Journal of Orthopedics & Rheumatology
Download PDF
Research Article
*Address for Correspondence: Grazyna Sobal, Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Austria,; E-mail: grazyna.sobal@meduniwien.ac.at
Citation: Sobal G, Menzel EJ, Dorotka R, Sinzinger H, Hacker M. Ex-vivo Evaluation of 99mTc Labeled Glucosamine Sulfate (99mTcGS) in Articular Cartilage. Relevance for Osteoarthritis Imaging. J Orthopedics Rheumatol. 2014;1(3): 9.
Copyright © 2014 Sobal G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Orthopedics & Rheumatology | ISSN: 2334-2846 | Volume: 1, Issue: 3
Submission: 06 October 2014 | Accepted: 13 November 2014 | Published: 18 November 2014
Reviewed & Approved by: Dr. Guangju Zhai, Department of Genetics, Memorial University of Newfoundland, Canada
Uptake of 99mTcGS in articular cartilage
When degenerated tissues were preincubated with cold GS (80 μg/ml) for 16 weeks to mimic in vivo treatment, followed by incubation with 99mTcGS the additional uptake of 99mTcGS (24 h - 72 h) was strongly reduced by 52.3-71.8% (Figure 5a) in degenerated cartilage tissue (grade III). Comparable effect was found in degenerated cartilage tissue (grade II) by 40, 3-64, 3% (Figure 5b). These prove the chondrotropic effects of GS during the treatment. During the incubation with cold GS degenerated cartilage seems to take up GS from incubation medium to repair the cartilage matrix. It is known that in degenerated matrix GAG are depleted and cartilage claims to rebuild GAG in the matrix. This repair mechanism could be observed already after preincubation with cold GS, therefore the additional uptake with 99mTcGS resulted in lower uptake as compared to incubation with labeled 99mTcGS alone, without treatment.
Autoradiography
Ex-vivo Evaluation of 99mTc Labeled Glucosamine Sulfate(99mTcGS) in Articular Cartilage. Relevance for Osteoarthritis Imaging
Grazyna Sobal1*, Ernst Johannes Menzel2, Ronald orotka3, Helmut Sinzinger1 and Marcus Hacker1
- 1Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Austria
- 2Institute of Immunology, Medical University of Vienna, Austria
- 3Department of Orthopaedic Surgery, Medical University of Vienna, Austria
*Address for Correspondence: Grazyna Sobal, Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Austria,; E-mail: grazyna.sobal@meduniwien.ac.at
Citation: Sobal G, Menzel EJ, Dorotka R, Sinzinger H, Hacker M. Ex-vivo Evaluation of 99mTc Labeled Glucosamine Sulfate (99mTcGS) in Articular Cartilage. Relevance for Osteoarthritis Imaging. J Orthopedics Rheumatol. 2014;1(3): 9.
Copyright © 2014 Sobal G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Orthopedics & Rheumatology | ISSN: 2334-2846 | Volume: 1, Issue: 3
Submission: 06 October 2014 | Accepted: 13 November 2014 | Published: 18 November 2014
Reviewed & Approved by: Dr. Guangju Zhai, Department of Genetics, Memorial University of Newfoundland, Canada
Abstract
Background: Glucosamine sulfate (GS) is widely used in the treatment of osteoarthritis, but no data concerning the local uptake of GS in articular cartilage exist. For this reason we performed radiolabeling of GS (Rottapharm, Monza, Italy) using the 99mTcO4-/t in method and investigated its uptake in articular cartilage.Methods: Radiolabeling of GS is difficult, because of the small MW of GS (450 Da) and of 99mTcO4 - for separation after radiolabeling. For uptake studies, articular surgery tissue from patients (n=6, 68-79a) undergoing knee arthroplasty (pieces of ~3 mg wet weight), or frozen tissue sections (10 μ) for autoradiography (10 μCi) were used. The uptake of 99mTcGS was monitored from 0.5 to 72 h of incubation to achieve saturation, by quantitation of radioactivity with a Gammacounter or autoradiography. To mimic in-vivo treatment, tissue pieces were preincubated with cold GS (80 μg/ml) for 16 weeks prior to incubation with 99mTcGS. The incubation was performed with tissues with different degree of degeneration.
