Journal of Orthopedics & Rheumatology
Download PDF
Review Article
Adipose Tissue-Derived Mesenchymal Stem Cells: A Novel Therapy in Orthopedics
Ahmad Salaheddine Naja1*Hassan Baydoun Mohammad EL1 Nassreddine Zouheir Naja2and Said S Saghieh1
- 1Orthopedic Surgery Division, American University of Beirut Medical Center, Lebanon
- 2Anesthesia Department, Makassed General Hospital, Lebanon
Address for Correspondence: Ahmad Salah Naja, Orthopedic Surgery Division, American University of Beirut Medical Center, Beirut, Lebanon, Tel: +961 3201173; E-mail: ahmadnaja90@hotmail.com
Citation: Naja AS, Mohammed HB, Naja NZ, Saghieh S. Adipose Tissue-Derived Mesenchymal Stem Cells: A Novel Therapy in Orthopedics. J Orthopedics Rheumatol. 2018; 5(1): 5.
Journal of Orthopedics & Rheumatology | ISSN: 2334-2846 | Volume: 5, Issue: 1
Submission: 12 March, 2018| Accepted: 25 April, 2018 | Published: 04 May, 2018
Copyright: © 2018 Naja AS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Mesenchymal stem cells can differentiate into a variety of cell types. They have the ability to release bioactive molecules, making them attractive tools in cell-based therapies. Adipose tissue is an ideal source of autologous mesenchymal stem cells for its abundance and easy access. Mesenchymal stem cells are routinely obtained enzymatically from lipoaspirate as stromal vascular fraction.
Objectives: The aim of this review is to investigate the advantages of adipose derived stem cells in orthopedic application.
Methods: Randomised controlled trials (RCTs) and non-RCTs, in addition to cohort and case control studies, as well as case series were included. Search language was restricted to English and time starting from year 2012. Only published papers were eligible.
Results: Thirteen articles were included in the review, they consisted of 1 RCT, 4 nRCTs, 1 prospective comparative study, 1 longitudinal cohort study, 2 case control studies, 3 case series and one case report. The number of patients injected with stem cells ranged between 12 and 1128. A total of 1482 patients were treated with adipose derived stem cells.
Conclusion: The use of adipose derived stem cells had shown to be safe and effective in bone and cartilage repair.
Keywords
Mesenchymal stem cells; Adipose tissue; Regenerative medicine; Orthopedic application
Introduction
Regenerative medicine is an emerging field of multidisciplinary science. This evolving field is taking over the recent medical era as it combines growth factors, biomaterials, and stem cells to repair failing organs relying on fast and efficient tissue growth [1,2]. Regenerative medicine is being applied in orthopedics. It is being used to repair bone, tendon and cartilage damage [3].
Regenerative medicine is divided into two different techniques, the first is cell therapy and the second is tissue engineering. In cell therapy, a cell suspension is injected into the damaged tissue or blood circulation. This is used to repair damaged tissues to regain their function. However, this approach is insufficient to replace organs or regenerate large tissue defects. An alternative approach is tissue engineering. Yet, it is more complex and it is being barely used in clinical practice due to the issue of vascularization [3].
Mesenchymal Stem Cells
Regenerative medicine applications require a reliable and ideal source of stem cells, besides the needed development of cytokines factors and the biomaterial support [1]. On the other hand, both clinicians and researchers still face the challenge of finding available stem cells useful for regenerative medical practices. MSCs are multipotent cells, available in numerous tissues in one’s body, such as bone marrow, muscle, adipose tissue, dental pulp, fetal organs, connective tissue, skin, placenta, blood, cord blood, synovium, periosteum, and perichondrium [2,4-23]. They are characterized by their ability to self-renew or differentiate ex vivo or in vitro into several cell lineages [1]. These cell types include adipocytes, chondrocytes, osteoblasts, and myoblasts [24-39]. In addition, MSCs have the ability to release bioactive molecules. Due to their proliferative and multi-potency differentiation, MSCs are considered to be strong pro-inflammatory process inhibitors that stimulate anti-inflammatory mechanisms, promising to serve in regenerative medicine and inflammatory disease treatment [25]. In this context, research is focusing on the study of the isolation, manipulation, and expansion of MSCs [25].
