Journal of Ocular Biology
Download PDF
Research Article
*Address for Correspondence: M. Nurhalim Shahib, Department of Biochemistry and Molecular Biology, Universitas Padjadjaran, Bandung, Indonesia, Tel: +62 817210770; Fax: +62 22 2037824; E-mail: nurhalimshahib@yahoo.com
Citation: Budiman, Feranthy ZA, Shahib MN. The Existence of mRNAs and miRNAs Expressions for Maintaining Cell Survival Networks Associated with the Human Transparent and Cataractous Lens. J Ocular Biol. 2015;3(1): 8.
Copyright © 2015 Budiman, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 3, Issue: 1
Submission: 30 July 2015 | Accepted: 26 August 2015 | Published: 31 August 2015
Reviewed & Approved by: Dr. Mark Willcox, Professor, School of Optometry and Vision Science, University of New South Wales, Sydney, Australia
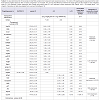
In Table 3, of 25 gene expressions showed that 5 genes were upregulated expressions (miR-205, Sirt3, Hsp60, miR-137, and Akt) (p<0,05), 7 genes were down-regulated expressions (p21, p27, Cox2, CygB, mirlet-7A, caspase3, and CryAA) (p<0,05), the remaining 13 (Hsp70, GSS, Ucp1, E-Cadherin, Sirt1, TNFα, p53, Rip1, beclin1, NFκB, Hstf, GSR, and Grp78) were not significant different (p>0,05). The two largest decreases in expression among the 7 genes were the p21 and Caspase 3. The largest increase among the 5 up-regulated genes was Sirt3. The Sirt3 expression was associated with cell aging signal which in this study different trend with Sirt1. Here, the Sirt1 was low expression. Gene maintaining cell survival was represented by Akt that was up-regulated. Cell proliferation was maintained by Cox2 that in this study was down-regulated. In this study, miR-205 and miR-137 up-regulated but mirlet-7A was down-regulated suggested mirlet-7A different mechanism from the other two miRNAs. Hsp60 gene expression is important for immune response that in this study increased. From these 13 gene expressions showed that the Ucp1 was ~ 8- fold increase in cataractous lens. This gene codes protein producing heat in mitochondria. In addition, Sirt1 was ~2,6-fold decrease, while NFκB was ~4,0-fold increase. CygB that was downregulated in cataractous lens should be noticed, it might be related to the hypoxic state of lens cell. Interestingly, p53, Caspase-3, Rip1, and beclin1 gene inducing cell death signal were low expressions.
The Existence of mRNAs and miRNAs Expressions for Maintaining Cell Survival Networks Associated with the Human Transparent and Cataractous Lens
Budiman1, Zoraya A. Feranthy1 and M. Nurhalim Shahib2*
- 1Department of Ophthalmology Faculty of Medicine, Universitas Padjadjaran/National Eye Center – Cicendo Eye Hospital, Bandung, Indonesia
- 2Department of Biochemistry and Molecular Biology, Universitas Padjadjaran, Bandung, Indonesia
*Address for Correspondence: M. Nurhalim Shahib, Department of Biochemistry and Molecular Biology, Universitas Padjadjaran, Bandung, Indonesia, Tel: +62 817210770; Fax: +62 22 2037824; E-mail: nurhalimshahib@yahoo.com
Citation: Budiman, Feranthy ZA, Shahib MN. The Existence of mRNAs and miRNAs Expressions for Maintaining Cell Survival Networks Associated with the Human Transparent and Cataractous Lens. J Ocular Biol. 2015;3(1): 8.
Copyright © 2015 Budiman, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 3, Issue: 1
Submission: 30 July 2015 | Accepted: 26 August 2015 | Published: 31 August 2015
Reviewed & Approved by: Dr. Mark Willcox, Professor, School of Optometry and Vision Science, University of New South Wales, Sydney, Australia
Abstract
Background: Human lens cells have a long lifespan and so must maintain cell survival networks with regulate biological processes including gene expression and regulation. The purpose of this study was to identify mRNA transcript differences in the transparent and cataractous human lens.Methods: The expression of 25 genes was examined at RNA level in human lenses, including miRNA-205, miRNA-137, and mirlet-7A. All genes were isolated from 9 subjects with senile cataractous lenses and 5 subjects with transparent lenses as a control. The gene expressions were determined by methods of Real Time PCR (qRT-PCR). All genes were normalized to internal control by using Gapdh gene expression, and the calculations were based on the 2-ΔΔCt method.
