group compared to 14.75 % in placebo group (Table 2)
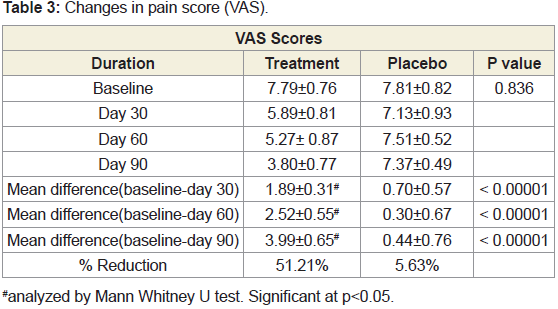
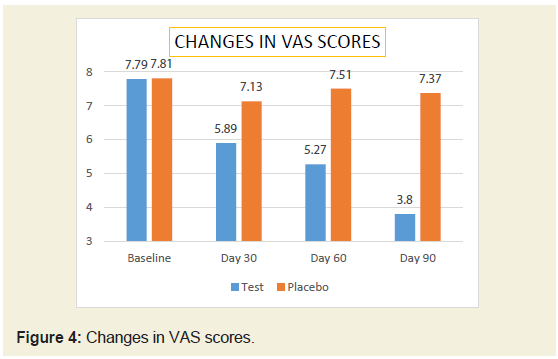
(Figure 3).Changes in pain score (VAS) between groups:
The baseline pain score was comparable between groups. After
treatment, there was significant (51.21% reduction) in VAS score in
JSP group compared to 5.63 % in placebo group (Table 3) (Figure 4).
In both the groups, the reduction in pain score was found to be
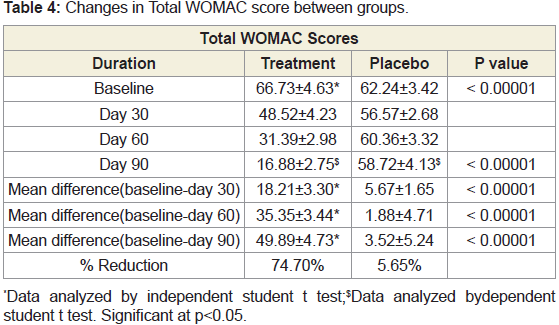
statistically significant at P <0.05 from baseline to the end of the study.Changes in WOMAC score between groups:
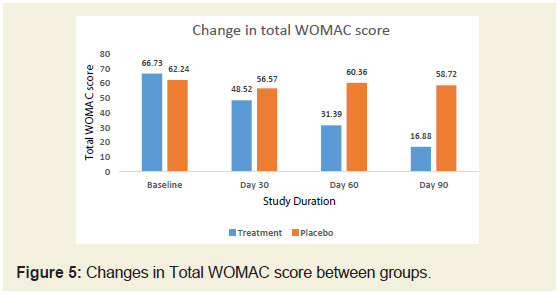
The baseline WOMAC score of JSP and placebo groups were
66.73 and 62.24 respectively. After treatment, on day 90, there was
significant (74.70%) reduction in WOMAC score in JSP group
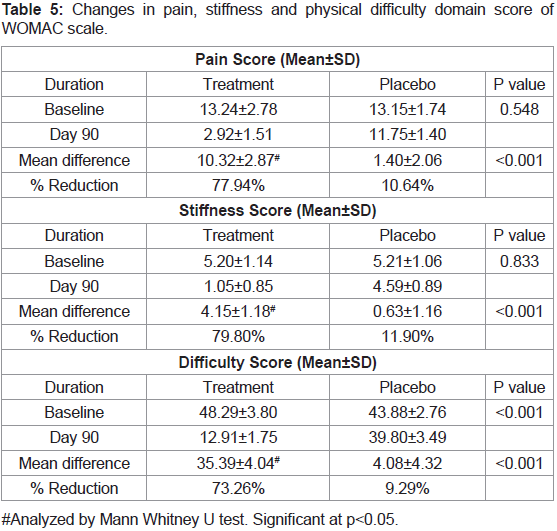
compared to 5.65% in placebo group (Figure 5).We studied different domain scores of WOMAC scale such as
pain, stiffness and physical difficulty scores. It was found that there
was around 80% reduction in scores of all these domains compared to
baseline in JSP group compared to around 10% reduction in placebo
group. Compared between groups, JSP statistically reduced the pain,
stiffness and physical difficulty in patients in 90 days treatment. In
both the groups, the reduction in WOMAC score was found to be
statistically significant at P <0.05 from baseline to the end of the study
(Tables 4 & 5).