Results: The best radiolabeling results were obtained using 25 mg of GS, 120-150 MBq of 99mTc, separation on G10 column, specific activity of 162.2-202.8 mCi/mM, n=6. The uptake was time-dependent and amounted to a maximum of 77.4. -71.7% at 48 h versus 72 h, at saturation. Saturation was achieved already after 24 h of incubation. When the tissue was preincubated with cold GS the subsequent uptake of 99mTcGS was strongly reduced by 52.3-71.8%, proving the chondrotropic effects of GS during preincubation. The reduction of 99mTcGS uptake was observed also in areas of different grades of degeneration from the same patient by 27.8-35.8%, proving the specificity of uptake. Autoradiographic studies paralleled the uptake in tissue pieces. We performed a statistical evaluation of differences between all investigated OA patients with different degree of cartilage degeneration. For these patients we evaluated the autoradiographic uptake at all experimental points of incubation times (1 h, 3 h, 6 h, 24 h, 48 h). 99mTcGS uptake in tissues of different extent of degeneration varied significantly during the whole time of incubation, the more degenerated regions showing a significantly higher 99mTcGS uptake. The non-specific uptake in the presence of 50-fold excess of cold GS was increasing with time up to a maximum of about 10%, at saturation.
Conclusions: 99mTcGS could be a suitable tracer to quantify degeneration in articular cartilage, because of its high uptake and specificity. It could be a potential candidate for imaging osteoarthritis in cartilage and possibly for monitoring therapeutic effects.
Introduction
Osteoarthritis (OA) is a degenerative joint disease resulting frequently in a degeneration and irreversible destruction of articular cartilage. Besides collagen (mainly type II), proteoglycans, especially aggrecan, belong to the most abundant components of cartilage. The disease progresses through several phases. In its early stage mainly cartilage proteoglycans (PG) are degraded. As a consequence the quality and quantity of glycosaminoglycan (GAG) side chains are altered. Especially in the superficial zone of OA cartilage, loss of fixed negative charge density (FCD) is manifested. The stationary phase of OA is characterized by the formation of osteophytes and joint space narrowing. The disease finally culminates in the obliteration of the joint space. In general, OA triggers accelerated but inadequate tissue repair. Osteoarthritis involves changes in both synthesis and degradation of aggrecan and other cartilage molecules [1]. PGdegrading enzymes and inflammatory mediators such as cytokines inducing catabolic processes are produced within the cartilage. These mediators and matrix degrading enzymes are generated by cartilage cells, i.e. chondrocytes which may even undergo irreversible phenotypic modulation during the progression of the disease.Changes in PG content and structure as a consequence of defective biosynthesis and turnover of glucosamine (GA) and other hexosamines occur early in OA. It is therefore tempting to investigate in vivo cartilage uptake of labeled GA as a possible marker for early pathological changes in OA, e.g. by scintigraphic imaging of cartilage. Tissue distribution of uniformly labeled 14C-GA after intravenous or oral administration was studied by Setnikar et al. [2,3]. Ten minutes after i.v. dosage, GA was already found in all organs and tissues, including cartilage. Interestingly, radioactivity in cartilage (femur) was significantly higher than in bone (sternum), suggesting a chondrotropic behavior for GA.
For studies investigating absorption, tissue distribution, excretion and incorporation of GA using scintillation counting, 14C-GA [2,3] or 3H-GA [4] are acceptable. For imaging techniques, however, to study in vivo cartilage degradation, scintigraphy using 99mTc-labeled glucosamine sulfate (99mTcGS) as tracer is the method of choice. Labeling with 99mTc results in the formation of a 99mTcGS-complex, that could be more resistant to metabolism and/or integration into plasma proteins than uniformly labeled 14C-GA or 3H-GA. As the molecular structure of GS is not directly labeled in these complexes, excreted CO2 or plasma proteins incorporating GS fragments are “silent”, both in conventional counting techniques and in scintigraphy. Cartilage associated radioactivity signals are therefore always originating in complex-bound GS.