Adipose Tissue Derived Stem Cells
Researchers investigated and tested clinically the disadvantages of the bone marrow derived mesenchymal stem cells (hBMSCs) by enzymatic lipoaspiration process [40]. The aforementioned procedure is painful and invasive [40]. Its cell population yield is low and the cell fate in vivo is uncertain, especially that the delivery efficiency is reduced [20,40-46]. In addition, the cryogenic preservation followed by thawing of enzymatically aspirated fat tissue from bone marrow lead to a relatively negligible cell survival ex vivo [40-46]. Cell deterioration, decreased multipotency, as well as diminished proliferation potential are exhibited, increasing the chance for viral recipient tissue infection and morbidity [13,26,28,33,47-49].
In order to overcome the above hurdles, alternative sources of MSCs were sought. Adipose tissue of abdomen, thigh and hips serves as an ideal source of mesenchymal stem cells because of its availability and accessibility [26,27,29,30,40,49]. Adipose tissue derived stem cells are being employed in regenerative medicine due to their ability to differentiate into bone, tendon, cartilage, muscles and other tissues.
Non-enzymatic Isolation
Adipose tissue, or lipoaspirate, obtained from liposuction is digested with collagenase to release MSCs called adipose tissuederived stem cell (ASCs). The ASCs undergo several rounds of centrifugation and dilution through which collagenase is washed off and the remaining product is the stromal vascular fraction (SVF) [35,50-54]. SVF has been used as a source of cells useful for tissue regeneration. The most common isolation technique to obtain SVF is the enzymatic digestion which consists of washing, treatment with enzymes, erythrocyte lysis, and cryopreservation or culture expansion [55-64]. This technique is costly and might jeopardize the safety as well as the efficacy of the lipoaspirates [4,65]. When compared to enzymatically derived MSCs, mechanically derived MSCs have superior differentiation potential with larger secretome content and more diverse exosome content without disruption of vascular stroma [66,67]. This method is purely mechanical with no chemical additives used; thus, showing promising results that power the method of nonenzymatic harvesting of stem cells derived adipose tissue [19,68-72].
Application of Adipose Derived Stem Cells in Orthopedics
The aim of this review is to investigate the advantages of adipose derived stem cells in orthopedic application. Randomised controlled trials (RCTs) and non-RCTs (nRCTs), in addition to cohort and case control studies, as well as case series were included. Search language was restricted to English and time starting from year 2012. Only published papers were eligible.