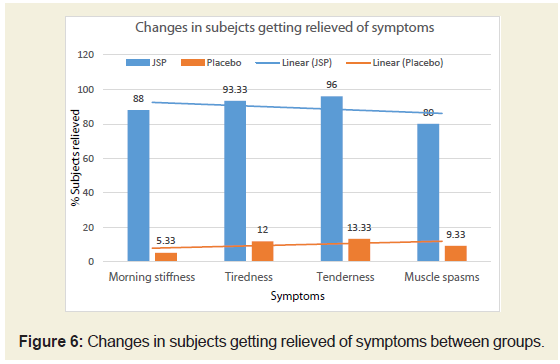
Changes in symptom between groups:
It was observed in the present study that subjects were
experiencing moderate to severe symptoms like morning stiffness,
tiredness, tenderness and muscle spasms on baseline. After 90 days of
treatment there were around 88%, 93.3%, 96% and 80%, respectively
subjects from JSP group experienced no symptoms such as morning
stiffness, tiredness, tenderness and muscle spasms. In both the groups,
the improvement in all the above mentioned symptoms was found to
be statistically significant at P <0.05 from baseline to the end of the
study. These subjects were relieved of symptoms compared to around
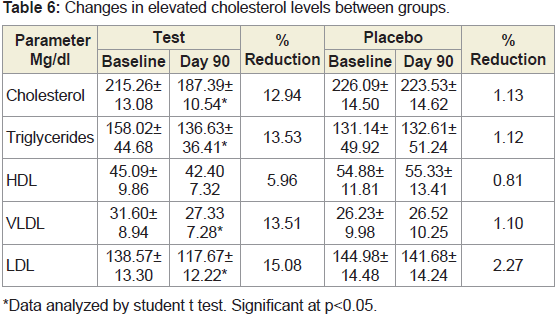
10% subjects from placebo group (Figure 6).Changes in lipid profile between groups:
Subjects with high cholesterol levels at baseline, showed significant
reduction in the total cholesterol and LDL cholesterol after treatment
of JSP after 90 days. There were no significant post treatment changes
in the lipid profile of subjects with normal lipid levels at baseline in
both groups (Tables 6).Changes in biochemical and hematological parameters:
There were no clinically significant changes in hematological and
biochemical parameters like liver function and kidney function test
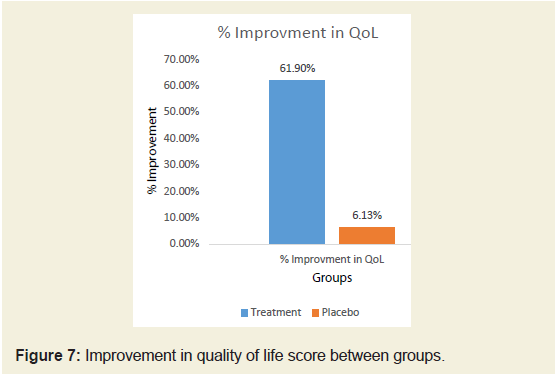
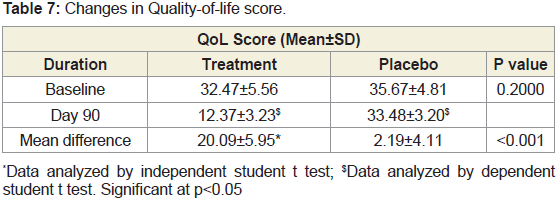
post treatment.Changes in Quality-of-life score between groups:
The average baseline QoL (Quality-of-life) Score was 34.07 in
both the groups. It was reduced to 12.37±3.23 in JSP group and 33.48
±3.20 in placebo group after 90 days of treatment. There is significant improvement in quality of life of subjects in JSP treated group
compared to placebo evident by reduced QoL (Quality-of-life) Score
(Tables 7) (Figure 7).Changes in dependency on rescue analgesic medication between groups:
We proposed percent responder subjects between groups as a
measure to depict the reduced dependency over analgesics in both
groups. The responder subject was defined as subjects showing
50% or more reduction in number of events in which the rescue
of analgesic medicines was used. This comparison was with the
baseline events of the analgesic consumption reported by subjects.
After 90 days treatment with JSP there were 92% responders to the
treatment compared to 8% in placebo group. There were statistically
more subjects showing reduction in the dependency on analgesic
medication in JSP than placebo.There were fifteen adverse events recorded (7 events from JSP
group and 8 events from placebo group). The mild adverse events like
nausea, hyperacidity, headache, wound cut were resolved completely
in 2-3 days. There was no need of rescue medication for the adverse
events needed.