Results: Seven genes had lower levels of expression in the samples from cataractous lenses (p21, p27, Cox2, CygB, Mirlet-7A, Caspase-3 and CryAA) and 5 genes had higher levels of expression in cataractous lenses (Mir-205, SIRT3, HSP60, miR137 and Akt). The remaining 13 were not significantly different.
The increasing of Sirt3, Hsp60, and Akt in cataractous lens suggested these genes protected and maintained lens cells survival and both miR-205 and miR-137 might also involve in lens cell survival networks. These two microRNA different profile from miR-7A that was down-regulated. Interestingly, increasing of Hsp60 indicated the inflammatory reaction or immune reaction might be involved in pathogenesis of cataract.
p21 and p27 were down-regulated in cataractous lens, suggested the role of these genes in maintaining cell senescent decreased. Interestingly, the CygB was down-regulated in cataractous lens, this is also indicating hypoxic may be one of the risk factor in cataract.
Conclusion: Collectively, our data showed that of 25 detected gene expressions in cataractous lens, 13 genes were different from transparent lens. Interestingly, the Hsp60 might be associated with inflammatory reaction or immune system and CygB might be associated with hypoxic state of lens cells, suggested both Hsp60 and CygB were important in pathogenesis of human cataract. In addition, miR-205 and miR-137 expressions that were increased strongly in cataractous lens and decreasing of mirlet-7A indicated that these three miRNAs involved in cell lens network regulation.
Keywords
Cataract; Transparent; Senescent; Senile cataractAbbreviations
ROS: Reactive Oxygen Species; HSTF: Heat Shock Transcription Factor; ERSS: Endoplasmic Reticulum Stress SignalIntroduction
To analyze the functional changes of human eye lens at the molecular level, we need to determine mRNA transcripts as molecular indicators for early detection of senile degenerative diseases, including of human eye lens cataract. For this purpose, we need to take both specific and the other related genes expressions of lens into account, and also to analyze the involvement of related signaling networks. The changes in gene expression in human lens can cause a variety of clinical impacts, including a cataract, which in this study, we attempt to learn more about the expressions and further develop the molecular network of 25 genes expression associated with the cataractous lens. Several stimuli such as oxidative, radiation and temperature variation contribute to the cataractous lens [1-3]. However, no information about inflammation and another injured stimuli, including hypoxic condition were reported, those all might be accumulating throughout the human life impacting eye lens functions. As protein synthesis is an important cell function, the existing mRNA transcripts play a central role in most cell responses, including in lens cells.In the current study, we identified 25 gene expressions at mRNA level including miRNA differentially expressed in transparent and cataractous lens. Micro RNAs (miRNAs) are a class of short endogenous RNA (~ 22 nt) that regulate a wide range of biological processes, including their expressions to maintain the eye lens functions. Over the past decade, both in vitro and in vivo analysis have shown that micro RNAs (miRNAs) is essential for gene expression regulation [4]. The regulation is to degraded the mRNA or repress the translation.
To explore the possibility that miRNAs might be partly of the several genes suppression network, we examined miRNA 205, mir-137 and mirlet-7A expression profiles in the cataractous and transparent lens, beside another 22 genes (Hsp70, TNFα, Grp78, Sirt1, Sirt3, CryAA, p27, Ucp1, NFκB, p53, Caspase-3, GSR, CygB, p21, Hstf, Hsp60, Cox2, Rip1, Beclin1, GSS, E-Cadherin, and Akt1) expressions. MiR-205 is now known to play a fundamental role in epithelial biogenesis and maintenance [5,6] and has been widely studied in a number of settings [7], however, there have not been reported the expression of miR-205, miR-137 in cataractous lens, and mirlet-7A has not yet concluded too.
