Journal of Ocular Biology
Download PDF
Overall, the disinfecting solutions removed any live bacteria or fungi from the comfilcon A lenses. However, for somofilcon A, there remained some live bacteria on lenses after disinfection, with 4-12 cfu for cleadew and 20-24 cfu for RepleniSH [Table 2]. However, there were no overall statistical differences between the disinfecting solutions.
Research Article
Ability of Two Multipurpose Disinfecting Solutions to Kill Microbe’s Adherent to Contact Lenses
Itoi M, Kalaiselvan P and Willcox M
School of Optometry and Vision Science, University of New South Wales, Sydney, NSW 2052, Australia
*Address for Correspondence:Mark Willcox, School of Optometry and Vision Science,
UNSW, Sydney, NSW 2052, Australia. Email Id: m.willcox@unsw.edu.au
Submission: 17 March, 2025
Accepted: 12 May, 2025
Published: 17 May, 2025
Copyright: © 2025 Itoi M, et al. This is an open access article distributed
under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided
the original work is properly cited.
Keywords: Contact Lens; Disinfection; Pseudomonas Aeruginosa; Fusarium Solanii; Stenotrophomonas Maltophilia
Abstract
Aim:The aim of this project was to determine the ability of two
contact lens disinfecting solutions to kill bacteria and fungi attached
to lenses.
Methods:Pseudomonas aeruginosa PA181, Stenotrophomonas maltophilia 006 and Fusarium solanii ATCC 36031 were allowed to adhere to contact lenses (comfilcon A and somofilcon A) for one hour. The lenses were washed and then placed into contact lens cases of the two disinfecting solutions (cleadew soft containing povidone-iodine, or OPTIFREE RepleniSH containing polyquaternium-1 and myristamindopropyl dimethylamine) and disinfected for the manufacturers recommended time. Lenses are then washed and any viable bacteria removed and grown on agar plates. Both contact lens types that had been worn for a minimum of 6 hours were also disinfected, and any remaining viable microbes were cultured.
Results:After adding microbes to the lenses in the laboratory study, no viable microbes grew from the comfilcon A lenses, but 1-6 colony forming units of bacteria could be grown from the somofilcon A lenses after either type of disinfecting solution was used. After wear, bacteria could be cultured from both lens types after disinfection, with slightly more bacteria being cultured from the front vs. the back surface of the comfilcon A lenses (average cfu/lens 15-190 vs. 5-10).
Conclusions:There was no difference in the ability of the disinfecting solutions to kill bacteria adherent to lenses. The finding of viable bacteria remaining on lenses after disinfection reinforces the need to rub and rinse lenses after wear to remove some of these adherent bacteria.
Methods:Pseudomonas aeruginosa PA181, Stenotrophomonas maltophilia 006 and Fusarium solanii ATCC 36031 were allowed to adhere to contact lenses (comfilcon A and somofilcon A) for one hour. The lenses were washed and then placed into contact lens cases of the two disinfecting solutions (cleadew soft containing povidone-iodine, or OPTIFREE RepleniSH containing polyquaternium-1 and myristamindopropyl dimethylamine) and disinfected for the manufacturers recommended time. Lenses are then washed and any viable bacteria removed and grown on agar plates. Both contact lens types that had been worn for a minimum of 6 hours were also disinfected, and any remaining viable microbes were cultured.
Results:After adding microbes to the lenses in the laboratory study, no viable microbes grew from the comfilcon A lenses, but 1-6 colony forming units of bacteria could be grown from the somofilcon A lenses after either type of disinfecting solution was used. After wear, bacteria could be cultured from both lens types after disinfection, with slightly more bacteria being cultured from the front vs. the back surface of the comfilcon A lenses (average cfu/lens 15-190 vs. 5-10).
Conclusions:There was no difference in the ability of the disinfecting solutions to kill bacteria adherent to lenses. The finding of viable bacteria remaining on lenses after disinfection reinforces the need to rub and rinse lenses after wear to remove some of these adherent bacteria.
Introduction
Daily wear of contact lenses requires the lenses to be cleaned and
disinfected when not being worn. There are several types of disinfecting
solutions, those that have an oxidative disinfecting process such as
hydrogen peroxide or iodine, and those that use disinfectants such as
polymeric poly-quaternary ammonium compounds (QACs). All of
these disinfecting solutions must pass standard tests to demonstrate
their ability to kill a set of microbes.