Study Characteristics
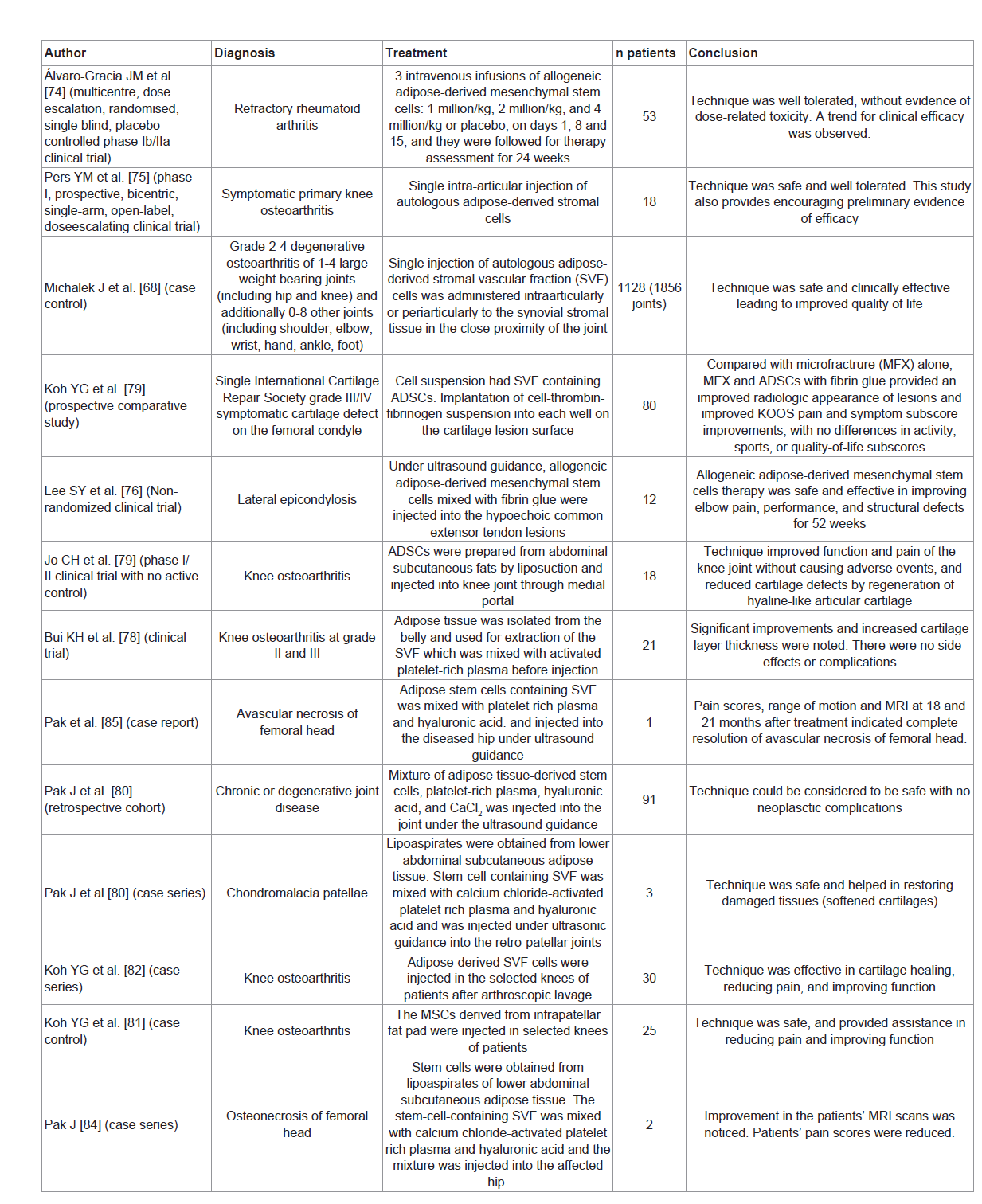
Thirteen articles were included in the review, they consisted of 1 RCT, 4 nRCTs, 1 prospective comparative study, 1 longitudinal cohort study, 2 case control studies, 3 case series and one case report [68,73-84]. The number of patients injected with stem cells ranged between 12 and 1128. A total of 1482 patients were treated with adipose derived stem cells. Table 1 summarizes the articles.
Rheumatoid Arthritis
Adipose derived stem cells have shown therapeutic effect in vitro [85]. They inhibit T-cell proliferation, produce inflammatory cytokines, and generate antigen-specific regulatory T-cells. Thus, they suppress responses of collagen II-reactive T-cells from patients with rheumatoid arthritis [85]. Alvaro-Gracia JM et al. conducted a randomized, placebo-controlled trial to assess the safety and tolerability of the intravenous administration of allogeneic expanded adipose-derived stem cells in patients with refractory rheumatoid arthritis. Three cohorts received treatment with doses of 1, 2 and 4 million cells/kg at days 1, 8 and 15 while the fourth cohort received placebo. They concluded that the intravenous administration of adipose derived stem cells was well tolerated and was not associated with dose-related toxicity. Signs for a potential therapeutic effect of these cells were observed [73].
Osteoarthritis
Intra-articular injection of adipose derived stem cells was shown to reduce synovitis, osteophyte formation, and cartilage degeneration in animal models [86,87]. Pers YM et al conducted a clinical trial to assess the safety and efficacy of intra-articular injection of autologous ASCs in patients with knee osteoarthritis. Eligible patients were allocated to three treatment groups (no placebo) with different doses (2, 10 and 50 million cells). They concluded that the technique was safe and well tolerated in patients with knee osteoarthritis [74].
Michalek J et al. carried out a case control study to evaluate the safety and clinical efficacy of autologous stromal vascular fraction (SVF) cells in patients with grade 2-4 degenerative osteoarthritis mainly of large joints (hip and knee) in addition to medium joints (elbow and wrist) as well as small joints (hand and foot). Single injection of adipose-derived SVF cells was given intra-articularly or peri-articularly to the synovial stromal tissue in close proximity of the joint. The technique was safe and clinically effective. This was demonstrated by the improved quality of life of patients [67].