For these purposes, both cataractous and control mRNAs were reverse transcribed under certain condition in which the reaction was normalized to certain equal amount of RNA extract. The response of eye lens cells to microenvironment changes is a useful symptom to study complex cellular process in aged cataractous lens. In this study, qualitative analysis of 25 different genes could be grouped into their response associated with their multicellular function, such as general cell survival (Akt and Cox2), cell senescent (p27, p21), cell adhesion (E-Cadherin), cell aging signals (Sirt1, Sirt3), cell death (p53, Rip1, Beclin1, Caspase-3), thermal induced signals (Ucp1, Hstf, Hsp70), oxidative signals or ROS production signals (CryAA, GSR, GSS), inflammation signal (Hsp60, TNFα, NFκB), hypoxic signal (CygB), endoplasmic reticulum stress (Grp78), and the three miRNAs (miR-205, miR-137, and mirlet-7A).
It has been known that the lens cells of the human eye are very long term survival, it can be decades of years. It has been reported [8,9], that in the center and in the fiber cells of the human eye lens in the old person contains the same cells as those when the person was born. Even though the fiber cells still contain many proteins while the cells have not enough organelles anymore. How lens cell maintains the existing protein molecules in long time period and contributing the maintenance of cell survival. The cellular architecture and arrangement of fiber cells and particularly their structure are critical for light transmission and lens transparency [10]. Although gene expressions at the protein level have been reported [2,11] in the eye lens cells, but the mechanism how cells maintain their protein is still unknown. In that regard, we intend to conduct the research on the role of RNA and miRNAs genes expression in lens cells both in transparent and cataractous lens and created a biological network that can explain the mechanism of the cell to be survived. Therefore, the purpose of this study is to analyze the presence of mRNA transcripts of 25 genes by Real Time PCR (qRT-PCR) associated with the multicellular functions.
Materials and Methods
Human lens specimensPatients were age 40-79 years old (n=9) diagnosed with cataract NC4 senilis up to NC6 (NC = nuclear cataract) and 5 non-cataract patients with age 40-79 years old were use as control (transparent lens). These 5 patients suffer from High Myopia (>10.00 D/seq) who needed refractive surgery intervention (clear lens extraction). This decision was made based on pre surgical diagnostic evaluations: less corneal thickness, decreased endothelial cell count, and narrow anterior chamber depth, leading to LASIK procedure and other refractive interventions could not be chosen. Lens material was very soft, for that the lens extraction were performed by making 4.5 mm scleral incision. These lens material were usually disposed, but in this study we collected them after gaining approval from the patient to be obtained as control (transparent lens) in our study. Both senile cataract and the control were not suffered from diabetic and another diseases causing lense cataract. Human lens specimens were obtained from lens during cataract surgery by means of the small incision cataract surgery (SICS) after informed consent at the National Eye Center-Cicendo Eye Hospital Bandung-Indonesia. By this method the cortex and the nucleus of the lens were taken and put into Eppendorf tube containing RNA later (Ambion, USA). The specimen has to be soaked completely in the RNA later solution. Then the lens specimens were stored at -20 °C. The next day the specimens were subjected to RNA preparation. This study was approved by the Research Ethic Board of Faculty of Medicine, Universitas Padjajaran, Bandung-Indonesia (the ethical number: 545/UN6.C2.1.2/KEPK/PN/2014).
RNA preparation
RNA extractions were carried out by homogenizing the subcapsular material of lens tissues containing fiber cells [9] in trizol reagent (Invitrogen, USA) from which total RNA was obtained. The homogenized solution was centrifuged at 14,000 g using column tube according to the manufacturer’s instructions. RNA pellets were recovered and purified by phenol-chloroform extraction and ethanol precipitation then was diluted with TE Buffer pH 8.0. Total RNA purity, quantity, and quality were checked by NanoDrop spectrophotometer ND-1000 (Thermo Scientific, USA). The RNA solution was ready for Real Time PCR (qRT-PCR) (Kappa Biosystem, USA).