These standard tests include the International Organisation for
Standardisation (ISO) 14729 “Ophthalmic Optics—Contact Lens
Care Products—Microbiological Requirements and Test Methods
for Products and Regimens for Hygienic Management of Contact
Lenses”[1]. In this set of tests, standard microbial strains are used
initially in suspension and the ability of the disinfectants to reduce
the viable number of these microbes, during the manufacturers
recommended disinfection time, is measured. If a solution fails to
meet thest and-alone disinfection criteria (at least a 3 log10 reduction
in bacteria and a 1 log10 reduction for fungi), it can be tested under the
regimen criteria as long as it met, at the manufacturer’s recommended
soaking time, stasis for the fungi and an average of 5 log10 reduction
in all bacteria, with at least 1 log10 occurring for each bacterium. The
regimen test includes adding the microbes to the contact lenses and
undergoing the manufacturers recommended cleaning steps such as
rubbing and rinsing. From an inoculum of 5 log10 organism, no more
than 10 viable organisms should remain viable on the lenses after the
regimen test.
Another test that is recommended is ISO 18259 “Method to Assess
Contact Lens Care Products with Contact Lenses in a Lens Case,
Challenged with Bacterial and Fungal Organisms”[2]. In this test,
specific bacteria or fungi are placed into a contact lens case containing
a contact lens, and the multipurpose disinfecting solution is added
for the manufacturer’s recommended disinfection time. After this
time, the lenses are removed and any viable microbial cells remaining
in the case are cultured. The other test that can be performed is ISO
19045-2:2024 “Method for evaluating disinfecting efficacy by contact
lens care products using trophozoites of Acanthamoeba species as the
challenge organisms”[3]. This test measures the ability of trophozoites
of Acanthamoeba to be killed by disinfecting solutions. There are no
pass criteria for each of these tests, but obviously the greater kill the
better.
With the exception of the regimen test, all others focus on the
solution rather than the lenses. Whilst the importance of a rub and
rinse with the disinfecting solution to remove adherent microbes has
been shown in laboratory studies [4],only 7% of contact lens wearers
reported they would rub and rinse their lenses after wear and before
adding to a disinfecting solution [5]. Therefore, the current study was
designed to examine whether two contact lens disinfecting solutions,
one oxidative solution containing iodine and one solution containing
QACs, were able to kill adherent microbes in the absence of a rub and
rinse regimen.
Materials and Methods
In vitro investigation:
Microbes and growth: Pseudomonas aeruginosa PA181, isolated
from a case of microbial keratitis and resistant to piperacillin with
intermediate resistance to imipenem and ceftazidime [6], and
Stenotrophomonas maltophilia 006, isolated from a contact lens caseat the time of microbial keratitis [7], were retrieved from the culture
collection of the School of Optometry and Vision Science, UNSW
Sydney. The strains were grown overnight in Trypticase soy broth
(TSB; Oxoid, Basingstoke, UK) at 30oC, and then resuspended in
sterile PBS pH 7.4 (NaCl 8 g L-1 , KCl 0.2 g L-1, Na2HPO4 1.15 g L-1,
KH2PO4 0.2 g L-1) to an optical density at 660nm of 0.1 (1x108 colony
forming units (CFU) mL-1), then diluted a further 100 fold in PBS to
yield final concentration of 1x106 CFU mL-1.
Fusarium solanii ATCC 36031 was also retrieved from the culture
collection, was grown on Sabouraud’s Dextrose Agar (SDA) (Oxoid,
Hampshire, UK) at 25°C for 10 to 14 days, and then harvested by
scraping off the agar surface. Approximately 1x 108 CFU mL-1 were
suspended in PBS and vortexed for 2 to 3 min. Retrospective plate
counts were performed to ensure that 1 x 108 CFUmL-1 were present.
This was then diluted further in PBS to achieve a final concentration
of 1x106 CFU mL-1.
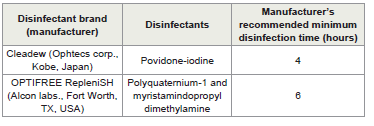
Contact lens disinfecting solutions: Two contact lens
disinfecting solutions were used [Table 1].