The present study aims at proving the chondrotropic character of 99mTcGS. We hypothesize that this tracer can be used in the diagnosis of early and advanced OA.
Materials and Methods
Radiolabeling procedureRadiolabeling of GS (Rottapharm, Monza, Italy) was done using our modified 99mTcO4 -/stannous chloride method [5] at pH 7.0 in 0.01 M citrate for 1 h at room temperature. Separation of labeled GS from unbound 99mTc was performed by gel chromatography over G 10.
Quality control
For quality control thin layer chromatography (ITLC) was used with the following solvent mixtures: 0.2 M saline/10% ethanol to separate colloid and ethanol/aluminium oxide IB-F to detect free 99mTc. The latter method was also used to evaluate the stability of the labeled 99mTcGS complex 3 h and 6 h after labeling at room temperature.
Molecular weight estimation of 99mTcGS complex
The molecular weight of the 99mTcGS complex was determined via molecular sieve chromatography using G10 columns (Sephadex, Bio- Rad, California, USA). As molecular weight standards glucose, sucrose and dextran blue were used. The elution position of these sugars and of 99mTcGS complex was detected in the eluted 1 ml fractions on the same gel column. Sugars were quantitated by a colorimetric method [6]. GS measurement in eluted fractions was performed according to the modified method by Elson and Morgan [7]. Molecular weight of the 99mTcGS complex was calculated [8].
Uptake studies
To study the uptake of labeled GS(99mTcGS) by cartilage, articular surgery tissue samples from individuals undergoing knee arthroplasty (n=6, 68-79a) were used. The cartilage sections from different patients were selected according to degeneration or regional variation of degeneration from the same patient. Tissues with different degree of degeneration were evaluated histologically by hematoxylin & eosin staining and uptake was compared. The uptake of 99mTcGS in pieces of ~3 mg wet weight by incubation at 37 °C from 0.5 h to 72 h was monitored by quantitation of radioactivity in the Gamma-counter. After 72 h saturation was achieved. Alternatively, 10 μ frozen tissue sections were incubated with 10 μCi 99mTcGSat 37 °C and uptake was evaluated by autoradiography (Instant Imager, Packard, Meriden, CT, USA). To mimic in vivo therapy with GS treatment, tissue pieces were preincubated with cold GS (80 μg/ml) for 16 weeks (at 37 °C in CGM Bullet medium) prior to incubation with 99mTcGS. This corresponds to a daily dose of 400 mg GS (i.e. one tablet of the drug). We performed inhibition experiments with cold GS both for higher (grade III) and mildly degenerated samples (grade II). The substrate was renewed every week. To determine the specificity of the binding of the 99mTcGS complex, the degree of uptake inhibition by a 50 fold excess of unlabeled free GS was verified.
Statistical analysisThe uptake data were analyzed for statistical significance using the t-test. Each data point on the plots (Figure 1) represents the mean of the values obtained from 6 independent experiments (6 patients grade II and 6 patients grade III) for each incubation time. Error bars indicating the standard error of means (±SEM) and p.
Figure 1: Autoradiographic uptake (total counts) in degenerated human articular cartilage from patients (n=6) with lower degeneration (grade II) versus patients (n=6) with heavier degeneration (grade III). Uptake intensity correlates with degree of degeneration during the whole time of incubation, the more degenerated regions showing a significantly higher 99mTcGS uptake.