Real time PCR (qRT-PCR)
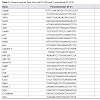
The expression levels of mRNA and microRNA were determined using the 1 step qRT-PCR kit according to the manufactures instructions (Kapa Biosystem, USA). The primers consisted of forward and reverse sequences for Hsp70, TNFα, Grp78, Sirt1, Sirt3, CryAA, p27, Ucp1, NFκB, p53, caspase-3, GSR, miR-205, miR-137, mirlet-7A, CygB, p21, Hstf, Hsp60, Cox2, Ripk1, beclin1, GSS, E-cadherine and Akt1 (Table 1). The primers were designed based on NCBI Referrence Sequence. The qRT-PCR reaction was subjected to reverse transcription (RT) for 5 minutes at 42 °C, followed by inactivation of the RT enzyme at 95 °C for 3 minutes and the cycle of PCR for 40 cycles. The circle consisted of 95 °C of denature (30 sec); 60 °C of annealing (20 sec) and 72 °C of extensions (20 sec). The result can be seen in the form of threshold cycle (Ct curve) by applying the relative changes in targeting genes expression [12]. Ct (threshold cycle) is reported as the PCR cycle number that crosses an arbitrary placed signal threshold [13]. The average Ct was calculated for both target gene and internal control (Gapdh) [14] and the ΔCt was equal to the difference in threshold cycles for target and Gapdh (Ct target–Ct Gapdh). Gene expressions was then calculated as 2-ΔΔCt. We categorized the Ct values varying from 15 to 40; 15-20 was very high expression; >20-25 was high expression: 25-30 was moderate expression; >30-35 was low expression, and >35-40 was very low or no expression. We also adapted the possibilities of genes expression under 15 of Ct value, called over-expression. All these categories were confirmed with gel electrophoretic data.
Results
In an effort to understand the changes of biological functions of eye lens and cell signaling networks, we establish gene expressions both in transparent and cataractous lenses by analysis the mRNA transcripts. The mRNA transcripts were determined by Real-Time PCR, which identified 26 genes expressions (including Gapdh) using 26 primers oligonucleotide synthesis (Table 1) and data evaluation results in threshold cycle (Ct) value. The mean Ct values for both cataractous and transparent lens were determined at the same time and the amount of the transcripts = 2-ΔΔCt. By using Gapdh as a reference gene [14] we determined ΔCt that is equal to the difference in threshold cycle (Ct) for target and reference gene [12]. The expression were studied and completed by analyzing the Ct values of 25 gene expressions compared to Gapdh expression of human lens cataract and transparent lens. In Table 2, the variations of expressions were divided into 5 categories. These categories were needed to know how far the gene expressions shift their profiles from transparent to the cataractous lens. Of the 25 gene expressions in cataractous lens, 2 genes (beclin1 and mirlet-7A) were very low expressions, 9 genes (Hsp70, E-cadherin, Caspase 3, Sirt1, p21, Ripk1, Cox2, CygB and p53) were low expressions, 5 genes (p27, mirlet-137, GSS, Ucp1 and NFκB) were moderate expressions, 4 genes (GSR, mir-205, Grp78, Akt) were high expressions, and 2 genes (Sirt3 and CryAA) were very high expressions, but it was not found the over expression gene as in the transparent lens. The profile of miRNA expressions was also shown that miR-205 and miR-137 were increased 40 and 13, 4 fold, respectively, but the miR-let-7A were 3 fold decrease. Of six thermal induced signal genes, 4 genes (Ucp1, Hstf, Hsp70, and Grp78) were not significant different, but they showed the relative fold changes trend decreased. Interestingly, the other two thermal genes (Hsp60 and CryAA) were significant different. Hsp60 was up-regulated whereas CryAA was down-regulated. Hsp60 was 7,8 fold increase in cataractous lens compared to transparent lens.Table 2: Ct value determined for 25 transcripts in total RNA samples from transparent and cataractous lenses. The order of 25 targeting genes was placed in the order from lowest to highest based on their Ct values compared to Ct of Gapdh. The ΔCt was the differences between the target gene and the Gapdh Ct value. Negative delta Ct (-ΔCt) indicated a higher expression than Gapdh and positive delta Ct (+ΔCt) indicated a lower expression than Gapdh. ΔΔCt = ΔCt (target gene) - ΔCt (Gapdh). Calculation of data was adapted from Livak and Schimttgen [12].