Intra-articular injection of adipose derived stem cells was shown to reduce synovitis, osteophyte formation, and cartilage degeneration in animal models [86,87]. Pers YM et al conducted a clinical trial to assess the safety and efficacy of intra-articular injection of autologous ASCs in patients with knee osteoarthritis. Eligible patients were allocated to three treatment groups (no placebo) with different doses (2, 10 and 50 million cells). They concluded that the technique was safe and well tolerated in patients with knee osteoarthritis [74].
Koh YG et al. carried out a prospective comparative study during which patients with a single International Cartilage Repair Society grade III/IV symptomatic cartilage defect on the femoral condyle were randomized into two groups. The first group received adipose derived stem cells with fibrin glue and micro fracture treatment whereas the second group received micro fracture treatment alone. The group that received stem cells and micro fracture with fibrin glue had improved radiologic, pain and symptom scores with no differences in activity, sports, or quality-of-life scores in comparison to the other group [78].
Jo CH et al. conducted a phase I/II clinical trial to assess the safety and the efficacy of intra-articular injection of autologous adipose tissue derived mesenchymal stem cells in patients with knee osteoarthritis. Phase I consisted of three cohorts; with three patients each. Patients in each dose group received 10, 50 and 100 million cells in 3 mL of saline. Three patients in each cohort were followed up for 28 days after injection. A safety review was done and phase II was started including 9 patients receiving 100 million cells. The authors concluded that intra-articular injection of 100 million adipose stem cells into the osteoarthritic knee improved function and reduced knee pain without causing adverse events. Radiological, histological and arthroscopic measures showed decreased articular cartilage defects by regeneration of hyaline-like articular cartilage [76].
Bui KH et al. performed a clinical trial to evaluate the efficiency and side-effects associated with the injection of SVF (obtained from adipose tissue of the belly) and platelet rich plasma in knee osteoarthritis. All patients had improved joint function and reduced pain scores. MRI findings showed significant improvements and increased thickness of cartilage layer. There were no complications with respect to infection or graft rejection [77].
Koh YG et al. described a series of elderly patients with knee osteoarthritis who received injections of adipose-derived stromal vascular cells after arthroscopic lavage. They concluded that the technique was effective in reducing pain, cartilage healing and improving function [81].
Pak J et al. carried out a retrospective cohort study in which patients were treated with autologous adipose derived stem cells with platelet-rich plasma for hip, knee, low back and ankle procedures. They concluded that the technique was safe when used as percutaneous local injections and there were no neoplastic complications [79].
Koh YG et al. conducted a case control study to assess the safety and effectiveness of mesenchymal stem cells (MSCs) derived from the infrapatellar fat pad when percutaneously injected into arthritic knees. Patients in the study group received a mean of 1.89 million stem cells with 3.0 mL of platelet-rich plasma and injected in the selected knees of patients while patients in the control group received platelet rich plasma that does not contain stem cells. There were no complications such as infection, fever, hematoma, tissue hypertrophy, adhesion formation observed among the study subjects. The authors concluded that the therapy reduced pain and improved patients’ function [80].
Lateral Epicondylosis
Lee SY et al. evaluated the safety and efficacy of allogeneic adipose-derived mesenchymal stem cells for the treatment of lateral epicondylosis. Allogenic adipose derived stem cells were mixed with fibrin glue and injected into the hypoechoic common extensor tendon lesions under ultrasound guidance. Patients had reduced pain scores, improved elbow performance scores and decreased tendon defects. Within 52 weeks after the injection [77].
Chondromalacia Patellae
Pak J et al. evaluated the injection of adipose derived stem cells in series of three patients with Chondromalacia Patellae. The stromal vascular fraction was separated from the lipoaspirates by centrifugation after treatment with collagenase. The stem-cell-containing stromal vascular fraction was mixed with calcium chloride-activated platelet rich plasma and hyaluronic acid. The mixture was injected under ultrasonic guidance into the retro-patellar joints of the patients. The pain scores improved 50-70% one month after the injection and 80-90% after three months. The pain improvement persisted over one year. There were no serious side effects. The repeated magnetic resonance imaging scans at three months showed restoration of the damaged tissues (softened cartilages) on the patellae-femoral joints [82].