Discussion
OA is characterized by deterioration of joint cartilage, change in
underlying bone, and synovitis. OA has a slow onset i.e. usually begin
in the later age of life (age ≥ 40 yrs.). OA has a prevalence rate of 22 %
to 39 % in India. Established literature shows high prevalence of OA
in women as compared to men. OA of the knee is a major cause of
mobility impairment, particularly among females. OA not only leads
to discomfort to the patients but it also affects the quality of life. There
are various rescue medications prescribed to patients such as oral and
topical analgesics, intra-articular corticosteroids, modified release
dosage forms of the oral analgesics, hyaluronic acid, glutathione,
chondroitin, vitamin, mineral and collagen supplementation etc.
along with non-pharmacological interventions like massage, exercise
and weight loss etc. Conventional OA medications cannot reverse the
damage caused to the OA joints due to lack of self-healing capacity of
articular cartilage. Conventional OA medications can cause serious
side effects and high costs. However, phytotherapy is safe and effective
for managing arthritis.
The Joint Support Product (JSP) is a novel formulation
containing the mixture of standardized and fortified extracts of
Boswellia, Curcumin, Tinospora, Guggul, Nirgundi etc. The present
randomized controlled trial was designed to evaluate the efficacy and
safety of JSP versus Placebo for the short-term treatment (90 days) of
symptomatic osteoarthritis of the knee. Parameters like inflammatory
markers (CRP and IL-6), pain score(VAS), WOMAC scores, change
in symptoms, lipid profile changes, biochemical and hematological
parameters quality of life and dependency on rescue medication were
studied.
Boswellic acids inhibit the synthesis of the pro-inflammatory
enzyme 5-lipoxygenase (5-LO), and in vitro studies show that they
can reduce pain and inflammation. In contrast to non-steroidal
antiinflammatory drugs (NSAIDS), which accelerates articular
damage in arthritic conditions, boswellic acids have been shown
to significantly reduce glycosaminoglycan degradation. [11,12]
Curcumin can reduce inflammation by decreasing the production
of interleukin-1 and IL-6 [13]. The 3-acetyl-11-keto-β-boswellic
acid (AKBA) is the most potent inhibitor of 5-lipoxygenase. The
T. cordifolia extract (TCE) and the compounds isolated from T.
cordifolia have been shown to possess immunomodulatory, antiproliferative,
and anti-angiogenic effects in various in vitro models.
The anti-inflammatory effects of eugenol were attributed to its effect
to prevent neutrophil/macrophage chemotaxis and prostaglandin
synthesis as well as cyclooxygenase II enzyme expressions. Moreover,
eugenol dimers exhibited a chemo preventive effect by inhibiting the
cytokines expression in macrophages [14-16]. It was also revealed that
eucalyptol acts by inhibiting a known sensor of noxious cold, called
the human transient receptor potential cation channel, belonging
to subfamily A, member 1 (TRPA1). Other studies, on the other
hand, have concluded that it may involve a non-opioid mechanism.
Inhalation of eucalyptus oil was effective in decreasing the pain and
blood pressure following total knee replacement surgery [17]. It has
been proposed that V. negundo leaf oil is a potent anti-inflammatory
agent that works by inhibiting COX-2 without interfering with COX-
1 pathways [18]. Constituents of the Piper species have shown in vitro
inhibitory activity against the 5-lipoxygenase and COX-1 enzymes
responsible for leukotriene and prostaglandin biosynthesis [19].
An increase in C-reactive protein levels indicates systemic
inflammation. Researchers have suggested earlier that CRP itself
might contribute to OA. Metabolic syndrome and obesity are risk
factors for OA and increase CRP levels. Hence, reducing CRP levels
could reduce inflammation and slow degenerative joint changes [20].
Pro-inflammatory mediators like IL-6 promote disease progression.
The incidence and severity of OA were associated with elevated levels
of IL-6. By inducing matrix-degrading enzymes, IL-6 contributes to
cartilage pathology [21]. After 90 days treatment of JSP there was
significant reduction (50%) in serum CRP levels whereas the placebo
group shows reduction of only 23.55%. There was 48.84% reduction
in IL-6 levels in JSP group compared to 14.75% in placebo group.
Thus, indicating a promising effect in reducing inflammation caused
by OA.
The effectiveness of the JSP can be attributed to the phytoconstituents
used in the formulation. Evidence suggests that Boswellia
reduces inflammation, prevents cartilage damage, and controls disease
progression [22]. It has been revealed that Boswellia significantly reduced pain and stiffness in patients of OA. Boswellic acid promotes
extracellular matrix formation in joints and chondrocyte production
for cartilage repair [23]. Boswellic acid treatment has potential role of
downregulating pro-inflammatory cytokines such as TNF, IL-1, IL-2,
IL-4, IL-6, and IFN involved in joint degenerative changes [24].