Results
Quality control
The radiochemical purity of 99mTcGS amounted to 95% (5% colloid + 99mTc free, as impurities). Using ITLC-SG-chromatography the radiolabeled complex remained at the start (Rf = 0.2) and free pertechnetate moved with the solvent front (Rf = 0.95). Using aluminium oxide IB-F TLC colloid remained at the start (Rf = 0.1) and the radiolabeled complex moved with the solvent front (Rf = 0.9). The tracers were stable over 6 h after labeling (95% of the radiochemical purity).Structure of 99mTc GS-complex
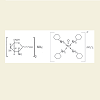
Different labeling procedures were compared. The best results were obtained using a concentration of 25 mg per ml GS, 120-150 MBq of 99mTcO4 - at a pH of 7.0 in 0.01 M citrate. The 99mTcGScomplex was separated by gel chromatography on Sephadex gel G 10 using PBS as elution buffer. 70.4 % of total radioactivity was bound by the complex. GS was chemically detected exclusively in the main elution peak. The specific activity obtained was 6 MBq per mg 99mTcGS-complex. A molecular weight of approximately 1000 for the labeled complex was computed from the relative elution position of 99mTcGS (Figure 2).
Figure 2: The chemical structure of the starting reagent (glucosamine sulfate) is given left. The proposed structure of 99mTcGS complex formed during the labeling procedure, right. The central 99mTc4+ ion is surrounded by four GA molecules forming electron pair bridges via their amino groups. The four ring structure containing amino groups represents symbolically the chemical structure of glucosamine residues (left). The molecular weight of the labeled 99mTcGS complex was verified by molecular sieve chromatography and corresponds to the suggested structure (MW:1000).
To estimate the degree of cartilage degeneration for ex-vivo uptake studies from patients undergoing knee arthroplasty we performed histopathological evaluation of tissue (Figure 3). We selected patients with mild degeneration (grade II) and heavier degeneration (grade III). The use of these samples from patients undergoing knee replacement was approved by the local ethic commission EK-Nr. 523/2008. The uptake in tissue pieces was found to be time-dependent, amounting to a maximum of about 67-78% at saturation, which was already obtained after 24 h of incubation at 37 °C (Figure 4). The reduction of 99mTcGS uptake was observed also in areas of different grade of degeneration from the same patient (Figure 4). 99mTcGS uptake in low degenerated tissue areas (grade II) was lower than in heavier degenerated tissue areas (grade III). Especially in the initial period of incubation up to 18 h we observed a significantly higher uptake of 99mTcGS up to 35.8% in heavier degenerated tissue areas, proving the specificity of uptake for advanced pathological changes(Figure 4).
Figure 3: Histopathological examination of degenerated human cartilage tissueby hematoxylin & eosin staining. We selected patient with mild degeneration (grade II) and more severe degeneration (grade III), showing severe erosion of the cartilage surface. Histological pictures of these sections represent magnification of 100x.
Figure 4: Uptake of 99mTcGS in articular cartilage tissue pieces from the same patient but in areas of different grade of degeneration. The reduction of 99mTcGS uptake was observed in low degenerated tissue areas (grade II) as compared to more severely degenerated tissue areas (grade III). Especially in the first period of incubation up to 6 h we observed significantly higher uptake of 99mTcGS in heavier degenerated tissue areas.
Figure 5a: Uptake of 99mTcGS in articular cartilage tissue pieces (grade III) after preincubation with cold GS (80 μg/ml) for 16 weeks versus uptake with 99mTcGS alone. Preincubation results in lower uptake as compared to incubation with labeled 99mTcGS, proving the chondrotropic effects of GS during the treatment.
Figure 5b: Uptake of 99mTcGS in articular cartilage tissue pieces (grade II) after preincubation with cold GS (80 μg/ml) for 16 weeks versus uptake with 99mTcGS alone. Preincubation results in lower uptake as compared to incubation with labeled 99mTcGS, proving the chondrotropic effects of GS during the treatment.