Adhesion of bacteria to lenses and disinfection: Contact lenses
(Biofinity, comfilcon A), and clariti 1 day (somofilcon A, both from
CooperVision, Pleasanton, CA, USA; -1.00 D) were used in the
study. Whilst the comfilcon A lenses are routinely used on a daily
wear schedule and so would be exposed to a disinfection cycle each
night when not being worn prior to disposal, the clariti lenses are
designed for daily disposable use, and so would not routinely be
exposed to a disinfection cycle as they are disposed on each day after
wear. However, as 20% of daily disposable lens users may actually
reuse their lenses and use a contact lens case [8], and reuse of daily
disposable lenses can increase the risk of corneal infection by 5.4
times [9], the authors thought it would be of interest to compare these
lenses.
Contact lenses were removed from their packaging solutions
and placed into 2 mL of PBS. The lenses were then rinsed in the PBS
three times, and then placed into a final concentration of 1x104 cfu of
each microbe separately. The microbes were allowed to adhere to the
lenses for one hour, then lenses were washed once in PBS to remove
loosely adherent microbes. The lenses were then placed into the
contact lenses cases supplied with each contact lens solution, and the
manufacturer’s recommended amount of each disinfectant added,
and the lenses disinfected for the manufacturer’s recommended
disinfection time.
After disinfection, the lenses were removed and placed into 2 mL
of PBS, then vortexed on high speed for 1 minute to release adherent
microbes from the lenses. An aliquot of the solution (50 μL) was then
plated in triplicate onto TSB containing agar at 15 g L-1, and lecithin
at 7 g L-1 and polysorbate 80 at 5 g L-1 as neutralising agents [10]. After
overnight incubation at 37oC, the number of CFUs was counted.
In vivo test:
Contact lenses (comfilcon A and somofilcon A) were retrieved
at the end of use (at least six hours wear for daily disposable lenses)
from established contact lens wearers, selected as they had previously
given informed consent to be contacted for future tests in prior
clinical trials. As these lenses would have been discarded, and were
not intended for further human use, the ethics committee of UNSW
Sydney did not require ethical approval of the study. Lenses were
collected, using sterile gloves, added to appropriate lens cases with 2
mL of sterile PBS and de-identified before further use.A sample size calculation was performed based upon the data
in a previous study that evaluated the number of staphylococci
(the most commonly isolated bacterium from the normal ocular
surface[11] in populations of lens wearers in Sydney Australia. There
were an average of 3.29 ± 3.27 cfu from a contact lens[12]. If contact
lens disinfecting solutions reduce this average number to ≤0.5 then
eleven contact lens wearers for each contact lens type were required.
Furthermore, prior studies had shown that 73% of contact lenses
were contaminated during wear [13]. Assuming this is reduced to
10% after disinfection, a sample size of eight contact lenses would be
needed t show a significant difference.
Upon receipt of the lenses in the laboratory, they were subjected
to disinfection as per the manufacturer’s recommendations. After
disinfection, lenses were washed once in PBS and then each side was
swabbed with a sterile cotton wool swab, by rubbing over the surface
three times. The side (concave or convex) that was swabbed first was
randomised. The cotton swabs were then added to sterile microtubes
containing sterile PBS and vortexed on high speed for one minute.
After vortexing, 100 μL of the solution was placed onto each of three
chocolate agar plates for aerobic, anaerobic and 5% CO2 enrichment
growth and SDA for fungal growth. The plates were incubated at 37oC
for 48 hours for aerobic and CO2 growth and 72 hours for anaerobic
growth, and 7 days at 21oC for fungal growth. After incubation, the
number of microbial colonies were counted and back calculated to
obtain the number of cfu/lens side. Gram staining was performed to
determine whether bacteria were gram-positive or gram-negative and
their cellular morphology (coccus or rod). Fungi were identified as
moulds or yeasts based upon colony morphology.
Results
In vitro investigation:
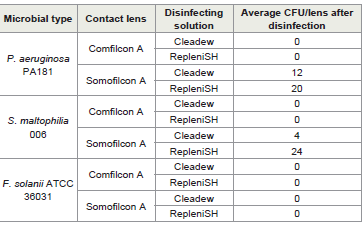
The number of CFU of each microbe recovered from the contact
lenses after disinfection is given in [Table 2].Overall, the disinfecting solutions removed any live bacteria or fungi from the comfilcon A lenses. However, for somofilcon A, there remained some live bacteria on lenses after disinfection, with 4-12 cfu for cleadew and 20-24 cfu for RepleniSH [Table 2]. However, there were no overall statistical differences between the disinfecting solutions.