Guggul's anti-inflammatory and antiarthritic properties are well
proven fact, Z-guggulsterone are associated with inhibition of GFAP
expression, as well as the proinflammatory cytokines IL-1, IL-6, and
TNF in osteoarthritis [25].
In one study, Tinospora treatment restored bone health by
reducing proinflammatory mediators such as TNF and interleukin-1,
shifting the balance of mediators of bone remodeling toward anti
osteoclastic activity [26].
VAS scores are based on self-reported measures of pain on
0-10 scale where zero represents “no pain” and ten as the “worst
pain”. It provides the basis of ranking the pain severity from
patient perception [27]. Many studies depicted role of curcumin in
management of osteoarthritis similar in efficacy to nonsteroidal antiinflammatory
drugs, and glucosamine but with much more safety.
[28] The phytonutrients from Vitex negundo are proven to ameliorate
nociception and inflammation by inducing peripheral and central
analgesic activity [29]. These are the important constituent’s in JSP.
Thus after treatment, there was significant (51.21% reduction) in
VAS score in JSP group compared to 5.63% in placebo group. Thus,
indicating beneficiary effect in pain management in OA.
The Western Ontario and McMaster Universities Arthritis Index
(WOMAC) is widely used clinically validated in the evaluation of
Hip and Knee Osteoarthritis. It is a self-administered questionnaire
consisting of 24 items divided into 3 subscales: pain (five questions),
stiffness (two questions), and physical function (17 questions)
[30]. Higher scores represent worse pain, stiffness, and functional
limitations. As OA interferes in daily activities through pain and
stiffness of joints its very valuable that treatment with JSP not only
lowers the total WOMAC score (74.70%) but individually all three
subsets are statistically reduced up to 80% after the treatment. Apart
from this after 90 days treatment of JSP, the patient’s experienced
no symptoms such as morning stiffness, tiredness, tenderness and
muscle spasms by around 88%, 93.3%, 96% and 80% respectively.
This gives the symptomatic relief and that can be contributed to
overall improvement in joint function and thus the improved quality
of life perception of the patients. This is also justified by the significant
improvement in quality of life of subjects in JSP treated group
compared to placebo evident by QoL (Quality-of-life) Score.
JSP has also shown an additional lipid lowering effect in subjects
having high baseline cholesterol levels but no significant effects were
observed in the lipid profile of subjects with normal cholesterol levels
at baseline in both groups.
Patients with OA often use rescue medications like analgesics for
the pain management but these drugs can potentially cause various
side effects like liver damage, nausea, dizziness, developing addiction
and tolerance, etc. after chronic use. After 90 days treatment with
JSP the subjects showed 50 % decrease in incidence of dependency
on rescue analgesics. Thus, indicating beneficial effect in pain
management during OA.
By virtue of the composition of the phytoconstituents in JSP, it
offered wholesome effectiveness in the management of OA including
analgesic, anti-inflammatory, regenerative activities with no adverse
events related to the JSP. Thus administration of JSP to OA patients
will reduce their dependency on the analgesics with symptomatic
relief as well as will work on the root cause of degenerative processes
at joints by providing chondroprotective action.
Treatment of JSP in patients with osteoarthritis led to reduction
in pain, stiffness and other related parameters to provide the
symptomatic relief and thus improved quality of life. Treatment with
JSP could reduce the inflammatory markers by 50% which can have
potential joint restorative action. After treatment with JSP, most of
the subjects showed reduced dependency on the analgesics as a rescue
in the treatment tenure. There were no evident side effects of the JSP
treatment. JSP has potential to provide symptomatic relief but also
helps in repair and restoration of degenerating joints in osteoarthritic
patients.
Acknowledgments
The authors would like to acknowledge the research team and
the back-office team involved in the research work. We would like to
acknowledge the support from all study site staff, investigators and
back-office team.
Declaration of conflict of interests and funding:
Dr. Shridhar Pandya and Dr. Chetan Savaliya are directors in
GPlife Healthcare Pvt. Ltd. Other authors declare non competing
interests. Authors received funding from GPlife Healthcare Pvt. Ltd.Compliance with Ethics Guidelines:
We conducted a randomized placebo-controlled trial involving
patients suffering from osteoarthritis of knee recruited from the
outpatient department of Lokmanya Medical Research Centre,
Lokmanya Hospital, Chinchwad, Pune; Atharva Multispecialty
Research Centre, New Sanghvi, Pune. The study was approved
by Institutional Ethics Committee, Lokmanya Medical Research
Centre, and was registered with the Clinical Trial Registry of India
(CTRI/2022/01/039179).