Autoradiographic studies paralleled the uptake by tissue pieces. An example of autoradiographic uptake of 99mTcGS in patients with different cartilage degeneration is shown in (Figure 6) and (Figure 7). We performed a statistical evaluation of differences between all investigated OA patients with different degree of cartilage degeneration. For these patients (n=6 for grade II and n=6 for grade III) we evaluated the autoradiographic uptake (counts in cartilage areas) at all experimental points of incubation times (1 h, 3 h, 6 h, 24 h, 48 h) and computed statistical data shown in (Figure 1). 99mTcGS uptake in tissue of different extent of degeneration from patients varied significantly. Uptake intensity correlates with degree of degeneration during the whole time of incubation, the more degenerated regions showing a significantly higher 99mTcGS uptake (Figure 1). The non-specific uptake in the presence of a 50 fold excess of cold GS was increasing with time up to a maximum of about 10 % at saturation (Figure 8).
Figure 6: Autoradiographic uptake studies with 99mTcGS (as an example) in degenerated human articular cartilage from patient with heavier degeneration (grade III). The dimensions of the autoradiographic images are 1.8:1 to the original size of the slices. The right side of individual slices represents the cartilage surface, left side the subchondral part of cartilage as shown in the histological image. We observed a continuously increasing uptake of the tracer during the whole incubation time.
Figure 7: Autoradiographic uptake studies with 99mTcGS (as an example) in degenerated human articular cartilage from patient with lower degeneration (grade II). The dimensions of the autoradiographic images are 1.6:1 to the original size of the slices. The right side of individual slices represents the cartilage surface, left side the subchondral part of cartilage as shown in the histological image. We observed also a continuously increasing uptake of the tracer during the whole incubation time. The uptake in this patient tissue was lower as compared to the uptake seen in a tissue sample with heavier degeneration (grade III).
Figure 8: Non-specific uptake in degenerated human articular cartilage in the presence of 50-fold excess of cold GS in patients with grade III. The dimensions of the autoradiographic images are 1.8:1 to the original size of the slices. The right side of individual slices represents the cartilage surface, left side the subchondral part of cartilage.
Discussion
In the treatment of OA the early recognition of the disease as well as early therapeutic intervention are essential. Among the plethora of drugs used to treat OA, GA belongs to the group of nutraceuticals. Originally only considered to constitute an exogenous source for building blocks in the biosynthesis of PGs, GA, together with chondroitin sulfate (CS) was introduced as safer alternative to antiinflammatory drugs [9]. The rationale for its use in OA therapy lies in the fact that it is a precursor of GAGs which constitute the side chains of PG representing major components of cartilage. It can reduce inflammation and the extent of cartilage degradation [10,11]. More recently it was found that the combination of GA with CS can enhance these effects [12]. GA is bioavailable after oral administration both systemically and at the site of supposed action, i.e. the joint, persists in circulation and its pharmacokinetics support once-daily dosage [13,14]. GA is known to effectively retard progression of OA, especially on long-term treatment. The drug was also shown to display structure-modifying effects such as reducing joint space narrowing [15-23]. In particular, two recent randomised trials of 2-3 years duration in knee OA patients indicate that these symptommodifying effects are sustained over long-term treatment [21,23]. Thus,joint space narrowing/radiological progression of OA was found to be significantly reduced on daily GA administration. Hovewer, the therapeutical efficacy of GA is controversial [24-26].Normally, GA and its acetylated bioactive derivative N-acetyl-GA is produced endogenously in sufficient amounts via biosynthesis from glucose. In the presence of exogenous GA, however, biosynthesis of GAGs is enhanced [27]. In general, exogenous GA is incorporated via NAc-GA or GA-6-phosphate into a nascent CS chain [24]. Not surprisingly, GA was found to stimulate PG production in human chondrocytes in vitro [28].
Labeling of GA (MW 180 D) is more difficult than that of larger polysaccharide entities such as CS(MW 25.000 D), since separation of the tracer from excess/free 99mTc may be hampered by the comparable MW of GS and the 99mTc label. Nevertheless, we achieved an acceptable degree of separation using molecular sieve chromatography on G10 gel, suggesting the formation of a tetravalent complex in which the central 99mTc4+ ion is surrounded by four GA molecules forming electron pair bridges via their amino groups (Figure 2). The molecular weight of the labeled 99mTcGS-complex was verified by molecular sieve chromatography and corresponds to the suggested structure (MW 1000 D).