Osteonecrosis of Femoral Head
Pak J et al. evaluated the effect of adipose tissue-derived stem cells and platelet-rich plasma on to the regeneration of medullary bone-like tissue and long-term reduction of pain in two patients with femoral head osteonecrosis. Stem cells were obtained from lipoaspirates of lower abdominal subcutaneous adipose tissue. The stromal vascular fraction was separated from the lipoaspirates by centrifugation after treatment with collagenase. The stem-cell-containing stromal vascular fraction was mixed with calcium chloride-activated platelet rich plasma and hyaluronic acid. The mixture was injected into the affected hip. The diseased hip was re-injected with calcium chlorideactivated platelet rich plasma on weekly basis for 4 weeks. The physical therapy testing, visual analog scale score, and Harris Hip score of the two patients improved after the treatment. Both patients showed improvement in their MRI scans, evidenced by positive T1 signal changes consistent with medullary bone regeneration. Moreover, the long-term reduction in hip pain was correlated with the MRI findings indicative of bone regeneration. The two cases demonstrated the presence of sustained, regenerated medullary bone-like tissue in severely necrotic femoral heads and suggest that this simple and minimally invasive procedure may be a promising therapy for patients with femoral head osteonecrosis [83].
Another report by Pak J et al. described a single case of early stage a vascular necrosis of the femoral head. Adipose derived stem cells containing stromal vascular fraction were mixed with platelet rich plasma and hyaluronic acid. The mixture was injected into the diseased hip under ultrasound guidance. The affected hip was reinjected weekly with additional platelet rich plasma for 4 weeks. The patient was followed-up MRI scans at 3, 18, and 21 months after treatment. Visual Analogue Scale, Walking Index, Functional Rating Index, Harris Hip Score, and Range of Motion assessments were also noted. The patient’s severe hip pain was considerably improved at 3 months after treatment. Pain scores, range of motion and MRI at 18 and 21 months post treatment indicated complete resolution of a vascular necrosis of the femoral head [84-87].
Conclusion
The use of adipose derived stem cells had shown promising results in bone and cartilage repair. Further clinical trials are needed to confirm the short and long term efficacy of adipose derived stem cells in various orthopedic applications.
References
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, et al. (2013) Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 132: 1017-1026.
- Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, et al. (2010) Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 16: 169-175.
- Schmitt A, van Griensven M, Imhoff AB, Buchmann S (2012) Application of stem cells in orthopedics. Stem Cells Int 2012: 394962.
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, et al. (2000) Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2: 477-488.
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, et al. (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76: 579-592.
- Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T (2002) Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med 195: 1549-1563.
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, et al. (2001) Surface protein characterization of human adipose tissue‐derived stromal cells. J Cell Physiol 189: 54-63.
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, et al. (2004) Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78: 1439-1448.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75: 389-397.
- Puissant B, Barreau C, Bourin P, Clavel C, Corre J, et al. (2005) Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 129: 118-129.
- Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42: 1539-1546.
- Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100: 1249-1260.
- Butler DL, Goldstein SA, Guilak F (2000) Functional tissue engineering: the role of biomechanics. J Biomech Eng 122: 570-575.
- Katz AJ, Llull R, Hedrick MH, Futrell JW (1999) Emerging approaches to the tissue engineering of fat. Clin Plast Surg 26: 587-603.
- Tremolada C (2015) Device and method for preparing tissue, particularly adipose tissue. United States Patent US 9,044,547.
- García-Contreras M, Messaggio F, Jimenez O, Mendez A (2015) Differences in exosome content of human adipose tissue processed by non-enzymatic and enzymatic methods. CellR4 3: e1423.
- Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23: 812-823.
- Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, et al. (2015) Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant 24: 1233-1252.
- Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, et al. (2014) Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant 23: 1489-1500.
- Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, et al. (2009) Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 23: 3494-3505.
- Daley GQ, Scadden DT (2008) Prospects for stem cell-based therapy. Cell 132: 544-548.
- Riazi AM, Kwon SY, Stanford WL (2009) Stem cell sources for regenerative medicine. Methods Mol Biol 482: 55-90.
- Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20: 121-133.
- Tremolada C, Colombo V, Ventura C (2016) Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep 2: 304-312.