In vivo test:
Unfortunately, the study was unable to obtain each type of contact
lens from eleven individuals; only eight people were willing to donate
comfilcon A lenses, and only four people were willing to donate
Table 2:Number of microbes recovered from contact lenses disinfected with two
disinfecting solutions
somofilcon A lenses. This partly reflects the lens types commonly
worn in the Sydney region, with only 22% of daily wearers using
comfilcon A [14] and most (≥80%) daily disposable wearers using
etafilcon A, delefilcon A, or comfilcon A (in-house data).
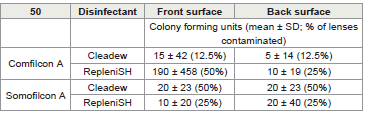
The numbers of viable bacteria (there were no fungi grown)
remaining on lenses after wear and after disinfection are given in
[Table 3].
No microbes could be grown from most contact lenses after
disinfection with either of the solutions. When microbes (bacteria
only) were grown the most commonly cultured were Gram-positive
cocci, which upon gram staining and on inspection of colony
morphology resembled staphylococci. Gram-negative bacteria were
rarely cultured; a gram-negative rod was cultured from a comfilcon A
lens that had been disinfected with RepleniSH in large numbers (1320
CFU/lens) from the front surface, and a Gram-negative coccus was
cultured from the back surface of a comfilcon A lens disinfected with
cleadew (40 CFU/lens). From somofilcon A lenses, a Gram-negative
coccus was cultured from the back surface (40 CFU/lens) after
disinfection with cleadew, and a Gram-negative rod (40 CFU/lens)
was cultured from the front surface after disinfection with RepleniSH.
Statistical analysis of either the CFU/lens or percentage of lenses with
contamination after disinfection did not find any difference between
the disinfecting solutions.
Discussion
This study has shown that two contact lens disinfecting solutions,
one oxidative (povidone-iodine) and one using QACs were generally
able to kill all bacteria that had adhered to lenses either in a laboratory
study or during wear.
The laboratory study showed that disinfection effectivity could be dependent on the lens polymer, with somofilcon A lenses having live bacteria remaining on them after disinfection. While the somofilcon A lenses are designed for daily disposable use and are not routinely disinfected, 20% of daily disposable lens users may reuse their lenses and store them in a contact lens case [8], and reusing daily disposable lenses can increase the risk of corneal infection by 5.4 times [9]. It would be interesting to determine if other polymers used for daily disposable lenses face a similar issue.
The laboratory study showed that disinfection effectivity could be dependent on the lens polymer, with somofilcon A lenses having live bacteria remaining on them after disinfection. While the somofilcon A lenses are designed for daily disposable use and are not routinely disinfected, 20% of daily disposable lens users may reuse their lenses and store them in a contact lens case [8], and reusing daily disposable lenses can increase the risk of corneal infection by 5.4 times [9]. It would be interesting to determine if other polymers used for daily disposable lenses face a similar issue.
The clinical test demonstrated that bacteria could be cultured
Table 3:Number of bacteria cultured from the front or back surface of contact
lenses after wear and disinfection
from some lenses after wear and subsequent disinfection. The study
did not include a rub and rinse procedure after lens removal. Had this
been in place, this may have removed all or most of these adherent
bacteria [4], and the authors remind readers of the need to reinforce
this procedure to all contact lens wearers if they reuse their lenses.
However, the authors do not recommend this for daily disposable
lens wearers, with reinforcing the requirement to discard lenses
after each day of wear being an appropriate message. For comfilcon
A lenses, there tended to be more bacteria remaining viable on the
front of contact lenses after disinfection, regardless of the disinfecting
agent. A previous study examining the number of microbes in contact
lens cases during daily wear of lenses had shown that OPTIFREE
RepleniSH left greater numbers and frequency of viable Gramnegative
bacteria such as S. Maltophilia in the lens cases [15]. This
is similar to the current data, where disinfection with OPTIFREE
RepleniSH left great numbers of viable Gram-negative bacteria on
lenses.
It would be useful in future studies to increase the numbers of
worn lenses collected, include other lens types, and other disinfecting
solutions. This may also help to determine whether the differences
could reach statistical significance. Using the data from the current
study, for the in vitro tests, 7 lenses in each group may be able to
show significant differences between CFU/lens with somofilcon A
lenses; for the in vivo tests, the minimum number of people required
to see differences in front surface contamination with comfilcon A
lenses with percentage contamination would be 21, but with CFU/
lens would be 108, per group. In conclusion, overall, the study has
demonstrated equivalence of the disinfecting ability of the two
contact lens disinfecting solutions on microbes adhered to lenses