As uniformly labeling of GA with 14C or 3H results in its metabolism we used instead a 99mTcGS-complex. According to our results, free 99mTc liberated from such a complex does not significantly bind to cartilage (max 5% uptake). Only GA integrated in the 99mTcGS complex is demonstrable since neither unbound GA nor its metabolized fragments are labeled. In addition, radiolabeling with a gamma emitting label such as 99mTc instead of 3H or 14C is necessary for prospective SPECT imaging studies in-vivo.
A clear indication for the significant in vitro stability of the 99mTcGS-complex derives from the fact that it can be separated from free 99mTc by gel chromatography. For in vivo cartilage imaging significant complex stability should be granted over a time period of at least 2-6 h to avoid erroneous scintigraphic signaling from liberated free 99mTc. Another even more convincing proof for longterm stability of the 99mTcGS-complex can be deduced from our finding that the complex in contrast to free 99mTc is strongly taken up by human cartilage in vitro, while the uptake of free 99mTc is negligible.
A further hint at long-term stability of our 99mTcGS-complex comes from the results of preincubation experiments. When tissues were preincubated with cold GS, uptake of 99mTcGS was strongly reduced. This finding proves that binding of complex-bound 99mTc was inhibited (Figures 5a and 5b). Quality control experiments using ITLC chromatography furthermore clearly show that the 99mTc-GA complex does not significantly dissociate over a time period of at least 6 h.
Both 99mTcCS as well as 99mTcGS can be used as scintigraphic probes for non-invasive diagnosis of early degenerative cartilage changes in OA, although each of the tracers acts via different pathways to delineate proteoglycan degradation. Charge effects, such as the reduced FCD, i.e. reduced concentration of negatively charged GAG-chains in degraded PG in OA cartilage cause both tracers to increasingly diffuse into the matrix. Katta et al. [29] recently showed that in vitro CS is able to diffuse into cartilage, the diffusion rate depending on the GAG content of the matrix. Our results provide the same evidence [30,31].
In contrast to CS, GA much more rapidly diffuses into the cartilage matrix because of its much smaller molecular weight (roughly 1.000 vs. 25.000). This fact results in faster diffusion of the tracer into the tissue, as compared to 99mTcCS. We have demonstrated that the maximal uptake of 99mTcCS was achieved in vitro after 24- 48 h, while for 99mTcGS between 6-24 h (depending on the degree of degeneration). This fact is important for the planned scintigraphic studies in patients suffering from OA.
While CS is able to bind to the cell surface of chondrocytes [32] via specific receptors, no such interaction is possible for GA. Nevertheless, GA is also taken up by the cartilage cells and integrated into GAG chains. The transport of GA through the plasma membrane is mediated by glucose transporter proteins [33]. Chondrocytes express several glucose transporters and actively take up and metabolize GA in contrast to N-acetyl-GA. There is an active competition between glucose and GA, GA inhibiting glucose transport in a noncompetitive mode [34]. However, for both tracers, the contribution of cellular participation to their uptake by cartilage is limited, due to the low population density of chondrocytes in cartilage. That the overall uptake of 99mTcGS is mediated via specific charged sites in cartilage is evident. Thus, even at saturation only 10% tracer is bound to the cartilage in the presence of a 50 fold excess of cold GS.
In contrast to 99mTc-CS, however, the 99mTc-GS-complex is positively charged. Therefore, a higher uptake of the complex by heavily degenerated cartilage, as shown by us in the present paper, cannot be due to the decrease in FCD of this cartilage, as seen for 99mTcCS, which is characterised by an overall negative charge of the molecule. Instead, the above mentioned repair mechanism combined with an increased leakness of the pathologically altered tissue towards a relatively small molecular complex, might explain the fact that heavily degraded cartilage binds more 99mTcGS than healthy or less degraded cartilage. In early OA i.e. before the onset of radiologic abnormalities, a significant decrease in uptake of GA is to be expected, however, as shown by others using other cationic cartilage targeting compounds such as 99mTc-NTP15-5 in a rabbit model of experimentally induced OA [35-37]. These tracers and related compounds are cartilage targeting, but not endogenous components of cartilage such as our 99mTcGS.