- Alexander RW (2012) Understanding adipose-derived stromal vascular fraction (AD-SVF) cell biology and use on the basis of cellular, chemical, structural and paracrine components: a concise review. J Prolotherapy 4: e855-e869.
- Doornaert MA, Declercq H, Stillaert F, Depypere B, Van de Walle I, et al. (2012) Intrinsic dynamics of the fat graft: in vitro interactions between the main cell actors. Plast Reconstr Surg 130: 1001-1009.
- Stolzing A, Jones E, McGonagle D, Scutt A (2008) Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 129: 163-173.
- Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341-347.
- Huang JI, Beanes SR, Zhu M, Lorenz HP, Hedrick MH, et al. (2002) Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg 109: 1033-1041.
- Tremolada C, Beltrami G, Magri A, Bianchi F, Ventura C, et al. (2014) Adipose mesenchymal stem cells and “regenerative adipose tissue graft” (Lipogems™) for musculoskeletal regeneration. Eur J Musculoskelet Dis 3: 57-67.
- Pallua N, Serin M, Wolter TP (2014) Characterisation of angiogenetic growth factor production in adipose tissue-derived mesenchymal cells. J Plast Surg Hand Surg 48: 412-416.
- De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, et al. (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12: 574-591.
- Lindroos B, Suuronen R, Miettinen S (2011) The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7: 269-291.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
- Kassem M, Abdallah BM (2008) Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res 331: 157-163.
- Ceserani V, Ferri A, Berenzi A, Benetti A, Ciusani E, et al. (2016) Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell 8: 3.
- Coleman SR (2001) Structural fat grafts: the ideal filler? Clin Plast Surg 28: 111-119.
- Coleman SR (2006) Structural fat grafting: more than a permanent filler. Plast Reconstr Surg 118 (3 Suppl): 108S-120S.
- Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, et al. (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22: 2063-2077.
- Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P (2004) Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun 314: 420-427.
- Mimeault M, Batra SK (2008) Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev 4: 27-49.
- Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R (2001) Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma 42: 863-870.
- D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14: 1115-1122.
- Muschler GF, Nitto H, Boehm CA, Easley KA (2001) Age-and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 19: 117-1125.
- Bonaros N, Rauf R, Schachner T, Laufer G, Kocher A (2008) Enhanced cell therapy for ischemic heart disease. Transplantation 86: 1151-1160.
- Centeno CJ, Bashir J (2015) Safety and regulatory issues regarding stem cell therapies: one clinic's perspective. PMR 7 (4 Suppl): S4-S7.
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, et al. (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54: 132-141..
- Striano RD, Chen H, Bilbool N, Azatullah K, Hilado J, et al. (2015) Case Study: Non-responsive knee pain with osteoarthritis and concurrent meniscal disease treated with autologous micro-fragmented adipose tissue under continuous ultrasound guidance. CellR4 3: e1690.
- Carvalho PP, Gimble JM, Dias IR, Gomes ME, Reis RL (2013) Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Methods 19: 473-478.
- Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, et al. (2013) Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther 4: 27.
- Aguena M, Fanganiello RD, Tissiani LA, Ishiy FA, Atique R, et al. (2012) Optimization of parameters for a more efficient use of adipose-derived stem cells in regenerative medicine therapies. Stem Cells Int 2012: 1-7.
- Yang XF, He X, He J, Zhang LH, Su XJ, et al. (2011) High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci 18: 59.
- Markarian CF, Frey GZ, Silveira MD, Chem EM, Milani AR, et al. (2014) Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett 36: 693-702.
- Philips BJ, Marra KG, Rubin JP (2012) Adipose stem cell-based soft tissue regeneration. Expert Opin Biol Ther 12: 155-163.
- Thirumala S, Gimble JM, Devireddy RV (2010) Cryopreservation of stromal vascular fraction of adipose tissue in a serum‐free freezing medium. J Tissue Eng Regen Med 4: 224-232.
- Kirkpatrick CJ, Melzner I, Göller T (1985) Comparative effects of trypsin, collagenase and mechanical harvesting on cell membrane lipids studied in monolayer-cultured endothelial cells and a green monkey kidney cell line. Biochim Biophys Acta 846: 120-126.
- Stadler G, Hennerbichler S, Lindenmair A, Peterbauer A, Hofer K, et al. (2008) Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy 10: 743-752.