Discussion
Analysis of 25 genes expression was determined based on the 2-ΔΔCt method variated from the lowest to the highest expression (Table 2). Further analysis, we observed 25 gene expressions, 5 genes (Mir-205, Sirt3, Hsp60, Mir-137 and Akt) were up-regulated, 7 genes (p21, Cox2, CygB, Mirlet-7A, caspase3, p27, and CryAA) were down-regulated, the remaining 13 (Hsp70, GSS, Ucp1, E-Cadherine, Sirt1, TNFα, p53, Ripk1, Beclin1, NFκB, Hstf, GSR, Grp78) were not significant different (Table 3).Of the 5 up-regulated genes, 2 genes were microRNA (miR-205 and miR-137) which have different role from another 3 genes (Sirt3, Hsp 60, and Akt). In human, expression of miR-205 and miR-137 were detected in many tissues [6] including cornea [15], but it has not been reported in human lens. In this study, the increasing of miR-205 and miR-137 in cataract lens, suggesting that these microRNAs were required for regulating the mRNA target in lens cells. However, we did not examine their mRNA target in lens cell.
It has been well known that microRNAs were associated with the majority of key cellular processes [4] and increasing of microRNAs may acts either as tumor suppressor or oncogen during the progression of tumor [5] depending on which kind of their gene targets [16]. However, we did not find any report about miR-205 and miR-137 activity in cataractous lens. Senile cataract is a clouding of the lens due to the inability of the eye lens to maintain its normal function caused by senescent, but the etiology has not been explained to date.
Vosgha et al. mentions the increasing of miR-205 is connected to the increasing of Akt activity in trophoblast. It through the suppressing Src Homology 2-containing Phosphoinositide 5’-Phosphatase 2 (SHIP2) [17]. miR-205 expression also have been reported to be changed in hypoxic trophoblast, the expression of miR-205 is elevated through suppression of mediator of RNA polymerase II transcription subunit (MED1) [18]. We suggested the increasing of miR-205 in lens cells might be relevance to those in trophoblast. Most notably, we found that miR-205 and miR-137 were up regulated in cataractous lens. Whereas the let-7A was found to be down regulated, suggesting the expression pattern of miR-205 and miR-137 in controlling their mRNA targets in eye lens might have different mechanism from that mirlet-7A.
Although several gene expressions mentioned above were responsive to signaling by external factors, such as oxidative, temperature and radiation [2], however, inflammatory reaction and hypoxic stresses were seldom reported. Further analysis was to explore Cytoglobin (CygB) expression. To date, there is no report about relationship between hypoxic in lens cells and cataract. We hypothesized that prolong hypoxic in lens cells might be also one of risk factors in pathogenesis of cataract. In order to know hypoxic state occurred in lens cells, we use Cytoglobin B (CygB) as an indicator of the hypoxic status of cataractous compared to the transparent lenses. Our result showed that CygB expression in cataractous lens was down-regulated (p<0,05) and it changed ~2,5-fold decrease from transparent lens. We suggested that cellular hypoxic was another mechanism involved in cataract pathogenesis, besides oxidative (ROS), radiation, and temperature or heat that have been known. CytoglobinB (CygB) is a gene maintaining storage of oxygen in cells [19]. Including in the anterior eye segment [20]. No publication was found to explore interaction of miR-205 and CygB mRNA inhuman lens or in human cataract. Another three up-regulated gene expressions were shown in Sirt3, Hsp60, and Akt of cataractous lens (p<0,05).
In this study, the cataractous lens showed different expression of sirtuin genes. Sirt3 was a very high expression, but Sirt1 was low expression (Table 2). We suggested Sirt3 serve as a protective mechanism against to the lens cell aging, the same function as those in other aged-organs [5,10,21-23]. In addition, it also did as a molecular signal that mitochondrial stress was prominent in cataractous lens. This may be relevant to the ROS synthesis [2] that plays an important role in pathogenesis of cataract. According to Kelly, Ucp1 is one of the molecular targets of lens Sirt3 [24]. We suggested the expression of Sirt3 was upregulated in cataractous lens might be associated with increasing of Ucp1 expression (8-fold increase) (Table 3). According to Salminen et al., the low expression of Sirt1 is related to the expression of NFκB that in our result was moderate expression in both transparent and cataractous lenses [25]. In Indonesia, at which incidence of infectious disease is still high, the moderate expression of NFκB (~4-fold increase), TNFα (~1,6-fold increase) and Hsp60 (~7,8-fold increase) (p<0,05) in cataractous lens might indicate a cellular response of those genes to a moderately prolonged inflammatory reaction. It has been known that Hsp60 involves in the immune system network [26]. The Hsp60 like the other Hsps family participate in the cellular response to stress. However the Hsp60 is more dominant play an important role in the central of the immune response [26]. Thus, the Hsp60 besides can halt the apoptotic signaling, it also has a protective effect on immune response to disease. Our result showed a significant increase of Hsp60 in cataractous lens and trend increasing of TNFα, and NFκB, we suspected that the inflammatory reaction or immune response might play a role in cataractous lens in Indonesia.