We show that the in vitro uptake of 99mTcGS depends on the degree of cartilage degeneration, i.e. PG depletion of the matrix. By autoradiographic uptake studies we have seen higher uptake in tissue at heavier degeneration (grade III) as compared to uptake of tissue with lower extent of degeneration (Figures 6 and 7). But not only uptake intensity was monitored. Also, the time curve of max uptake (saturation) was correlated with degeneration and whereas at grade III the saturation was attained after 6 h of incubation, in contrast at grade II the saturation onset was observed only at 24 h.
Conclusion
Uptake of 99mTcGS in degenerated cartilage in OA is tissue specific and correlates with tissue degeneration. Therefore, we believe that 99mTcGS could be a suitable tracer to quantify degeneration in articular cartilage, due to its high uptake and sensitivity. As we found that uptake is degeneration dependent, we hope to take clinical advantage for early diagnosis of OA. In addition it could be a promising tracer to monitor therapeutic effects in OA.Acknowledgements
We are grateful to Rottapharm (Monza, Italy) for supplying of GS and Mr. Guenther Brand (Department of Orthopaedic Surgery, Medical University of Vienna) for the preparation of tissue sections.References
- Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, et al. (2006) Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage 14: 101-113.
- Setnikar I, Giachetti C, Zanolo G (1984) Absorption, distribution and excretion of radioactivity after a single intravenous or oral administration of [14C]-glucosamine to the rat. Pharmatherapeutica 3: 538-550.
- Setnikar I, Giacchetti C, Zanolo G (1986) Pharmacokinetics of glucosamine in the dog and in man. Arzneimittelforschung 36: 729-735.
- Severson AR (1983) Inhibition of bone resorption and increased incorporation of 3H-glucosamine into hyaluronate in bone organ cultures treated with dibutyryl cyclic AMP and colchicine. Exp Cell Biol 51: 70-76.
- Sobal G, Menzel J, Sinzinger H (2009) Optimal 99mTc radiolabeling and uptake of glucosamine sulfate by cartilage. A potential tracer for scintigraphic detection of osteoarthritis. Bioconjug Chem 20: 1547-1552.
- Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168: 167.
- Elson LA, Morgan WT (1933) A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J 27: 1824-1828.
- Andrews P (1964) Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J 91: 222-233.
- Daher RJ, Chahine NO, Greenberg AS, Sgaglione NA, Grande DA (2009) New methods to diagnose and treat cartilage degeneration. Nat Rev Rheumatol 5: 599-607.
- Dingle JT (1999) The effect of nonsteroidal anti-inflammatory drugs on human articular cartilage glycosaminoglycan synthesis. Osteoarthritis Cartilage 7: 313-314.
- Petersen SG, Saxne T, Heinegard D, Hansen M, Holm L, et al. (2010) Glucosamine but not ibuprofen alters cartilage turnover in OA patients in response to physical training. Osteoarthritis Cartilage 18: 34-40.
- Lipiello L, Woodward J, Karpman R, Hammad, A (2000) In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res 381: 229-240.
- Persiani S, Roda E, Rovati LC, Locatelli M, Giacovelli G, et al. (2005) Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthritis Cartilage 13: 1041-1049.
- Persiani S, Rotini R, Trisolino G, Rovati LC, Locatelli M, et al. (2007) Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage 15: 764-772.
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, et al. (2001) Long-term effects of glucosamine sulfateon osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357: 251-256.
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, et al. (2002) Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 162: 2113-2123.
- Pavelka K, Bruyere O, Rovati LC, Olejarova M, Giacovelli G, et al. (2003) Relief in mild-to-moderate pain is not a confounder in joint space narrowing assessment of full extension knee radiographs in recent osteoarthritis structure-modifying drug trials. Osteoarthritis Cartilage 11: 730-737.