- Hu CB, Myers KE, Peterson RC (2000) Devices for harvesting and homogenizing adipose tissue containing autologous endothelial cells. United States Patent US 6,020,196.
- Domenis R, Lazzaro L, Calabrese S, Mangoni D, Gallelli A, et al. (2015) Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther 6: 2.
- Schafer ME, Hicok KC, Mills DC, Cohen SR, Chao JJ (2013) Acute adipocyte viability after third-generation ultrasound-assisted liposuction. Aesthet Surg J 33: 698-704.
- Zhu M, Cohen SR, Hicok KC, Shanahan RK, Strem BM, et al. (2013) Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg 131: 873-880.
- Zimmerlin L, Rubin JP, Pfeifer ME, Moore LR, Donnenberg VS, et al. (2013) Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy 15: 102-108.
- Ferguson RE, Cui X, Fink BF, Vasconez HC, Pu LL (2008) The viability of autologous fat grafts harvested with the LipiVage system: a comparative study. Ann Plast Surg 60: 594-597.
- Ansorge H, Garza JR, McCormack MC, Leamy P, Roesch S, et al. (2014) Autologous fat processing via the revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J 34: 438-447.
- Riordan NH, Ichim TE, Min WP, Wang H, Solano F, et al. (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7: 29.
- Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, et al. (2015) Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant.
- Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, et al. (2001) Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec 264: 51-62.
- Striano RD, Battista V, Bilboo N (2017) Non-Responding Knee Pain with Osteoarthritis, Meniscus and Ligament Tears Treated with Ultrasound Guided Autologous, Micro-Fragmented and Minimally Manipulated Adipose Tissue. J Regenerative Med 6: 17-26.
- Franceschini M, Castellaneta C, Mineo G (2016) Injection of autologous micro-fragmented adipose tissue for the treatment of post-traumatic degenerative lesion of knee cartilage: a case report. CellR4 4: e1765.
- Pelletier JP, Martel-Pelletier J, Abramson SB (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44: 1237-1247.
- Liu Y, Mu R, Wang S, Long L, Liu X, et al. (2010) Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther 12: R210.
- Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, et al. (2017) Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis 76: 196-202.
- Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, et al. (2016) Adipose mesenchymal stromal cell‐based therapy for severe osteoarthritis of the knee: A phase I dose‐escalation trial. Stem Cells Transl Med 5: 847-856.
- Lee SY, Kim W, Lim C, Chung SG (2015) Treatment of lateral epicondylosis by using allogeneic adipose‐derived mesenchymal stem cells: a pilot study. Stem Cells 33: 2995-3005.
- Jo CH, Lee YG, Shin WH, Kim H, Chai JW, et al. (2014) Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof‐of‐concept clinical trial. Stem Cells 32: 1254-1266.
- Pham PV, Bui KH, Duong TD, Nguyen NT, Nguyen TD, et al. (2014) Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 1: 02.
- Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH (2015) Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 32: 97-109.
- Pak J, Chang JJ, Lee JH, Lee SH (2013) Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord 14: 337.
- Koh YG, Choi YJ (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19: 902-907.
- Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE (2013) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23: 1308-1316.
- Pak J, Lee JH, Lee SH (2013) A novel biological approach to treat chondromalacia patellae. PloS One 8: e64569.
- Pak J (2012) Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician 15: 75-85.
- Pak J, Lee JH, Jeon JH, Lee SH (2014) Complete resolution of avascular necrosis of the human femoral head treated with adipose tissue-derived stem cells and platelet-rich plasma. J Int Med Res 42: 1353-1362.
- Gonzalez MA, Gonzalez‐Rey E, Rico L, Büscher D, Delgado M (2009) Treatment of experimental arthritis by inducing immune tolerance with human adipose‐derived mesenchymal stem cells. Arthritis Rheum 60: 1006-1019.
- ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, et al. (2012) Antiinflammatory and chondroprotective effects of intraarticular injection of adipose‐derived stem cells in experimental osteoarthritis. Arthritis Rheum 64: 3604-3613.
- Mokbel AN, El Tookhy OS, Shamaa AA, Rashed LA, Sabry D, et al. (2011) Homing and reparative effect of intraarticular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord 12: 259.