Interestingly, besides Sirt3 which was very high expression, Akt was also upregulated in cataractous lens. Analysis of Akt expression promoting cataract development through the specific loss of gene (PTEN) has been reported by Sellitto [27]. This excellent report suggested that Akt play an important role in maintaining cell survival, including in the ocular lens [27,28]. Our data showed that Akt gene was highly expressed (97,14 times Gapdh expression) in transparent lens, suggested its role in maintaining lens cell survival was very important. In this study, the expression of caspase3 was very low expression, suggested in the cataractous lens the apoptosis pathway might be very weak or not active. In relevance to this condition, p21 and p27 of transparent lens were moderately and highly expressed respectively, suggesting cell senescent was maintained in the transparent cell. In this study both of them (p21 and p27) were downregulated in cataractous lens, suggesting the lens failed to maintain cell senescent, cell then might be shifting to be cloudy (cataractous lens). Senescent lens cells are characterized by an inhibited of cell proliferation, and by an enlarged morphology of epithelial cells into lens fiber cells [8,9,29]. It has been known that p21 and p27 play a role in cellular senescent [30,31], however, of the lens cataract have not been reported.
In this study, lens cells of cataractous lens that have very low expression of p53, Beclin1, and Rip1 indicated cell death signal were not active or very slow. However, both transparent and cataractous lens showed high expression of Akt, suggesting this gene maintained the survival signals. In this aspect, there were no any differences between the expression of p53, Beclin1, and Rip1 in transparent and cataractous lens (p>0,05) (Table 2). Akt pathway maintains cell survival and prevent apoptosis by inactivating several apoptosis effectors [32], including Caspase-3, similar inhibit autophagy [33]. Our data confirmed this cell death signal by showing low expression of becl1. In normal lens, the caspase-3 activity was required for organelles destruction during cell lens differentiation at which the epithelial cell was transformed into fiber cells maintaining its transparency. It is known that the mitochondrial cell death pathway is required for the initiation of lens fiber cell differentiation [34]. According to this model, executioner caspases (specifically caspase-3) are required for the proper elongation of fiber cells [35]. In this study, the caspase-3 was 14,9-fold decrease (p<0,05) in cataractous lens compared to transparent lens (Table 3). Eye lens cell, by deregulating the proliferative signaling and delaying cell death, become master plans of their own homeostasis. An understanding of how the expression of human eye lens-genes is induced is not only fundamental maintaining cell survival networks interest but also of clinical importance of studying pathogenesis of cataract. How lens cells induce permanent cell cycle arrest and maintain cellular senescence in response to daily stimulation signals and many others stimuli in whole human life remain poorly understood.