- Bruyere O, Pavelka K, Rovati LC, Deroisy R, Olejarova M, et al. (2004) Glucosamine sulfate reduces osteoarthritis progression in postmenopausal women with knee osteoarthritis: evidence from two 3-year studies. Menopause 11: 138-143.
- Taniguchi S, Ryu J, Seki M, Sumino T, Tokuhashi Y, et al. (2012) Long-term oral administration of glucosamine or chondroitin sulfate reduces destruction of cartilage and up-regulation of MMP-3 mRNA in a model of spontaneous osteoarthritis in Hartley guinea pigs. J Orthoped Res 30: 673-678.
- Tiraloche G, Girard C, Chouinard L, Sampalis J, Moquin L, et al. (2005) Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum 52: 1118-1128.
- Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2010) Effects of glucosamine or chondroitin sulfate on the esteoarthritis progression: a meta-analysis. Rheumatol Int 30: 357-363.
- Wen ZH, Tang CC, Chang YC, Huang SY, Hsieh SP, et al. (2010) Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis Cartilage 18: 1192-1202.
- Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, et al. (2014) Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis [Epub ahead of print].
- Mroz PJ, Silbert JE (2004) Use of 3H-glucosamine and 35S-sulfate with cultured human chondrocytes to determine the effect of glucosamine concentration on formation of chondroitin sulfate. Arthritis Rheum 50: 3574-3579.
- Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO 3rd, et al. (2008) The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum 58: 3183-3191.
- McAlindon TE, LaValley MP, Gulin JP, Felson DT (2000) Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 283: 1469-1475.
- Roden L (1956) Effect of hexosamines on the synthesis of chondroitin sulfuric acid in vitro. Ark Kemi 10: 345-352.
- Bassleer C, Rovati L, Franchimont P (1998) Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage 6: 427-434.
- Katta J, Jin Z, Ingham E, Fisher J (2009) Chondroitin sulfate: an effective joint lubricant? Osteoarthritis Cartilage 17: 1001-1008.
- Sobal G, Dorotka R, Menzel J, Sinzinger H (2013) Uptake studies with chondrotropic (99m)Tc-chondroitin sulfate in articular cartilage. Implications for imaging osteoarthritis in the knee. Nucl Med Biol 40: 1013-1017.
- Sobal G, Menzel J, Sinzinger H (2009) Uptake of 99mTc-labeled chondroitin sulfate by chondrocytes and cartilage: a promising agent for imaging of cartilage degeneration? Nucl Med Biol 36: 64-71.
- Sobal G, Sinzinger H (2002) Binding of [99mTc]chondroitin sulfate to scavenger receptors on human chondrocytes as compared to binding of oxidized [125I]LDL on human macrophages. J Recept Signal Transduct Res 22: 459-470.
- Uldry M, Ibberson M, Hosokawa M, Thorens B (2002) GLUT2 is a high affinity glucosamine transporter. FEBS Lett 524: 199-203.
- Shikhman AR, Brinson DC, Valbracht J, Lotz MK (2009) Differential metabolic effects of glucosamine and N-acetylglucosamine in human articular chondrocytes. Osteoarthritis Cartilage 17: 1022-1028.
- Ollier M, Maurizis JC, Nicolas C, Bonafous J, de Latour M, et al. (2001) Joint scintigraphy in rabbits with 99mtc-N-[3-(triethylammonio)propyl]-15ane-N5, a new radiodiagnostic agent for articular cartilage imaging. J Nucl Med 42: 141-145.
- Giraud I, Rapp M, Maurizis JC, Madelmont JC (2000) Application to a cartilage targeting strategy: synthesis and in vivo biodistribution of (14)C-labeled quaternary ammonium-glucosamine conjugates. Bioconjug Chem 11: 212-218.
- Sarda-Mantel L, Le Guludec D (2009) Molecular imaging of cartilage. J Nucl Med 50: 1391-1393.