In the tropical country which the temperature varies from 25-32 °C makes the people dealing directly with photooxidative throughout the year that can have an impact on the eye lens. It is not known whether there is any connection with the case of cataracts that are quite high in Indonesia. Photooxidative and other types of stressful stimuli including heat can increase the cellular expression of heat shock genes with impacting to heat shock protein (Hsps) activities [2]. These genes have been characterized as a family of heat shock genes which coding Hsp protein also known as stress proteins that protecting the cell [3], located in different compartment of cell. It has been shown that a decrease in Hsps gene expression leads to structural changes in the lens that might play apart in the development of lens cataract. These genes are regulated by heat shock transcription factor (Hstf) coded by Hstf gene. We showed that Hstf expression was 4-fold decrease in cataractous lens compared to the transparent lens (Table 3). Consistent with this result, Hsp70, CryAA, and Grp78 were also decreased. Hsp70 has been reported to decline with age [36]. Interestingly, Hsp60 that is also Hsp family, its expression was higher in cataractous than in the transparent lens (Table 2). In this perspective, the Hsp60 might not be activating at the same target molecules as another Hsp genes. The Hsp60 might be increased in order to provide a cellular protective action on ocular stress such as inflammatory reaction or immune reaction. CryAA is a gene coding for CryAA protein related to the heat shock protein family and have chaperone-like family [37] at which in our result showing significant decreased in cataractous lens (p < 0,05). However, one of the heat shock genes family called Grp78 that is affiliated with many cells response to stimuli, has not been reported yet in human lens, especially in human lens cataract. Grp78 gene codes for Grp78 protein that primary located in Endoplasmic Reticulum (ER). This protein plays an important role in ER (Endoplasmic Reticulum) stressed signaling (ERSS) [38], that in this study both cataractous and transparent lens was highly expressed (Table 2), suggesting endoplasmic reticulum involved in maintaining cellular networks in human lens. E-Cadherin gene expressions, a key cell to cells adhesion molecules, were expressed moderately in transparent lens and low expression in cataractous lens. Cataract is a process during which eye lens cells lose their transparency, where light scattering changes give rise to visual loss. The cells change their morphology, including cellcell attachment via E-Cadherin and opacity of lens via CryAA that were both of them decreased in cataractous lens.
Beclin1 was low expression, suggesting autophagy might be very slow or was not induced, but p27 was high expressions in transparent and it was moderate expressed in cataractous lens (p<0,05) (Table 2), indicating the ability of maintaining cell senescent decrease. In lens cells, both transparent and cataractous lens did not show necrotic signal induction, it was showed by low expression of Rip1.
Conclusion
In cataractous lens, 5 genes (miR-205, Sirt3, Hsp60, miR-137, and Akt) were up-regulated expressions, indicated that Sirt3, Hsp60, and Akt protected and maintained lens cells survival, and the two miRNAs (miR-205 and miR-137) might also involve in those lens cell signal regulation. Increasing of Hsp60 might be associated with inflammatory or immune reaction in lens cells. Further analysis showed that 7 genes (p21, p27, Cox2, CygB, mirlet-7A, caspase3, and CryAA) were down-regulated in cataractous lens. Decreasing of p21 and p27 indicated the ability to maintain cell senescent decreased in cataractous lens and this condition was relevance to decreasing of Cox2 and CryAA. Decreasing of mirlet-7A might be associated with suppression of survival signal network. Interestingly, CygB was also down-regulated in cataractous lens suggested hypoxic state of lens might be occur and might act as a risk factor in cataractous lens. Based on the increasing of the Hsp 60 and decreasing of CygB, suggested that inflammatory reaction or immune response and hypoxic state of lens cell might play a role in cataract pathogenesis.References
- Truscott RJ (2005) Age related nuclear cataract-oxidation is the key. Exp Eye Res 80: 709-725
- Urbak L, Vorum H (2010) Heat shock proteins in the human eye. Int J Proteomics 2010.
- Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22: 631-677.
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281-297.
- Qin AY, Zhang XW, Liu L, Yu JP, Li H, et al. (2013) MiR-205 in cancer: An angel or a devil?. Eur J Cell Biol 92: 54-60.
- Vosgha H, Salajegheh A, Smith RA, Lam AK (2014) The important roles of mir-205 in normal physiology, cancers and as a potential therapeutic target. Curr Cancer Drug Targets 14: 621-637.
- McKenna DJ, Patel D, McCance DJ (2014) MiR-24 and miR-205 expression is dependent on HPV onco-protein expression in keratinocytes. Virology 448: 210-216.
- Korlimbinis A, Berry Y, Thibault D, Schey KL, Truscott RJ (2009) Protein aging: Truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res 88: 966-973.
- Brennan LA, Kantorow WA, Chauss D, McGreal R, He S, et al. (2012) Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis 18: 1773-1786.
- Kurzak JR, Zoltoski RK, Sivertson C (2004) Fiber cells organization in crystalline lenses. Exp Eye Res 78: 673-687.
- Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, et al. (2006) The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci 47: 3951-3959.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402-408.
- Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46: 69-81.
- Barber RD, Harmer DW, Coleman RA, Clark BJ (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21: 389-395.
- Wu C, Lin H, Wang Q, Chen W, Luo H, et al. (2012) Discrepant expression of microRNAs in transparent and cataractous human lenses. Invest Ophthalmol Vis Sci 53: 3906-3912.
- Dong H, Lei J, Ding L, Wen Y, Ju H, et al. (2013) MicroRNA: Function, detection, and bioanalysis. Chem Rev 8: 6207-6033.
- Yu J, Peng H, Ruan Q, Fatima A, Getsios S, et al. (2010) MicroRNA-205 protects keratinocyte migration via the lipid phosphate SHIP2. FASEB J 24: 3950-3959.
- Mouillet JF, Chu T, Nelson DM, Mishima T, Sadovsky Y (2010) Mir-205 silences MED1 om hypoxic primary human trophoblast. FASEB J 24: 2030-2039.
- Burmester T, Ebner B, Weich B, Hankeln T (2002) Cytoglobin: A novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol 19: 416-421.
- Ostojic J, Grozdanic S, Syed NA, Hargrove MS, Trent JT 3rd, et al. (2008) Neuroglobin and cytoglobin distribution in the anterior eye segment: A comparative immunohistochemical study. J Histochem Cytochem 56: 863-872.
- Park SH, Ozden O, Jiang H, Cha YI, Pennington JD, et al. (2011) SIRT3, mitochondrial ROS, ageing, and carcinogenesis. Int J Mol Sci 12: 6226-6239.
- Brown K, Xie S, Qiu X, Mohrin M, Shin J, et al. (2013) SIRT3 reverses aging-associated degeneration. Cell Rep 3: 319-327.
- Kincaid B, Bossy-Wetzel E (2013) Forever young: SIRT3 a Shield against mitochondrial meltdown, aging, and neurogeneration. Front Aging Neurosci 5: 48.
- Kelly GS (2010) A review of the Sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Altern Med Rev 15: 245-263.
- Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 14: 3834-3859.
- Quintana FJ, Cohen JR (2011) The HSP60 immune system network. Trends Immunol 32: 89-95.
- Sellitto C, Li L, Gao J, Robinson ML, Lin RZ, et al. (2013) AKT activation promotes PTEN hamartoma tumor syndrome–associated cataract development. J Clin Invest 123: 5401.
- Martinez G, de Longh RU (2010) The lens epithelium in ocular health and disease. Int J Biochem Cell Biol 42: 1945-1963.
- Lovicu FJ, McAvoy JW (2005) Growth factor regulation of lens development. Dev Biol 280: 1-14.
- Garcia-Fernandez RA, Garcia-Palencia P, Suarez C, Sanchez MA, Gil-Gomez G, et al. (2014) Cooperative role between p21cip1/waf1 and p27kip1 in premature senescence in glandular proliferative lesions in mice. Histol Histopathol 29: 397-406.
- Majumder PK, Grisanzio C, O’Connell F, Barry M, Brito JM, et al. (2008) A prostatic intraepithelial neoplasia-dependent p27Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell 14: 146-155.
- Zhu X, Guo K, Lu Y (2011) Selenium effectively inhibits 1,2-dihydroxynaphtalene-induced apoptosis in human lens epithelial cells through activation of PI3-K/Akt pathway. Mol Vis 17: 2019-2027.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Weber GF, Menko AS (2005) The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem 280: 22135-22145.
- Zandy AJ, Lakham S, Zhang T, Flavell RA, Basnett S (2005) Role of the executioner caspases during lens development. J Biol Chem 280: 30263-30272.
- Bagchi M, Katar M, Maisel H (2001) A heat shock transcription factor like protein in the nuclear matrix compartment of the tissue cultured mammalian lens epithelial cell. J Cell Biochem 80: 382-387.
- Caspers GJ, Leunissen JA, de Jong WW (1995) The expanding small heat-shock protein family, and structure predictions of the conserved “alpha-crystallin domain”. J Mol Evol 40: 238-248.
- Ozcan L, Tabas I (2012) Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med 63: 317-328.